Abstract
The infusion of animal-derived antibodies has been known for some time to trigger the generation of antibodies directed at the foreign protein as well as adverse events including cytokine release syndrome. These immunological phenomena drove the development of humanized and fully human monoclonal antibodies. The ability to generate human(ized) antibodies has been both a blessing and a curse. While incremental gains in the clinical efficacy and safety for some agents have been realized, a positive effect has not been observed for all human(ized) antibodies. Many human(ized) antibodies trigger the development of anti-drug antibody responses and infusion reactions. The current belief that antibodies need to be human(ized) to have enhanced therapeutic utility may slow the development of novel animal-derived monoclonal antibody therapeutics for use in clinical indications. In the case of murine antibodies, greater than 20% induce tolerable/negligible immunogenicity, suggesting that in these cases humanization may not offer significant gains in therapeutic utility. Furthermore, humanization of some murine antibodies may reduce their clinical effectiveness. The available data suggest that the utility of human(ized) antibodies needs to be evaluated on a case-by-case basis, taking a cost-benefit approach, taking both biochemical characteristics and the targeted therapeutic indication into account.
Key words: immunogenicity, human anti-mouse antibody, cytokine release syndrome
Introduction
The ability of antibodies to bind with precision to particular biological targets has been harnessed over the last 30 years, resulting in significantly enhanced therapeutic options for patients in numerous disease indications. Originally, all therapeutic antibodies were polyclonal, but discovery of hybridoma technology allowed large volumes of antibodies with a single specificity to be produced. This technology was largely limited to production of murine-derived (usually mouse or rat) antibodies; as such, 80% of all monoclonal antibodies (mAbs) in clinical development in the 1980s were of murine origin.1 Murine-derived antibodies, however, have historically been associated with undesirable properties including short serum half-life and the ability to trigger human anti-mouse antibody (HAMA) or human anti-rat antibody (HARA) development.2,3 Initial advances in the understanding of antibody structure and molecular biology have allowed some murine antibodies to be engineered as chimeric or humanized forms, which resulted in a reduction in these issues for some antibodies.2,3 Further improvements in antibody development technology resulted in phage display libraries and transgenic animals that allowed generation of human antibodies without the need for murine antibodies as starting material.
Human(ized) antibodies are generally viewed as safer alternatives to murine antibodies and are often developed instead of their murine counterpart, should one exist. This trend is evidenced by the small proportion of murine-derived antibodies in development or approved. Analysis of antibody development trends described by Reichert1 suggests that, although 80% of all mAbs in clinical development during the 1980s were of mouse origin, this number dropped to 7% during the 2000s.1 To further address the safety of human(ized) antibodies, we reviewed publically-available data for 38 human(ized) and 43 rodent-derived antibodies that have been tested in humans (Table 1). While there are certainly caveats when comparing antibodies that have been utilized in contrasting indications, particularly with patients in some cases undergoing different background therapies, the collected data suggest that human(ized) and rodent-derived antibodies triggered similar levels of acute phase and infusion reactions. While rodent-derived antibodies appeared to trigger anti-drug-antibody production at higher frequency, this phenomenon was usually negligible, resulting in little to no affect on overall clinical objectives. Interestingly, in some cases increased anti-drug-antibody production resulted in enhanced therapeutic outcome.
Table 1.
Human(ized) and rodent derived antibodies that have been tested in humans
| Antibody INN (Trade name) | Antibody type (Generation Technique) | Target | Observed adverse events | Anti-drug antibodies | References |
| Antibodies targeting cytokines | |||||
| Adalimumab (Humira) | Human (phage display) | TNF | Infections, fever, diarrhea, rash | ++++ Neutralizing | Bender, et al. 2007;48 Coenen, et al. 200749 |
| Golimumab (Simponi) | Human (transgenic mouse) | TNF | Infusion reactions, nausea, infections | + Non-neutralizing | Shealy, et al. 2010,50 Kay, et al. 2010,51 Kay, et al. 200852 |
| Certolizumab pegol (Cimzia) | Humanized Fab | TNF | Abdominal pain, diarrhea, injection site reactions, infection | + Neutralizing | Baker 2009,53 Lichtenstein, et al. 201054 |
| Briakinumab | Human (phage display) | IL12/IL23p40 | Infections, fever, diarrhea, malignancies | Unknown | Gandhi, et al. 201055 |
| Ustekinumab (Stelara) | Human (transgenic mouse) | IL12/IL23p40 | Fatigue, headache, cardiac toxicity, infections | + Neutralizing | Gandhi, et al. 2010,55 Cingoz 200956 |
| Canakinumab (Ilaris) | Human (transgenic mouse) | IL1 | Infections | None Described | Dhimolea 2010,57 Lachmann, et al. 200958 |
| Tocilizumab/Atlizumab (Actemra) | Humanized | IL6 receptor | Infusion reactions, infections, malignancy, anaphylaxis | + Neutralizing | Sharma, et al. 200859 |
| Lerdelimumab | Human (phage display) | TGFα | Eye based infusion-Cataracts, pain, conjunctivitis | + Non-neutralizing | Khaw, et al. 200760 |
| B-E8 | Murine | IL6 | Headache, vomiting, fever, thrombocytopenia | + Non-neutralizing | Rossi, et al. 2005,61 Emilie, et al. 199462 |
| CB6 | Murine | TNF | Infections, headache, vomiting, fever, infusion reactions | +++++ | Fisher, et al. 199363 |
| B-N10 | Murine | IL10 | Infusion reactions | +++++ Neutralizing | Llorente, et al. 200064 |
| Afelimomab | Murine Fab | TNF | Infections, headache, vomiting, fever, infusion reactions | ++ Non-neutralizing | Panacek, et al. 2004,65 Reinhart, et al. 200166 |
| Nerelimomab | Murine | TNF | Serum sickness, hypotension | +++++ | Cohen and Carlet 199667 |
| Antibodies targeting T cells | |||||
| Zanolimumab | Human (transgenic mouse) | CD4 | Infusion reactions, infections, malignancies | + Non-neutralizing | Mestel, et al. 200868 |
| Ipilimumab | Human (transgenic mouse) | CTLA-4 (CD152) | Infusion reactions Anemia/diarrhea Autoimmune enterocolitis Antibody-induced lupus nephritis | None Described | Ansell, et al. 2009,69 Weber, et al. 2009,70 Weber 2009,71 Sanderson, et al. 200572 |
| Tremelimumab | Human (transgenic mouse) | CTLA-4 (CD152) | Fever, diarrhea, chills, endocrine disorders, anti-thyroid disorders | None Described | Kirkwood, et al. 2010,73 Camacho 200874 |
| Alemtuzumab (Campath 1H) | Humanized | CD52 | Infusion reactions, infections, malignancies | +++ Neutralizing | Waldmann and Hale 20053 |
| Teplizumab | Humanized | CD3 | Cytokine release, fever, anemia, vomiting, nausea, arthralgia, headache | +++ Neutralizing | Herold, et al. 200215 |
| Vedolizumab | Humanized | Alpha4 Beta7 Integrin | 92% of subjects experienced AEs (Grade 1), hypersensitivity | ++ Neutralizing | Baumgart 2010,75 Soler, et al. 200976 |
| Visilizumab | Humanized | CD3 | Headache, cytokine release syndrome, fever, rigors, infections | +++ Non-neutralizing | Baumgart, et al. 201077 |
| Zolimomab aritox | Murine, conjugated to ricin toxin | CD5 | Rash, liver toxicity, diarrhea, nausea/vomiting | + Non-neutralizing | Martin, et al. 199678 |
| Muromonab (Orthoclone) | Murine | CD3 | Cytokine release syndrome, pulmonary edema, coagulation disorders | ++++ Neutralizing | Sgro 199579 |
| T10B9 | Murine | αβTCR | Fever, chills | ++ | Waid, et al. 199780 |
| BMA-031 | Murine | αβTCR | Headache, joint pain, muscle stiffness diarrhea | +++ Neutralizing | Knight, et al. 199481 |
| Telimomab & Telimomab Aritox | Murine, conjugated with ricin toxin | T65 antigen | Urticaria, diarrhea, cough, hypotension | None Described | Dillman, et al. 1982,82 Schroff, et al. 198483 |
| 33B.1 | Murine | IL2 receptor | Chills, fever, diarrhea, renal dysfunction | +++++ Neutralizing | Soulillou, et al. 199084 |
| B-F5 | Murine | CD4 | Cytokine release syndrome, nausea, headache, diarrhea | +++ | Rumbach, et al. 1994,85 Racadot, et al. 199386 |
| BTI-322 | Murine | CD2 | Infusion related nausea, vomiting, diarrhea, hypertension, tachycardia | None Detected | Przepiorka, et al. 199887 |
| Antibodies targeting B cells/Antibody isotypes | |||||
| Ofatumumab (Arzerra) | Human (transgenic mouse) | CD20 | Infections (occurred in 70%), infusion reactions, bronchospasm | None Described | Lemery, et al. 2010,88 Wierda, et al. 201089 |
| Belimumab (Benlysta) | Human (phage display) | BlyS | Moderate infusion reactions: headache, rash | + Neutralizing | Ding 2008,90 Furie, et al. 20091 |
| Omalizumab (Xolair) | Humanized | IgE | Anaphylaxis, malignancies, infections, injection site reactions | + Non-neutralizing | Rodrigo, et al. 2010,92 Easthope and Jarvis 200193 |
| Ocrelizumab | Humanized | CD20 | Infections, malignancies | Inversely related to dose, lower doses= +++ Neutralizing | Genovese, et al. 200894 |
| Epratuzumab-90Y | Humanized, radiolabeled | CD22 | Fever, rash, diarrhea, infusion reactions | None Described | Leonard, et al. 2005,95 Leonard, et al. 2004,96 Morschhauser, et al. 201097 |
| RFB4-Ricin A | Murine, conjugated to ricin A toxin | CD22 | Pulmonary edema, tachycardia, fever, infection, vascular leak syndrome | ++ | Vitetta, et al. 199198 |
| Ibritumomab tiuxetan (Zevalin) | Murine, conjugated to tiuxetan | CD20 | Fatigue, nausea, chills, diarrhea, thrombocytopenia, neutropenia | ++ | Wang, et al. 200999 |
| Tositumomab-131I (Bexxar) | Murine, radiolabeled | CD20 | Infusion reactions, hypotension, rigors, fever, wheezing, edema, arthralgia & infections. | + Neutralizing | Kaminski, et al. 2001100 |
| General immune targets (not developed initially to target T cell, B cell or cancer targets) | |||||
| Eculizumab (Soliris) | Humanized | Complement C5 | Infections, fever, nausea, diarrhea | + Non-neutralizing | Dubois and Cohen 2009101 |
| Natalizumab (Tysabri) | Humanized | Alpha4 Beta1 & Alpha4 Beta7 | Infusion reactions, headache, fever, malignancy, infection (PML) | + | Selewski, et al. 2010,102 Johnson 2007,103 Stuve and Bennett 2007104 |
| Efalizumab (Raptiva) | Humanized | CD11a | Infections: sepsis, viral meningitis, PML | ++ | Vincenti, et al. 2007105 |
| Rovelizumab | Humanized | CD18 | Infusion reactions (30%); Infections common | Unknown | Rusnak, et al. 2001106 |
| Denosumab (Prolia) | Human (transgenic mouse) | RANKL | Infections, arthralgia, infusion reactions | ++ Non-neutralizing | Ellis, et al. 2008107 |
| Abciximab (ReoPro) | Chimeric | GPIIb/IIIa | Bleeding disorders | ++ | Brener, et al. 2003108 |
| LM-CD45 (YTH 54.12 & YTH 24.5) | Murine | CD45 | Fever, chills, bronchospasm, urticaria, infections | Unknown | Brenner, et al. 2003,109 Krance, et al. 2003110 |
| MDX-11, PM81 | Murine | CD15 | Fever, chills, Hypotension | + Non-neutralizing | Ball, et al. 1995111 |
| HRS-3/9 | Murine, bispecific | CD30, CD16 | Fever, allergic exanthema, hypotension | +++ Anaphylaxis-inducing | Hartmann, et al. 1997112 |
| Enlimomab | Murine | CD54 (ICAM) | Headache, fever, pneumonia, sepsis, cardiac failure | +++++ Neutralizing | Schneider, et al. 1998113 |
| Vepalimomab | Murine | VAP-1 | Headache, fever, eczema | ++ Neutralizing | Vainio, et al. 2005114 |
| Odulimomab | Murine | CD11a | Infection, thrombocytopenia, neutropenia | +++ | Hourmant, et al. 1996115 |
| ETI-104 | Murine, conjugated to double stranded DNA | Human Complement Receptor 1 | Infusion reactions, headache | +++++ | Iking-Konert, et al. 2004116 |
| Antibodies targeting cancer-associated antigens | |||||
| Panitumumab | Human (transgenic mouse) | EGF receptor | Infusion reactions, pulmonary fibrosis, dermatological toxicity with infectious sequelae | None described | Van Cutsem, et al. 2008,117 Van Cutsem, et al. 2007,118 Cohenuram and Saif 2007119 |
| Zalutumumab | Human (transgenic mouse) | EGF receptor | Infusion reactions, electrolyte imbalances, infections, rash | Unknown | Rivera, et al. 2009120 |
| Ramucirumab | Human (phage display) | VEGF receptor-2 | Dose limiting AE induction, hypertension, liver toxicity, gastrointestinal AE | None described | Spratlin, et al. 2010121 |
| Bevacizumab (Avastin) | Humanized | VEGF | Infusion reactions, hypersensitivity reactions, gastrointestinal perforation, wound healing concerns | None described | Lubner, et al. 2010122 |
| Necitumumab | Human (phage display) | EGF receptor | Rash, grade 3 skin reactions | Unknown | Kuenen, et al. 2010123 |
| Trastuzumab (Herceptin) | Humanized | HER-2 | Heart attack, infusion reactions, infections | + Non-neutralizing | Package insert124 |
| Pertuzumab | Humanized | HER-2 | Infusion reactions (in 50% of patients, grade 3–4), hemolytic uremic syndrome | None Described | Agus, et al. 2005125 |
| Farletuzumab | Humanized | Folate Receptor | AEs occurred in 80% of patients: infusion reactions, hypersensitivity, nausea | Unknown | Ebel, et al. 2007,126 Spannuth, et al. 2010127 |
| Figitumumab | Human (transgenic mouse) | IGF-1R | Fever, hyperglycemia, nausea, diarrhea | None Described | Olmos, et al. 2010,128 Karp, et al. 2009129 |
| L-6 | Chimeric | Tumor Associated Antigen L6 | Fever, nausea, chills | ++ | O'Donnell, et al. 1998130 |
| Anti-CEA-radiolabeled | Chimeric | Carcino-embryonic Antigen | Hematological toxicity | ++ Neutralizing | Buchegger, et al. 1995,131 Behr, et al. 1997132 |
| Anti-CEA-radiolabeled (Iodine 131) | Murine | Carcino-embryonic Antigen | Hematological toxicity | +++++ Neutralizing | Buchegger, et al. 1995131 |
| Anti-CEA-radiolabeled (Rhenium 188) | Murine | Carcino-embryonic Antigen | None described | + | Juweid, et al. 1998133 |
| EMD 559000 | Murine | Epidermal Growth Factor Receptor | Injection reactions | +++++ Non-neutralizing | Faillot, et al. 1996134 |
| Capromab | Murine | Prostate Specific Membrane Antigen | Infusion reactions, Grade 2 leukopenia | + | Deb, et al. 1996135 |
| XMME-OO1-RTA | Murine | Melanoma antigens | Profound fatigue, myalgia, arthralgia, edema. | ++++ | Gonzalez, et al. 1991,136 Spitler, et al. 1987137 |
| KS1/4 MTX-Mab, conjugated | Murine | EpCam | Fever, anorexia, nausea, vomiting, diarrhea, abdominal pain, acute immune complex mediated reaction | ++++ | Elias, et al. 1994,138 Elias, et al. 1990139 |
| Nofetumomab streptavidin | Murine, conjugated to streptavidin | EpCam | Diarrhea, nausea, vomiting, hematological toxicity | ++++ Neutralizing | Knox, et al. 2000140 |
| BIWA1 | Murine | CD44v6 | Fever, Infusion reaction | +++++ Transient | Stroomer, et al. 2000141 |
| MDX-210 | Murine, bispecific | Her-2, CD64 | Fever, malaise, hypertension | ++++ Neutralizing Transient | Valone, et al. 1995142 |
| OC/TR | Murine, bispecific | Ovarian Carcinoma Antigen, CD3 | Infusion reactions, cytokine release | ++++ Increased HAMA (associated with increased efficacy) | Miotti, et al. 199932 |
| Ertumaxomab | Murine, bispecific | Her-2/neu (Mouse), CD3 (Rat) | Infusion reactions, headache, vomiting, rigor, fever, liver toxicity | ++ | Jager, et al. 2009,143 Kiewe, et al. 2006144 |
| Catumaxomab (Removab) | Murine, bispecific | EpCam, (Mouse) CD3 (Rat) | Infusion reactions, headache, vomiting, rigor, fever, liver toxicity | +++++ | Chelius, et al. 2010,9 Sebastian, et al. 2009,145 Seimetz, et al. 201010 |
| Naptumomab estafenatox | Murine Fab, conjugated to Staph. Enterotoxin A | Oncofetal Trophoblast Glycoprotein Antigen 5T Superantigen SAE/E120 | Cytokine release, fever, nausea, diarrhea, chills and hypotension | +++ | Borghaei, et al. 2009146 |
| Metuximab-I131 (Licartin) | Murine Fab radiolabeled | Hepato-cellular Carcinoma Antigen HaB18G/CD147 | Fever, nausea, vomiting, anorexia, stomach ache, diarrhea, infection | + | Chen, et al. 2006147 |
| Gavilimomab | Murine | CD147 | Liver failure, myalgia, fever, hypotension | + | Deeg, et al. 2001148 |
| Pemtumomab-90Y | Murine, radiolabeled | Mucin 1 | Nausea, fatigue, arthralgia, myalgia. abdominal pain, rash, diarrhea, vomiting | +++++ HAMA (associated with increased efficacy) | Oei, et al. 2008,149 Verheijen, et al. 2006150 |
| Anatumomab mafenatox | Murine Fab, conjugated to Staph. Enterotoxin A | 5T4 Oncofetal Antigen | Fever, hypotension, nausea, vomiting | ++++ | Shaw, et al. 2007151 |
| Lym-1 | Murine | HLA-DR10 | Fever, nausea, vomiting, pruritus, urticaria, bronchospasm | ++ HAMA associated with increased efficacy | Azinovic, et al. 2006,31 DeNardo, et al. 2003,5 Kuzel, et al. 1993152 |
| Antibodies targeting infectious antigens | |||||
| Nebacumab (Centoxin) | Human | Endotoxin | Hypotension, anaphylaxis | Unknown | Derkx, et al. 1999153 |
| T88 | Human (Trioma-based fusion technology | Lipopolysaccharide | Hypotension, anaphylaxis | Unknown | Daifuku, et al. 1992,154 Albertson, et al. 2003155 |
| Raxibacumab | Human (phage display) | B. anthracis PA | Infections, headache | Unknown | Migone, et al. 2009156 |
| Edobacomab | Murine | Core Lipid A Region of Bacterial Endotoxin | Limited toxicity described | +++ | Harkonen, et al. 1988157 |
General clinical responses have been described based on the available literature. The ability to induce anti-drug-antibodies is described as a percentage of patients treated, using the following scale, 0–20 (+); 21–40 (++), 41–60 (+++), 61–80 (++++), 81–100 (+++++). In addition, where applicable, the ability of these antibodies to neutralize the monoclonal in question is also described. AE, adverse events; BlyS, B lymphocyte stimulator; CD, cluster of differentiation; CTLA, cytotoxic T lymphocyte antigen 4; EGF, epidermal growth factor; EpCAM, epithelial cell adhesion molecule; GP, glycoprotein; HER, human epidermal growth factor receptor; HLA-DR, human leukocyte antigen DR; ICAM, intracellular adhesion molecule; Ig, Immunoglobulin; IGF-1R, insulin-like growth factor-1 receptor; IL, interleukin; PA, protective antigen; PML, progressive multifocal leukoencephalopathy; RANKL, receptor activator of nuclear factor kappaB ligand; TCR, T-cell receptor; TGF, transforming growth factor; TNF, tumor necrosis factor; VAP, vascular adhesion protein; VEGF, vascular endothelial growth factor; Y, yttrium.
The data show that the assumption that safety gains will be made through human(ization) may be somewhat oversimplified. Reductions in acute phase reactions or anti-drug-antibody production have not been consistently observed. Attempts to reduce the pharmacological activity of certain agents through humanization, such as mitogenic abilities of antibodies targeting T cells, have resulted in only modest safety gains. A recent study highlighted the ability of humanized antibodies to stimulate CD4+ T cells through epitopes located within antibody complementarity determining regions (CDRs).4 On the other hand, events such as cytokine release syndrome (CRS) and antidrug-antibody development may even play critical roles in the mechanism of action of certain monoclonal antibodies.5 Taken together, these findings suggest that interactions between an antibody and the host are highly variable and difficult to predict6–8 and that the fact that an antibody is human or has been humanized does not necessarily translate to a safer, less immunogenic or more effective therapy. While the industry trend has been to favor development of human(ized) antibodies for much of the early 21st century, investigation into the relative benefits of human(ized) antibodies combined with enhanced understanding of disease processes may support the re-emergence of murine-derived antibody therapies. Indeed, the recent European approval of the murine-derived tri-functional antibody catumaxomab supports this position.9,10
Adverse Drug Reactions: Human(ized) Biologics and Immunogenicity
Highly specific and successful therapeutic mAbs have been developed for many disease conditions. Originally, mAbs were generated in mice against tumor antigens,11 and their development provided unique tools to help combat malignancies, including lymphomas and leukemia.11 During this time, development expanded to include those that could be utilized for immune modulation. The first antibody approved by the United States Food and Drug Administration, OKT3, was a highly mitogenic murine anti-CD3 antibody approved for treatment of organ transplant rejection. While immune-modulating murine mAbs such as OKT3 have exhibited excellent therapeutic efficacy, their therapeutic utilization was limited by the association with adverse events arising from immunogenic responses to the therapeutic agent.12–16
Immunogenicity usually refers to the ability of a biologic agent to trigger an anti-drug-antibody response; however, we also include CRS resulting from either acute phase reactions or related to the pharmacological activity of an antibody as a immunogenic event often observed in patients treated with mAbs.17–21
The precise mechanism(s) through which immunogenicity occurs are poorly defined, but it is clear that adverse events are associated with both the pharmacological activity of an antibody, as well as less specific responses.18 Foreign proteins such as murine-derived antibodies are, upon infusion, internalized by cells with antigen presentation capabilities. Subsequent immune responses result in T-cell-dependent antidrug-antibody production.17–21 This process was thought to occur for all murine antibodies; however, subsequent analysis has shown that 20% of murine-derived therapeutic antibodies induce negligible/tolerable levels of HAMA.19
It was hoped that the generation of humanized and fully human antibodies would circumvent the induction of antidrug antibodies altogether, but this has not been the case. For example, humanized mAbs anti-CD3 teplizumab14,16 and anti-CD52 alemtuzumab and human anti-tumor necrosis factor adalimumab, are all capable of triggering human anti-human antibodies (HAHA).7 Alemtuzumab has been shown to cause HAHA responses in 63% of rheumatoid arthritis patients and up to 23% of multiple sclerosis patients in Phase 1 and 2 studies, respectively.7,8,22 Adalimumab can have up to 89% HAHA incidence, even though it is a fully human antibody.4,7,8,13,14,16,17,23–30 The generation of anti-drug-antibodies is usually regarded as a negative outcome, with reductions in efficacy an obvious concern. However, in some instances the formation of high HAMA titers have correlated with increased therapeutic efficacy of certain antibodies.31,32 For instance, increased survival was observed in non-Hodgkin lymphoma patients administered Lym-1 who developed high levels of HAMA.31 The precise mechanism through which HAMA may mediate increased anti-cancer activity remains to be completely defined. However, the formation of idiotypic antibodies against the therapeutic antibody, which may also bind to the targeted epitope, may enhance antibody-mediated tumor destruction through mechanisms such as antibody-dependent cell cytotoxicity or complement-dependent cytotoxicity.5,32
It may also be argued that the formation of anti-drug antibodies is acceptable if clinical objectives have been met, e.g., rabbit anti-thymocyte globulin and alemtuzumab, which are agents utilized in transplantation.33,34 Both are associated with significant anti-drug antibody responses,8,35 but, since both are used as short course immune induction agents, the short term anti-drug globulin response has not been described as a significant clinical concern.
One critical element of mAb therapy is the development of CRS,17 which is the result of excessive secretion of pro-inflammatory mediators. While in some cases CRS may play a critical role in mAb-based therapeutics, it is usually considered to be a significant safety concern. Sensitization to murine components of antibodies has been described as a major cause for CRS; however, CRS is rarely caused by IgE-mediated anaphylaxis. Usually CRS is mild and self-limiting, with acute phase responses, also referred to as first dose reactions, thought to result from antibody-Fc receptor interactions.17,23 However, CRS can be the result of pharmacological interactions, such as those observed in patients treated with TGN1412 and can be life-threatening.24–26 Another example includes the cross-linking ability of OKT3, which may culminate in substantial T-cell activation and cytokine release.16 This response was originally thought to be mostly due to Fc binding and subsequent antigen presentation of OKT3 to T cells, but humanization and the reduction of Fc interactions have led to only modest increases in the safety of anti-CD3 antibodies.13,14,27 Alemtuzumab may also trigger significant CRS, even at low doses.7 CRS in this case has been argued to be associated with alemtuzumab's interaction with its CD52 ligand and its triggering of danger signals associated with cellular depletion and target cell lysis.28 Finally, T-cell epitopes embedded within antibody CDRs, such as those described for golimumab, are also capable of stimulating cytokine release through their T-cell stimulating potential.4
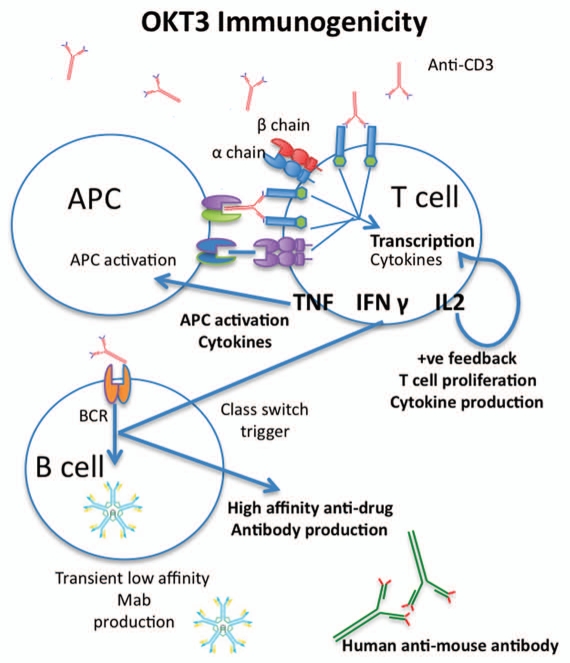
Anti-drug-antibody responses and CRS may appear as separate issues, but CRS may actually potentiate the anti-globulin response, with cytokine being released through target cross-linking (as observed with OKT3; Fig. 1) and possibly danger signals resulting from cellular lysis (as observed for alemtuzumab). Furthermore, IFNγ produced by CD4 T cells activated by CDR epitopes found on some fully human antibodies may also support B-cell production of human anti-human antibodies (HAHA).4 The ability of CRS to support anti-drug globulin responses has been described clinically in renal transplant patients undergoing renal transplantation, whereby chemical immune suppression, with cyclosporine significantly reduces HAMA induction observed in OKT3 treated patients.36
Figure 1.
Immunogenicity of monoclonal antibodies. There are numerous mechanisms that may account for the immunogenicity of monoclonal antibodies. Importantly, many of these phenomena act synergistically, potentiating life-threatening immune responses. In the case of anti-CD3 antibodies such as OKT3, the antibody can bind to the CD3 receptor freely, or in the context of antigen-presenting cells (APCs) through Fc receptor binding. This presentation cross-links the CD3 molecule on the T-cell surface, triggering immunoreceptor tyrosine-based activation motif (ITAM)-mediated T-cell activation. In the case of some human antibodies, complementarity-determining regions may also be presented to the T cells in the context of major histocompatibility complex. No matter the form of T-cell presentation, all trigger ITAM-mediated cytokine production. Some cytokines, including interleukin (IL)-2, act in a positive feedback loop on the T cells, causing further T-cell activation, proliferation and cytokine production. Other cytokines, such as tumor necrosis factor (TNF), mediate febrile responses and, in conjunction with interferon (IFN)γ, activate APCs, which may produce their own set of pro-inflammatory cytokines. In addition to APC activation, IFNγ also stimulates affinity maturation in B cells, also known as class switching. Initially, B cells will produce transient low-affinity antibodies, with little to no clinical relevance. However, cytokines such as IFNγ drive B cells to produce antibodies with high affinity. In the context of protein therapeutics, these antibodies are usually IgG antibodies that may exhibit long serum half lives and neutralizing properties.
The clinical relevance of immunogenicity is far-reaching and highly antibody dependent. Cytokine release is a serious event that can cause death, while the formation of anti-drug antibodies may have much milder consequences. For mAbs, anti-drug globulin responses seem in most cases to be either mild or, at minimum, short-lived.29,30 Indeed, the generation of anti-alemtuzumab antibodies in multiple sclerosis (MS) patients have been mostly described as short-lived and not preclusive to re-dosing; however, the anti-drug antibodies have also been found to reduce the efficacy of alemtuzumab in some patients.8 In addition, HAHA development in adalimumab-treated patients may be neutralizing in up to 89% of patients.4
These examples suggest that the immunogenicity of certain mAbs is not simply explained by the process through which it was derived, with both animal-derived and fully human therapeutic antibodies capable of eliciting anti-drug and cytokine responses. The overall impact of immunogenicity will be ultimately determined by clinical goals and the response observed in treated patients; the immunogenicity data will aid in the assessment of safety versus the overall benefits of disease reduction associated with biologics.
Monoclonal Antibody Therapy in Oncology
Murine antibody humanization may enhance the serum half-life of mAbs, a feature that is often considered to be an important element for enhancing therapeutic efficacy. For example, such an effect was observed upon humanization of the rat antibody CAMPATH-1G, which yielded alemtuzumab (Campath-1H). Alemtuzumab was first approved for chronic lymphocytic leukemia in 2001.3 Comparisons of alemtuzumab to its rat predecessor showed that the humanized form of this antibody exhibited a longer half-life and enhanced therapeutic effect in lymphocytic leukemia patients.3 The increase in half-life, however, has also been suggested to play a significant role in the high levels of infection, malignancies and autoimmune disease development observed in alemtuzumab-treated patients.3,6,22,37,38 While these risks become less of a concern in cases of terminal cancer, they may pose a significant issue during treatment of patients requiring longer-term administration, including those with early stage cancers or chronic disorders such as type 1 diabetes (T1D), MS and other autoimmune diseases.
The recent approval of the rat/mouse hybrid tri-functional antibody, catumaxomab, suggests that the ability of mAbs to destroy malignant cells is of significant importance. In this case, the product was approved in the European Union due to the overall benefit of catumaxomab to patients suffering malignant ascites, even though it is associated with numerous adverse events, including anti-drug antibody development and CRS.10 The induction of CRS may play a significant role in the therapeutic utility of catumaxomab. The induction of pro-inflammatory cytokines has been suggested to play an important role in switching cytokine profiles from those that may favor tumor development to one that drives immune-mediated tumor destruction, suggesting that CRS may be an important event in the efficacy of future anti-cancer monoclonal antibody therapies.
In addition, the precise reasons for the observation of the enhanced effectiveness of murine Lym-1 in patients who developed HAMA31,30 remain to be defined, but the results highlight the need for further research into the properties of HAMA besides the well-known ability of these antibodies to neutralize therapeutic agents. The data suggest that the generation of anti-drug antibodies should not preclude the testing of such agents in certain disease conditions.
Monoclonal Antibody Therapy in T-Cell-Mediated Autoimmune Disease
Scientific and clinical evidence supporting the use of mAb therapies in autoimmune diseases such as T1D and MS is mounting.13–15,27,39,40 Data suggest that therapies that specifically target activated pathogenic T cells while leaving other elements of the immune system unaltered are likely to have the greatest success in treating T-cell-mediated autoimmune disease. T1D and MS are considered to be chronic diseases in which the severity of symptoms increases over time and currently available therapies fail to effectively inhibit disease progression in the majority of patients. As a result, patients will eventually succumb to the disease. Studies of the pathogenesis of T1D and MS clinically and in animal models have uncovered a unique phenomenon, known as epitope spreading,41,42 in which a cascade of responses to different auto-antigens arise over the course of the disease.43,44 Experimental and clinical data have shown that early intervention in this cascade (before irreparable damage to the targeted organ has occurred) can have dramatic and long-term positive effects.
In animal models, short course immune induction therapy (SCIIT) with mAbs against murine αβ T-cell receptor (TCR) or CD3 is capable of preventing diabetes development40,45 and is effective therapy in the experimental autoimmune encephalomyelitis model of MS.46 Importantly, these findings have recently been translated to human clinical disease. Clinical trials using SCIIT in T1D patients have also shown promise,13,14,27 with humanized anti-CD3 resulting in reduced insulin requirements, in some cases lasting many years. Unfortunately, the humanized anti-CD3 therapies tested were still associated with anti-drug antibody development, CRS or reactivation of Epstein-Barr virus.13,14,27 These data validate development of mAbs that target T cells as methods for modifying these autoimmune diseases, but support the need to generate therapeutics with less severe adverse events. Humanization of CD3 antibodies has not necessarily provided this increase in safety, suggesting that increased understanding of host-biologic interactions is required. With this in mind, it is possible that other T-cell targets will provide similar therapeutic outcomes without the same adverse events. Other T-cell antibodies in clinical development, such as alefacept (anti-CD2)47 or TOL101 (anti-αβTCR antibody), may provide this T-cell targeting profile. Preliminary data with alefacept suggest that targeting CD2 may only offer moderate T-cell manipulation and questions remain regarding safety concerns, which will require further analysis.47 Further work is required to validate using anti-αβTCR to target T cells. Unlike the CD3 proteins, the αβTCR antibody lacks intracellular immuno-receptor tyrosine-based activation motifs (ITAMS), which are in part responsible for the mitogenic effects of anti-CD3 antibodies. As such, targeting the αβTCR may provide a unique method for inactivating T cells.
Conclusion
Many factors should be taken into consideration when making the decision to humanize a murine mAb. The inherent immunogenicity of the murine protein plays a critical role, but, as evidenced by studies of humanized anti-CD3 anti-bodies and a number of fully human antibodies, engineering of human/humanized antibodies is not guaranteed to completely reduce or prevent immunogenic issues including anti-drug antibody development. The therapeutic target may also need to be considered, for example, targeting the major TCR signaling protein CD3 results in mitogenic effects, which is not surprising. The indication also plays a substantial role in the decision of whether or not to humanize an antibody. In an indication such as cancer, in which long half-lives have been shown to play a beneficial role in tumor clearance, humanization can have benefits. Alemtuzumab has demonstrated the favorable effects of long half-life in cancer indications. In contrast, in indications in which short course immune induction therapy has been shown to be significantly advantageous, e.g., T1D or MS, the rapid clearance of non-human antibodies may prove to be a beneficial feature.
Another complicated factor in mAb development may reside in the contribution of cytokine release and anti-drug-antibody development. It appears, at least in some cancer indications, that both of these factors may play significant roles in increasing the efficacy of certain biologics.
In conclusion, the utility of humanized antibodies hinges on the immunogenicity of the original antibody in combination with the characteristics of the targeted therapeutic indication. Moving immediately to testing fully human antibodies may not only fail to provide the safety and efficacy desired, but may also result in discounting future testing of targets that hold significant therapeutic promise. In some cases, a humanized murine antibody may provide a unique therapeutic advantage in one disease setting, while its murine predecessor may show increased efficacy in others. Important steps in the future will be to identify and design predictive models that may aid in the resolution of appropriate forms of certain biologics for therapeutic use.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/13601
References
- 1.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal therapeutics. Nat Rev Drug Discov. 2010;9:767–767. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 2.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20:460–470. doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann H, Hale G. CAMPATH: from concept to clinic. Philos Trans R Soc Lond B Biol Sci. 2005;360:1707–1711. doi: 10.1098/rstb.2005.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding FA, Stickler MM, Razo J, Dubridge RB. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNardo GL, Bradt BM, Mirick GR, DeNardo SJ. Human antiglobulin response to foreign antibodies: therapeutic benefit? Cancer Immunol Immunother. 2003;52:309–316. doi: 10.1007/s00262-002-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H) J Clin Invest. 2009;119:2052–2061. doi: 10.1172/JCI37878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau T, Coles A, Wing M, Isaacs J, Hale G, Waldmann H, et al. Transient increase in symptoms associated with cytokine release in patients with multiple sclerosis. Brain. 1996;119:225–237. doi: 10.1093/brain/119.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt ME, Maddison PJ, Bulpitt KJ, Hazleman BL, Urowitz MB, Sturrock RD, et al. CAMPATH-1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis. An intravenous dose-escalation study. Arthritis Rheum. 1995;38:1589–1594. doi: 10.1002/art.1780381110. [DOI] [PubMed] [Google Scholar]
- 9.Chelius D, Ruf P, Gruber P, Ploscher M, Liedtke R, Gansberger E, et al. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs. 2010;2:309–319. doi: 10.4161/mabs.2.3.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAMxanti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–467. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26:1774–1777. doi: 10.1200/JCO.2007.15.7438. [DOI] [PubMed] [Google Scholar]
- 12.Herold KC. Treatment of type 1 diabetes mellitus to preserve insulin secretion. Endocrinol Metab Clin North Am. 2004;33:93–111. doi: 10.1016/j.ecl.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOK-T3gamma1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 16.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 17.Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs. 2010;2:233–255. doi: 10.4161/mabs.2.3.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke JB. Mechanisms of adverse drug reactions to biologics. Handb Exp Pharmacol. 2010:453–474. doi: 10.1007/978-3-642-00663-0_16. [DOI] [PubMed] [Google Scholar]
- 19.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Muller PY, Brennan FR. Safety assessment and dose selection for first-in-human clinical trials with immunomodulatory monoclonal antibodies. Clin Pharmacol Ther. 2009;85:247–258. doi: 10.1038/clpt.2008.273. [DOI] [PubMed] [Google Scholar]
- 21.Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol. 2009;20:722–729. doi: 10.1016/j.copbio.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 23.Chung DT, Korn T, Richard J, Ruzek M, Kohm AP, Miller S, et al. Anti-thymocyte globulin (ATG) prevents autoimmune encephalomyelitis by expanding myelin antigen-specific Foxp3+ regulatory T cells. Int Immunol. 2007;19:1003–1010. doi: 10.1093/intimm/dxm078. [DOI] [PubMed] [Google Scholar]
- 24.Puellmann K, Beham AW, Kaminski WE. Cytokine storm and an anti-CD28 monoclonal antibody. N Engl J Med. 2006;355:2592–2593. doi: 10.1056/NEJMc062750. [DOI] [PubMed] [Google Scholar]
- 25.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 26.Takita M, Matsumura T, Kami M. Cytokine storm and an anti-CD28 monoclonal antibody. N Engl J Med. 2006;355:2593. [PubMed] [Google Scholar]
- 27.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 28.Somerfield J, Hill-Cawthorne GA, Lin A, Zandi MS, McCarthy C, Jones JL, et al. A novel strategy to reduce the immunogenicity of biological therapies. J Immunol. 2010;185:763–768. doi: 10.4049/jimmunol.1000422. [DOI] [PubMed] [Google Scholar]
- 29.Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462. doi: 10.1038/nrd818. [DOI] [PubMed] [Google Scholar]
- 30.Shankar G, Shores E, Wagner C, Mire-Sluis A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol. 2006;24:274–280. doi: 10.1016/j.tibtech.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Azinovic I, DeNardo GL, Lamborn KR, Mirick G, Goldstein D, Bradt BM, et al. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother. 2006;55:1451–1458. doi: 10.1007/s00262-006-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JW, et al. Level of anti-mouse-antibody response induced by bi-specific monoclonal antibody OC/TR in ovarian-carcinoma patients is associated with longer survival. Int J Cancer. 1999;84:62–68. doi: 10.1002/(sici)1097-0215(19990219)84:1<62::aid-ijc12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 34.Safdar N, Smith J, Knasinski V, Sherkow C, Herrforth C, Knechtle S, et al. Infections after the use of alemtuzumab in solid organ transplant recipients: a comparative study. Diagn Microbiol Infect Dis. 2010;66:7–15. doi: 10.1016/j.diagmicrobio.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Book BK, Pescovitz MD, Agarwal A, Hardwick LL, Henson SL, Milgrom ML, et al. In vitro monitoring of in vivo development of human anti-thymoglobulin antibodies by ELISA. Transplant Proc. 2006;38:2869–2871. doi: 10.1016/j.transproceed.2006.08.124. [DOI] [PubMed] [Google Scholar]
- 36.Broeders N, Wissing KM, Crusiaux A, Kinnaert P, Vereerstraeten P, Abramowicz D. Mycophenolate mofetil, together with cyclosporin A, prevents anti-OKT3 antibody response in kidney transplant recipients. J Am Soc Nephrol. 1998;9:1521–1525. doi: 10.1681/ASN.V981521. [DOI] [PubMed] [Google Scholar]
- 37.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 38.Jones JL, Coles AJ. Campath-1H treatment of multiple sclerosis. Neurodegener Dis. 2008;5:27–31. doi: 10.1159/000109935. [DOI] [PubMed] [Google Scholar]
- 39.Chatenoud L. Restoration of self-tolerance is a feasible approach to control ongoing beta-cell specific autoreactivity: its relevance for treatment in established diabetes and islet transplantation. Diabetologia. 2001;44:521–536. doi: 10.1007/s001250051658. [DOI] [PubMed] [Google Scholar]
- 40.Chatenoud L. The use of CD3-specific antibodies in autoimmune diabetes: a step toward the induction of immune tolerance in the clinic. Handb Exp Pharmacol. 2008:221–236. doi: 10.1007/978-3-540-73259-4_10. [DOI] [PubMed] [Google Scholar]
- 41.Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderlugt CL, Begolka WS, Neville KL, Katz-Levy Y, Howard LM, Eagar TN, et al. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunol Rev. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 43.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 45.Sempe P, Bedossa P, Richard MF, Villa MC, Bach JF, Boitard C. Anti-alpha/beta T cell receptor monoclonal antibody provides an efficient therapy for autoimmune diabetes in nonobese diabetic (NOD) mice. Eur J Immunol. 1991;21:1163–1169. doi: 10.1002/eji.1830210511. [DOI] [PubMed] [Google Scholar]
- 46.Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, et al. Treatment with non-mitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 47.Scheinfeld N. Alefacept: a safety profile. Expert Opin Drug Saf. 2005;4:975–985. doi: 10.1517/14740338.4.6.975. [DOI] [PubMed] [Google Scholar]
- 48.Bender NK, Heilig CE, Droll B, Wohlgemuth J, Armbruster FP, Heilig B. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatol Int. 2007;27:269–274. doi: 10.1007/s00296-006-0183-7. [DOI] [PubMed] [Google Scholar]
- 49.Coenen MJ, Toonen EJ, Scheffer H, Radstake TR, Barrera P, Franke B. Pharmacogenetics of anti-TNF treatment in patients with rheumatoid arthritis. Pharmacogenomics. 2007;8:761–773. doi: 10.2217/14622416.8.7.761. [DOI] [PubMed] [Google Scholar]
- 50.Shealy D, Cai A, Staquet K, Baker A, Lacy ER, Johns L, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha. MAbs. 2010;2:428–439. doi: 10.4161/mabs.2.4.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kay J, Rahman MU. Golimumab: A novel human anti-TNFalpha monoclonal antibody for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis. Core Evid. 2010;4:159–170. doi: 10.2147/ce.s6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–975. doi: 10.1002/art.23383. [DOI] [PubMed] [Google Scholar]
- 53.Baker DE. Certolizumab pegol for the treatment of Crohn's disease. Expert Rev Clin Immunol. 2009;5:683–691. doi: 10.1586/eci.09.48. [DOI] [PubMed] [Google Scholar]
- 54.Lichtenstein GR, Thomsen OO, Schreiber S, Lawrance IC, Hanauer SB, Bloomfield R, et al. Continuous therapy with certolizumab pegol maintains remission of patients with Crohn's disease for up to 18 months. Clin Gastroenterol Hepatol. 2010;8:600–609. doi: 10.1016/j.cgh.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi M, Alwawi E, Gordon KB. Anti-p40 antibodies ustekinumab and briakinumab: blockade of interleukin-12 and interleukin-23 in the treatment of psoriasis. Semin Cutan Med Surg. 2010;29:48–52. doi: 10.1016/j.sder.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Cingoz O. Ustekinumab. MAbs. 2009;1:216–221. doi: 10.4161/mabs.1.3.8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 59.Sharma R, Sharma CL, Mahajan A. Biological agents targeting beyond TNFalpha. Indian J Crit Care Med. 2008;12:181–189. doi: 10.4103/0972-5229.45079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khaw P, Grehn F, Hollo G, Overton B, Wilson R, Vogel R, et al. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114:1822–1830. doi: 10.1016/j.ophtha.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 61.Rossi JF, Fegueux N, Lu ZY, Legouffe E, Exbrayat C, Bozonnat MC, et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant. 2005;36:771–779. doi: 10.1038/sj.bmt.1705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emilie D, Wijdenes J, Gisselbrecht C, Jarrousse B, Billaud E, Blay JY, et al. Administration of an anti-interleukin-6 monoclonal antibody to patients with acquired immunodeficiency syndrome and lymphoma: effect on lymphoma growth and on B clinical symptoms. Blood. 1994;84:2472–2479. [PubMed] [Google Scholar]
- 63.Fisher CJ, Jr, Opal SM, Dhainaut JF, Stephens S, Zimmerman JL, Nightingale P, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. The CB0006 Sepsis Syndrome Study Group. Crit Care Med. 1993;21:318–327. doi: 10.1097/00003246-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 65.Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 66.Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Mestel DS, Beyer M, Mobs M, Steinhoff M, Sterry W, Assaf C. Zanolimumab, a human monoclonal antibody targeting CD4 in the treatment of mycosis fungoides and Sezary syndrome. Expert Opin Biol Ther. 2008;8:1929–1939. doi: 10.1517/14712590802528696. [DOI] [PubMed] [Google Scholar]
- 69.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 71.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 73.Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 74.Camacho LH. Novel therapies targeting the immune system: CTLA4 blockade with tremelimumab (CP-675,206), a fully human monoclonal antibody. Expert Opin Investig Drugs. 2008;17:371–385. doi: 10.1517/13543784.17.3.371. [DOI] [PubMed] [Google Scholar]
- 75.Baumgart DC. Veto on vedolizumab (MLN0002) for Crohn's disease. Inflamm Bowel Dis. 2010;16:537–538. doi: 10.1002/ibd.21078. [DOI] [PubMed] [Google Scholar]
- 76.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- 77.Baumgart DC, Targan SR, Dignass AU, Mayer L, van Assche G, Hommes DW, et al. Prospective randomized open-label multicenter phase I/II dose escalation trial of visilizumab (HuM291) in severe steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:620–629. doi: 10.1002/ibd.21084. [DOI] [PubMed] [Google Scholar]
- 78.Martin PJ, Nelson BJ, Appelbaum FR, Anasetti C, Deeg HJ, Hansen JA, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88:824–830. [PubMed] [Google Scholar]
- 79.Sgro C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review. Toxicology. 1995;105:23–29. doi: 10.1016/0300-483x(95)03123-w. [DOI] [PubMed] [Google Scholar]
- 80.Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown S, Kryscio R, et al. Treatment of renal allograft rejection with T10B9.1A31 or OKT3: final analysis of a phase II clinical trial. Transplantation. 1997;64:274–281. doi: 10.1097/00007890-199707270-00017. [DOI] [PubMed] [Google Scholar]
- 81.Knight RJ, Kurrle R, McClain J, Racenberg J, Baghdahsarian V, Kerman R, et al. Clinical evaluation of induction immunosuppression with a murine IgG2b monoclonal antibody (BMA 031) directed toward the human alpha/beta-T cell receptor. Transplantation. 1994;57:1581–1588. [PubMed] [Google Scholar]
- 82.Dillman RO, Shawler DL, Sobol RE, Collins HA, Beauregard JC, Wormsley SB, et al. Murine monoclonal antibody therapy in two patients with chronic lymphocytic leukemia. Blood. 1982;59:1036–1045. [PubMed] [Google Scholar]
- 83.Schroff RW, Farrell MM, Klein RA, Oldham RK, Foon KA. T65 antigen modulation in a phase I monoclonal antibody trial with chronic lymphocytic leukemia patients. J Immunol. 1984;133:1641–1648. [PubMed] [Google Scholar]
- 84.Soulillou JP, Cantarovich D, Le Mauff B, Giral M, Robillard N, Hourmant M, et al. Randomized controlled trial of a monoclonal antibody against the interleukin-2 receptor (33B3.1) as compared with rabbit antithymocyte globulin for prophylaxis against rejection of renal allografts. N Engl J Med. 1990;322:1175–1182. doi: 10.1056/NEJM199004263221702. [DOI] [PubMed] [Google Scholar]
- 85.Rumbach L, Racadot E, Bataillard M, Galmiche J, Henlin JL, Trutmann M, et al. [Open therapeutic trial of anti-T CD4 monoclonal antibody in multiple sclerosis] Rev Neurol (Paris) 1994;150:418–424. [PubMed] [Google Scholar]
- 86.Racadot E, Rumbach L, Bataillard M, Galmiche J, Henlin JL, Truttmann M, et al. Treatment of multiple sclerosis with anti-CD4 monoclonal antibody. A preliminary report on B-F5 in 21 patients. J Autoimmun. 1993;6:771–786. doi: 10.1006/jaut.1993.1063. [DOI] [PubMed] [Google Scholar]
- 87.Przepiorka D, Phillips GL, Ratanatharathorn V, Cottler-Fox M, Sehn LH, Antin JH, et al. A phase II study of BTI-322, a monoclonal anti-CD2 antibody, for treatment of steroid-resistant acute graft-versus-host disease. Blood. 1998;92:4066–4071. [PubMed] [Google Scholar]
- 88.Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, et al. US Food and Drug Administration approval: Ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010;16:4331–4338. doi: 10.1158/1078-0432.CCR-10-0570. [DOI] [PubMed] [Google Scholar]
- 89.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding C. Belimumab, an anti-BLyS human monoclonal antibody for potential treatment of inflammatory autoimmune diseases. Expert Opin Biol Ther. 2008;8:1805–1814. doi: 10.1517/14712598.8.11.1805. [DOI] [PubMed] [Google Scholar]
- 91.Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10:109. doi: 10.1186/ar2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab versus placebo as add on therapy to corticosteroids for children and adults with asthma: A systematic review. Chest. 2010 doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 93.Easthope S, Jarvis B. Omalizumab. Drugs. 2001;61:253–260. doi: 10.2165/00003495-200161020-00008. [DOI] [PubMed] [Google Scholar]
- 94.Genovese MC, Kaine JL, Lowenstein MB, Del Giudice J, Baldassare A, Schechtman J, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:2652–2661. doi: 10.1002/art.23732. [DOI] [PubMed] [Google Scholar]
- 95.Leonard JP, Coleman M, Ketas J, Ashe M, Fiore JM, Furman RR, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 96.Leonard JP, Coleman M, Ketas JC, Chadburn A, Furman R, Schuster MW, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 97.Morschhauser F, Kraeber-Bodere F, Wegener WA, Harousseau JL, Petillon MO, Huglo D, et al. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: results of a multicenter, phase I/II study in non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:3709–3716. doi: 10.1200/JCO.2009.27.7863. [DOI] [PubMed] [Google Scholar]
- 98.Vitetta ES, Stone M, Amlot P, Fay J, May R, Till M, et al. Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res. 1991;51:4052–4058. [PubMed] [Google Scholar]
- 99.Wang M, Oki Y, Pro B, Romaguera JE, Rodriguez MA, Samaniego F, et al. Phase II study of yttrium-90-ibritumomab tiuxetan in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:5213–5218. doi: 10.1200/JCO.2009.21.8545. [DOI] [PubMed] [Google Scholar]
- 100.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 101.Dubois EA, Cohen AF. Eculizumab. Br J Clin Pharmacol. 2009;68:318–319. doi: 10.1111/j.1365-2125.2009.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Selewski DT, Shah GV, Segal BM, Rajdev PA, Mukherji SK. Natalizumab (Tysabri) AJNR Am J Neuroradiol. 2010;31:1588–1589. doi: 10.3174/ajnr.A2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson KP. Natalizumab (Tysabri) treatment for relapsing multiple sclerosis. Neurologist. 2007;13:182–187. doi: 10.1097/01.nrl.0000263760.53418.5b. [DOI] [PubMed] [Google Scholar]
- 104.Stuve O, Bennett JL. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev. 2007;13:79–95. doi: 10.1111/j.1527-3458.2007.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K, et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7:1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 106.Rusnak JM, Kopecky SL, Clements IP, Gibbons RJ, Holland AE, Peterman HS, et al. An anti-CD11/CD18 monoclonal antibody in patients with acute myocardial infarction having percutaneous transluminal coronary angioplasty (the FESTIVAL study) Am J Cardiol. 2001;88:482–487. doi: 10.1016/s0002-9149(01)01723-4. [DOI] [PubMed] [Google Scholar]
- 107.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 108.Brener SJ, Ellis SG, Schneider J, Apperson-Hansen C, Topol EJ. Abciximab-facilitated percutaneous coronary intervention and long-term survival—a prospective single-center registry. Eur Heart J. 2003;24:630–638. doi: 10.1016/s0195-668x(02)00812-6. [DOI] [PubMed] [Google Scholar]
- 109.Brenner MK, Wulf GG, Rill DR, Luo KL, Goodell MA, Mei Z, et al. Complement-fixing CD45 monoclonal antibodies to facilitate stem cell transplantation in mouse and man. Ann NY Acad Sci. 2003;996:80–88. doi: 10.1111/j.1749-6632.2003.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 110.Krance RA, Kuehnle I, Rill DR, Mei Z, Pinetta C, Evans W, et al. Hematopoietic and immunomodulatory effects of lytic CD45 monoclonal antibodies in patients with hematologic malignancy. Biol Blood Marrow Transplant. 2003;9:273–281. doi: 10.1053/bbmt.2003.50024. [DOI] [PubMed] [Google Scholar]
- 111.Ball ED, Selvaggi K, Hurd D, Herzig R, Clark L, Malley V, et al. Phase I clinical trial of serotherapy in patients with acute myeloid leukemia with an immunoglobulin M monoclonal antibody to CD15. Clin Cancer Res. 1995;1:965–972. [PubMed] [Google Scholar]
- 112.Hartmann F, Renner C, Jung W, Deisting C, Juwana M, Eichentopf B, et al. Treatment of refractory Hodgkin's disease with an anti-CD16/CD30 bispecific antibody. Blood. 1997;89:2042–2047. [PubMed] [Google Scholar]
- 113.Schneider D, Berrouschot J, Brandt T, Hacke W, Ferbert A, Norris SH, et al. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Eur Neurol. 1998;40:78–83. doi: 10.1159/000007962. [DOI] [PubMed] [Google Scholar]
- 114.Vainio PJ, Kortekangas-Savolainen O, Mikkola JH, Jaakkola K, Kalimo K, Jalkanen S, et al. Safety of blocking vascular adhesion protein-1 in patients with contact dermatitis. Basic Clin Pharmacol Toxicol. 2005;96:429–435. doi: 10.1111/j.1742-7843.2005.pto_05.x. [DOI] [PubMed] [Google Scholar]
- 115.Hourmant M, Bedrossian J, Durand D, Lebranchu Y, Renoult E, Caudrelier P, et al. A randomized multicenter trial comparing leukocyte function-associated antigen-1 monoclonal antibody with rabbit antithymocyte globulin as induction treatment in first kidney transplantations. Transplantation. 1996;62:1565–1570. doi: 10.1097/00007890-199612150-00006. [DOI] [PubMed] [Google Scholar]
- 116.Iking-Konert C, Stocks S, Weinsberg F, Engelbrecht R, Bleck E, Perniok A, et al. First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythematosus: safety and proof of principle of the antigen-heteropolymer ETI-104. Ann Rheum Dis. 2004;63:1104–1112. doi: 10.1136/ard.2003.016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Cutsem E, Siena S, Humblet Y, Canon JL, Maurel J, Bajetta E, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol. 2008;19:92–98. doi: 10.1093/annonc/mdm399. [DOI] [PubMed] [Google Scholar]
- 118.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 119.Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18:7–15. doi: 10.1097/CAD.0b013e32800feecb. [DOI] [PubMed] [Google Scholar]
- 120.Rivera F, Salcedo M, Vega N, Blanco Y, Lopez C. Current situation of zalutumumab. Expert Opin Biol Ther. 2009;9:667–674. doi: 10.1517/14712590902932871. [DOI] [PubMed] [Google Scholar]
- 121.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II consortium study. J Clin Oncol. 2010;28:3491–3497. doi: 10.1200/JCO.2010.28.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuenen B, Witteveen PO, Ruijter R, Giaccone G, Dontabhaktuni A, Fox F, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res. 2010;16:1915–1923. doi: 10.1158/1078-0432.CCR-09-2425. [DOI] [PubMed] [Google Scholar]
- 124.Trastuzumab product label. 1998. www.accessdata.fda.gov/drugsatfda_docs/label/1998/trasgen092598lb.pdf.
- 125.Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 126.Ebel W, Routhier EL, Foley B, Jacob S, McDonough JM, Patel RK, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 127.Spannuth WA, Sood AK, Coleman RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin Biol Ther. 2010;10:431–437. doi: 10.1517/14712591003592069. [DOI] [PubMed] [Google Scholar]
- 128.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karp DD, Pollak MN, Cohen RB, Eisenberg PD, Haluska P, Yin D, et al. Safety, pharmacokinetics and pharmacodynamics of the insulin-like growth factor type 1 receptor inhibitor figitumumab (CP-751,871) in combination with paclitaxel and carboplatin. J Thorac Oncol. 2009;4:1397–1403. doi: 10.1097/JTO.0b013e3181ba2f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O'Donnell RT, DeNardo SJ, Shi XB, Mirick GR, DeNardo GL, Kroger LA, et al. L6 monoclonal antibody binds prostate cancer. Prostate. 1998;37:91–97. doi: 10.1002/(sici)1097-0045(19981001)37:2<91::aid-pros5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 131.Buchegger F, Mach JP, Pelegrin A, Gillet M, Vogel CA, Buclin T, et al. Radiolabeled chimeric anti-CEA monoclonal antibody compared with the original mouse monoclonal antibody for surgically treated colorectal carcinoma. J Nucl Med. 1995;36:420–429. [PubMed] [Google Scholar]
- 132.Behr TM, Sharkey RM, Juweid ME, Dunn RM, Vagg RC, Ying Z, et al. Phase I/II clinical radioimmunotherapy with an iodine-131-labeled anti-carcinoembryonic antigen murine monoclonal antibody IgG. J Nucl Med. 1997;38:858–870. [PubMed] [Google Scholar]
- 133.Juweid M, Sharkey RM, Swayne LC, Griffiths GL, Dunn R, Goldenberg DM. Pharmacokinetics, dosimetry and toxicity of rhenium-188-labeled anti-carcinoembryonic antigen monoclonal antibody, MN-14, in gastrointestinal cancer. J Nucl Med. 1998;39:34–42. [PubMed] [Google Scholar]
- 134.Faillot T, Magdelenat H, Mady E, Stasiecki P, Fohanno D, Gropp P, et al. A phase I study of an anti-epidermal growth factor receptor monoclonal antibody for the treatment of malignant gliomas. Neurosurgery. 1996;39:478–483. doi: 10.1097/00006123-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289–1297. [PubMed] [Google Scholar]
- 136.Gonzalez R, Salem P, Bunn PA, Jr, Zukiwski AA, Lamb R, Benjamin RS, et al. Single-dose murine monoclonal antibody ricin A chain immunotoxin in the treatment of metastatic melanoma: a phase I trial. Mol Biother. 1991;3:192–196. [PubMed] [Google Scholar]
- 137.Spitler LE, del Rio M, Khentigan A, Wedel NI, Brophy NA, Miller LL, et al. Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res. 1987;47:1717–1723. [PubMed] [Google Scholar]
- 138.Elias DJ, Kline LE, Robbins BA, Johnson HC, Jr, Pekny K, Benz M, et al. Monoclonal antibody KS1/4-methotrexate immunoconjugate studies in non-small cell lung carcinoma. Am J Respir Crit Care Med. 1994;150:1114–1122. doi: 10.1164/ajrccm.150.4.7921445. [DOI] [PubMed] [Google Scholar]
- 139.Elias DJ, Hirschowitz L, Kline LE, Kroener JF, Dillman RO, Walker LE, et al. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunconjugate in patients with non-small cell lung carcinoma. Cancer Res. 1990;50:4154–4159. [PubMed] [Google Scholar]
- 140.Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6:406–414. [PubMed] [Google Scholar]
- 141.Stroomer JW, Roos JC, Sproll M, Quak JJ, Heider KH, Wilhelm BJ, et al. Safety and biodistribution of 99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in head and neck cancer patients. Clin Cancer Res. 2000;6:3046–3055. [PubMed] [Google Scholar]
- 142.Valone FH, Kaufman PA, Guyre PM, Lewis LD, Memoli V, Deo Y, et al. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol. 1995;13:2281–2292. doi: 10.1200/JCO.1995.13.9.2281. [DOI] [PubMed] [Google Scholar]
- 143.Jager M, Schoberth A, Ruf P, Hess J, Lindhofer H. The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res. 2009;69:4270–4276. doi: 10.1158/0008-5472.CAN-08-2861. [DOI] [PubMed] [Google Scholar]
- 144.Kiewe P, Hasmuller S, Kahlert S, Heinrigs M, Rack B, Marme A, et al. Phase I trial of the trifunctional anti-HER2 x anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12:3085–3091. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 145.Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, et al. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM x Anti-CD3): results of a phase 1/2 study. J Immunother. 2009;32:195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 146.Borghaei H, Alpaugh K, Hedlund G, Forsberg G, Langer C, Rogatko A, et al. Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4116–4123. doi: 10.1200/JCO.2008.20.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006;65:435–444. doi: 10.1016/j.ijrobp.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 148.Deeg HJ, Blazar BR, Bolwell BJ, Long GD, Schuening F, Cunningham J, et al. Treatment of steroid-refractory acute graft-versus-host disease with anti-CD147 monoclonal antibody ABX-CBL. Blood. 2001;98:2052–2058. doi: 10.1182/blood.v98.7.2052. [DOI] [PubMed] [Google Scholar]
- 149.Oei AL, Moreno M, Verheijen RH, Sweep FC, Thomas CM, Massuger LF, et al. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer. 2008;123:1848–1853. doi: 10.1002/ijc.23725. [DOI] [PubMed] [Google Scholar]
- 150.Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, Lopes A, Soper JT, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 151.Shaw DM, Connolly NB, Patel PM, Kilany S, Hedlund G, Nordle O, et al. A phase II study of a 5T4 oncofoetal antigen tumour-targeted superantigen (ABR-214936) therapy in patients with advanced renal cell carcinoma. Br J Cancer. 2007;96:567–574. doi: 10.1038/sj.bjc.6603567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kuzel T, Rosen ST, Zimmer AM, Silverstein EA, Spies S, Saletan SL, et al. A phase I escalating-dose safety, dosimetry and efficacy study of radiolabeled monoclonal antibody LYM-1. Cancer Biother. 1993;8:3–16. doi: 10.1089/cbr.1993.8.3. [DOI] [PubMed] [Google Scholar]
- 153.Derkx B, Wittes J, McCloskey R. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin Infect Dis. 1999;28:770–777. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 154.Daifuku R, Haenftling K, Young J, Groves ES, Turrell C, Meyers FJ. Phase I study of antilipopolysaccharide human monoclonal antibody MAB-T88. Antimicrob Agents Chemother. 1992;36:2349–2351. doi: 10.1128/aac.36.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Albertson TE, Panacek EA, MacArthur RD, Johnson SB, Benjamin E, Matuschak GM, et al. Multicenter evaluation of a human monoclonal antibody to Enterobacteriaceae common antigen in patients with Gram-negative sepsis. Crit Care Med. 2003;31:419–427. doi: 10.1097/01.CCM.0000045564.51812.3F. [DOI] [PubMed] [Google Scholar]
- 156.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 157.Harkonen S, Scannon P, Mischak RP, Spitler LE, Foxall C, Kennedy D, et al. Phase I study of a murine monoclonal anti-lipid A antibody in bacteremic and nonbacteremic patients. Antimicrob Agents Chemother. 1988;32:710–716. doi: 10.1128/aac.32.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]