Abstract
Neural crest cells (NCCs) are migratory cells that delaminate from the neural tube early in development and then disseminate throughout the embryo to give rise to a wide variety of cell types that are key to the vertebrate body plan. During their journey from the neural tube to their peripheral targets, NCCs progressively differentiate, raising the question of when the fate of an individual NCC is sealed. One hypothesis suggests that the fate of a NCC is specified by target-derived signals emanating from the environment they migrate through, while another hypothesis proposes that NCCs are already specified to differentiate along select lineages at the time they are born in the neural tube, with environmental signals helping them to realize their prespecified fate potential. Alternatively, both mechanisms may cooperate to drive NCC diversity. This review highlights recent advances in our understanding of prespecification during trunk NCC development.
Key words: neural crest cell, multipotent, prespecification, neuropilin, semaphorin, migration, cell fate
Introduction
Since the identification of stem cells more than 40 years ago, much research effort has focused on identifying the molecular and cellular mechanisms that direct undifferentiated multipotent precursors to attain their proper fate. Neural crest cells (NCCs) are a transient population of embryonic stem cells that are specific to vertebrates and instrumental in shaping the body plan. In addition to their anatomical significance, they provide a useful model to answer key questions in stem cell biology, in particular how fate potential is realized in the context of the organism.
NCCs are derived from the neuroectoderm in the dorsal neural folds of the vertebrate embryo. NCC specification in the neuroectoderm is initiated in response to the concerted action of several morphogens, including BMPs, FGFs and WNTs, which then induce the expression of NCC-specifying transcription factors, such as SNAIL1/2, SOX9, SOX10, PAX3, AP2, MSX1, ZIC1 and FOXD3 (reviewed in ref. 1). These transcription factors induce the expression of cell adhesion and morphology-changing molecules that promote delamination from the neural tube in a process known as epithelial-to-mesenchymal transition (EMT). NCCs then follow precise migration paths to their final targets under the control of adhesion molecules and secreted guidance cues before they differentiate into a diverse array of target-appropriate cell types.
Fate-mapping studies in chick and mice have defined the derivatives of NCCs emigrating from different regions along the antero-posterior axis and led to their classification into different groups. Cranial NCCs delaminate from the neural tube before the otic vesicle and give rise to neurons, glia and melanocytes, as well as cranial bones and connective tissue in the head. Cardiac NCCs delaminate from the neural tube between the otic vesicle and third somite and give rise to autonomic neurons and their supporting glia, the smooth muscle coat of the great vessels and also melanocytes. Vagal and sacral NCCs emigrate between somites 1–7 and posterior to somite 28 to give rise to enteric neurons, glia and melanocytes. Finally, trunk NCCs delaminate from somite seven to 28 and give rise to sensory neurons, sympathetic neurons, glia, chromaffin cells and melanocytes.
Two models have been put forward to explain the origin of NCC diversity. The first suggests that NCCs remain multipotent until they are specified by target-derived signals emanating from the environment they migrate through (“environmental conditioning;” Fig. 1A). The alternative model suggests that NCCs are already specified to differentiate along distinct lineages at the time they become a NCC or before they disperse through the body (“prespecification;” Fig. 1B). However, rather than being mutually exclusive, both models together may explain best how NCCs give rise to a vast array of different cell types in a coordinated manner. Here, we discuss several recent publications that address the question of how and when the fate of the trunk NCCs is sealed, with particular emphasis on evidence for prespecification.
Figure 1.
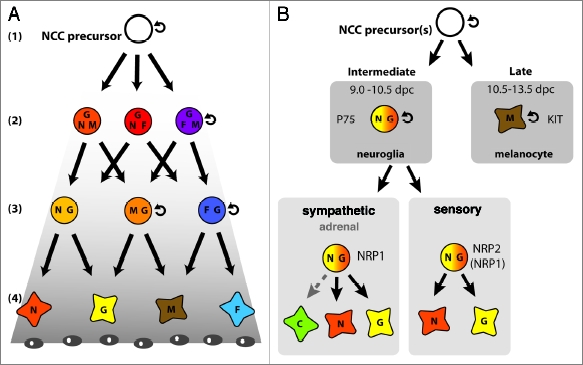
Two alternative models to explain NCC diversity: environmental conditioning of multipotent NCC precursors versus specification of NCCs prior to delamination. (A) The “progressive fate restriction model” predicts a hierarchical succession of NCC fate potential from pluripotent (1) via multipotent (2) to bipotent (3) and finally unipotent (4) progenitors. In this model, NCC precursors and some of their immediate progeny are able to self-renew, like stem cells (curved arrow). However, under the influence of signals emanating from cells in their target environment, for example morphogen gradients (indicated in grey), NCCs progressively differentiate into neurons (N), glia (G), melanocytes (M) and myofibroblasts/smooth muscle cells (F). Based on the timing of first NCC emergence and melanocyte differentiation, the events represented in this cartoon should take place between embryonic day (E) 8.5 and 10.5 in the mouse. (B) The “prespecification model” suggests that NCC precursors are fate-restricted before delamination. This model takes into account that neuroglial NCCs exit the neural tube between E9.0 and 10.5 in the mouse and migrate along a ventral path, whereas melanocytes emigrate between E10.5 and 13.5 and migrate dorsolaterally. This model also explains why p75NTR +ve neuroglial and KIT +ve melanocyte lineages emerge at different times and follow different migratory paths, and why the expression of the NCC guidance receptors NRP1 and NRP2 correlates with NCC fate. Thus, NRP2 is expressed in NCCs contributing to the sensory ganglia, while NRP1 is also expressed in NCCs forming sympathetic ganglia. The stippled arrow indicates that it is not known if NRP1 is expressed in the NCC precursors of chromaffin cells. Note that this cartoon depicts only the intermediate neuroglial and late melanocyte wave, not the early NCC wave. N, neuron; G, glia; M, melanocyte; C, adrenal chromaffin cell.
Fate Restriction of Multipotent NCCs in Response to Environmental Signals
NCC plasticity was first observed in the quail-chick chimera system, devised by Nicole Le Douarin.2,3 She observed that quail vagal NCCs, which are usually fated to become enteric neurons, differentiated into sympathetic neurons when grafted into the chick trunk. By culturing single NCCs from quail trunk explants and observing their clonal expansion, Cohen and Konigsberg (1975) found that individual NCCs had the ability to give rise to both pigmented and unpigmented progeny.4 Seiber-Blum and Cohen (1980) subsequently extended this finding to show that some NCCs in quail neural tube explants formed clones with neuronal and melanocytic progeny.5 By demonstrating that individual NCCs derived from such multipotent clones gave rise to both melanocytes and adrenergic neurons when transplanted to the chick trunk, Bronner-Fraser and colleagues (1980) provided support for the idea that the multipotency observed in vitro was retained in vivo.6 Using mouse neural tube explants, Stemple and Anderson (1992) then identified NCCs with the ability to self-renew and give rise to glia, neurons and myofibroblasts.7 Trentin and colleagues (2004) further substantiated the concept of the multipotent trunk neural crest stem cell in the chick by identifying a precursor that differentiated into all trunk NCC derivatives, including glia, neurons, myofibroblasts and melanocytes.8
As NCCs have the ability to give rise to multiple types of progeny, environmental signals must progressively restrict their fate potential. Thus, a model was proposed in which multipotent NCCs become progressively restricted to become oligopotent, bipotent and then unipotent progenitors under the influence of environmental signals (“environmental conditioning;” Fig. 1A). Importantly, however, the presence of unipotent precursors in the in vitro studies described above suggested that, at the onset of NCC migration, the NCC population is already a heterogeneous mix of cells with different proliferation and differentiation potentials.9
In support of the model for progressive and conditioned fate acquisition, oligopotent NCCs have been identified in chick and zebrafish embryos by single cell tracing.10–12 By labeling either neuroepithelial precursors of NCCs or migrating trunk NCCs, Bronner-Fraser and Fraser (1988 and 1989) provided compelling evidence that individual NCCs can give rise to multiple lineages, with single NCCs giving rise to neuronal and non-neuronal phenotypes in both sympathetic and sensory ganglia.10,11 Although this was not emphasized in their publication, this study also identified other NCCs that gave rise to only a single type of progeny, suggesting that they were prespecified.10 Indeed, using single cell tracing in zebrafish, Raible and Eisen (1994) observed that only 20% of labeled premigratory NCCs (i.e., NCCs that have delaminated from the neural tube, but are not yet migrating) gave rise to multiple derivatives, while the remainder gave rise to single derivatives.13 Thus, similar to earlier in vitro studies, this work suggested that migratory NCCs are a heterogenous mix of fate-specified and multipotent precursors, but that prespecified NCCs predominate.
The Model of NCC Prespecification
A growing body of in vivo evidence suggests that the fate of most trunk NCCs is already decided when they still reside within the neural tube, perhaps at the time when they cease to be neuroepithelial stem cells and commit to the NCC lineage (Fig. 1B). We therefore devote the remainder of this review to discussing studies that support the idea of trunk NCC prespecification. We will approach this topic by discussing current knowledge of regional NCC specification along the dorsoventral (DV) axis and the correlation of NCC fate with the timing of delamination. We will then discuss the relationship between NCC specification, choice of migration path and site of differentiation.
NCC Specification Along the DV Axis Correlates with the Time of Delamination
Cell tracing studies in chick, mouse and zebrafish revealed that trunk NCCs originating from the same axial level differentiate along diverse lineages that colonize their target regions in a ventral to dorsal order (Fig. 2A).10,13–18 Thus, the earliest wave of NCCs in the trunk colonizes the sympathetic ganglia and adrenal anlagen, the next wave colonizes the sensory ganglia, and the final wave gives rise to melanocytes. Strikingly, the timing of NCC delamination correlates with the order of dorsoventral target colonization (Fig. 2A).
Figure 2.
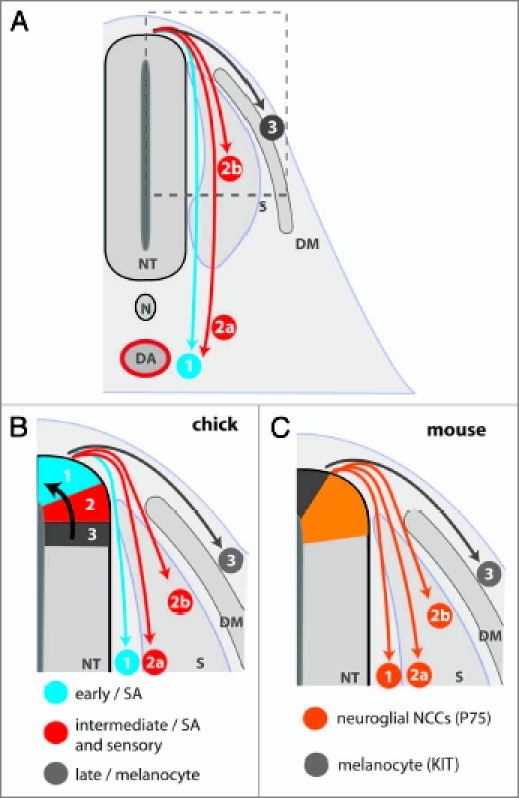
NCC derivatives are colonized in a ventral-dorsal pattern. (A) Schematic representation of a transverse section through the embryo trunk. NCC derivatives are colonized in a ventral to dorsal order. (1) The NCC precursors of the sympathetic ganglia emigrate from the neural tube first and migrate toward the dorsal aorta (DA). (2) The next wave of neural crest cells either migrate through the sclerotome to form sympathetic neurons (2a) or stall within the sclerotome to form the sensory ganglia (2b). (3) Melanocyte precursors emigrate last and scatter beneath the epidermis. (B and C) Schematic representation of the relationship between NCC migration and precursor localization in the neural tube. (B) Spatiotemporal fate map of NCC derivatives in the chick. NCC subpopulations emigrate successively from the neural tube, due to the progressive dorsal relocation of prespecified NCC precursors (black arrow). Thus, the dorsal tip is occupied by successive waves of sympathoadrenal, sensory and finally melanocyte precursors. The earliest wave of NCCs migrates to the dorsal aorta to seed the sympathetic nervous system (1). The intermediate wave of NCCs migrates through the sclerotome to form sympathetic neurons (2a) or stops within the sclerotome to form sensory neurons (2b). The final wave of NCCs migrates into the skin to form melnaocytes (3). (C) Relationship of NCC fate and precursor localization in the mouse neural tube. The KIT-expressing precursors of melanocytes are located at the dorsal tip of the neural tube throughout the period of NCC emigration, while the neuroglial precursors expressing p75 are located in a more ventral domain. The mechanism responsible for the ordered delamination of NCC subpopulations from the mouse neural tube has not yet been determined. The successive waves of NCCs detailed in (B) are represented numerically. NT, neural tube; DA, dorsal aorta; N, notochord; S, sclerotome; DM, dermomyotome.
Henion and Weston (1997) first observed the temporal order of trunk NCC specification in explants of quail neural tubes.14 By tracing single cells, they found that NCCs preferentially differentiated into neurons and glia within the first 6 h after explanting the neural tube into a tissue culture dish. In contrast, NCCs emigrating after 6 h gave rise to melanocytes. The work of Luo and colleagues (2003) extended this finding by showing that two tyrosine kinases distinguish NCC subpopulations that emerged from explanted quail neural tubes.15 Thus, TRKC-positive cells give rise to neurons and glia, while KIT-positive cells give rise to melanocytes. These studies suggested that NCC precursors with a neuroglial versus melanocyte fate arise in different developmental time windows.
Schilling and Kimmel (1994) first provided evidence of this temporal emigration in vivo by labeling single cells in different domains of the zebrafish neural keel, which overlies the neural tube and gives rise to NCCs in this organism.16 By observing the fate of individual NCCs they discovered that the position of a cranial NCC precursor relative to the DV axis predicted its time of emigration and also its fate. Hence, lateral NCC precursors emigrated first and gave rise to neuroglial structures, whereas medial NCC precursors disseminated later to form melanocytes. This work therefore suggests that the time at which a NCC is born influences or perhaps even dictates its fate.
Observations by Wilson and colleagues (2004) suggest that the spatial coordinates of NCC precursors within the mouse neural tube are also linked to the timing of delamination and cell fate. Thus, KIT and the low-density neurotrophin receptor p75NTR are expressed in distinct domains in the mouse neural tube, with p75-positive cells emigrating first to give rise to neuroglial structures, and KIT-expressing cells disseminating later to give rise to melanocytes (Fig. 2C).17
To provide support to the idea that NCC prespecification occurs within the neural tube, Krispin and colleagues (2010) labeled neuroepithelial progenitor cells before they become bona fide NCCs and then correlated cell position within the neural tube with timing of delamination and fate. Specifically, they describe that the first NCCs to delaminate were derived from precursors that were positioned closest to the dorsal ridge and were of sympathetic lineage. Concomitant with the delamination of sympathetic NCC precursors, the sensory NCC precursors, which were previously located in a more ventral stripe, moved dorsally into the ridge. Once in the dorsal ridge, the sensory NCCs delaminated, and their place was then taken by dorsally relocating melanocyte precursors. Accordingly, they propose the existence of a spatiotemporal fate map for NCC precursors (Fig. 2B).19 This sequential ventral-to-dorsal relocation of NCC progenitors within the neural tube correlates temporally with the narrowing and eventual loss of Foxd3 expression, a gene implicated in neuroglial, but not melanocyte specification.20 Finally, the emigration of the melanocyte NCCs exhausted the NCC precursor pool within the neural tube (Fig. 2B).
Figure 3.
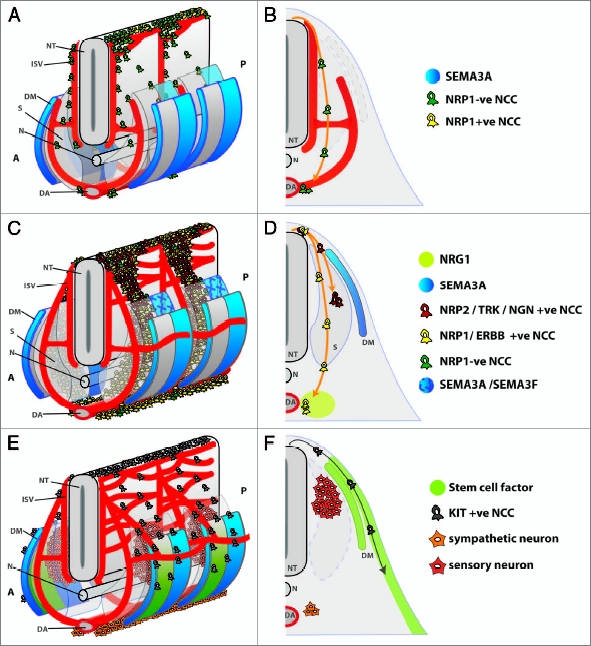
Schematic representation of NCC migration paths in the mouse. (A and B) The first wave of NCCs delaminates from the neural tube (NT) between E8.5 and 9.0 in the mouse and migrates towards the dorsal aorta (DA) to seed the sympathetic chain. (B) Half of a transverse section through the embryonic trunk shows the preferential migration of these NCCs in the intersomitic furrow alongside blood vessels (ISV). A small proportion of NCCs also migrate in the boundary between the anterior and posterior halves of the developing sclerotome (S). (C and D) The intermediate wave of NCCs delaminates between E9.0 and 10.5 in the mouse. These NCCs express NRP1 and are repelled by SEMA3A in the dermomyotome and posterior sclerotome and therefore migrate through the midst of the anterior sclerotome. ERBB2/3-expressing sympathoadrenal NCCs traverse the sclerotome to accumulate at the dorsal aorta in response to attractive NRG1 signals from surrounding mesenchyme. In contrast, sensory NCCs stall within the sclerotome, close to the neural tube. These cells likely express NRP2 in addition to NRP1. The NRP2 ligand SEMA3F is expressed in the posterior sclerotome to ensure that these NCCs remain in the anterior sclerotome. (D) Half of a transverse section through the anterior sclerotome shows the migration path of sympathoadrenal (yellow) and sensory (red) NCCs and highlights molecules that help to segregate their migration paths. (E and F) The late wave of NCCs delaminates between E10.5 and 14.5 in the mouse and gives rise to melanocytes. Stem cell factor in the dermomyotome (DM) and dermis attract KIT-expressing melanocyte precursors. By 10.5 dpc, sensory and sympathetic neurons have begun to differentiate to form the dorsal root ganglia and sympathetic ganglia. (F) Half of a transverse section through the anterior somite shows the characteristic migration path of melanocyte NCCs (grey) in relation to the position of the sensory and sympathetic ganglia. A, anterior; P, posterior; N, notochord. Blood vessels are shown in red.
While these findings support the idea that timing of emigration reflects NCC specification along a particular lineage, earlier studies disagree with particular aspects of this prespecification model. Firstly, Ahlstrom and Erickson (2009) labeled single cells in the dorsal neural tube to observe NCC delamination by time-lapse microscopy and found that delamination is not confined to the dorsal ridge, but instead took place along the entire dorsal neural tube.21 Secondly, the chick data presented by Krispin and colleagues (2010) also differ from observations in the mouse. Thus, Wilson and colleagues (2004) found that presumptive NCC precursors of melanocytes expressing KIT were located in the dorsal most aspect of the neural tube, even though they delaminate after the ventrally located neuroglial precursors expressing p75NTR (Fig. 2C).17 A detailed study of mouse NCC delamination with markers similar to those employed in the chick is now warranted to help establish how similar or perhaps different, the mechanisms of fate determination are across species.
Coordination of Cell Fate and Migratory Pathway
To reach their targets, trunk NCCs migrate along three independent pathways that reflect the timing of their delamination and their appropriate fate.22–24 Even though there are subtle differences how NCCs navigate their environment in different species, NCCs that delaminate first from the neural tube preferentially migrate on a ventral path, in close association with intersomitic blood vessels (Fig. 3A).25 Their destination is the dorsal aorta, where they seed the future sympathetic ganglia.10 It is not yet known if the migration along this vascular path is a consequence of neural crest cells and blood vessels sharing a common substrate or if blood vessels secrete chemoattractive signals or migratory substrates to guide the NCCs. In support of the former possibility, we observed that a small number of mouse NCCs in the early wave trail the boundary between the anterior and posterior half of each somite at the same time as blood vessels invade this area.26 In support of the latter possibility, there are several different classes of molecules expressed by blood vessels, which may mediate a cross-talk of blood vessels and NCCs, for example endothelins,27 ephrins28 and artemin.29
Serbedzija and colleagues (1990) observed that some chick NCCs in the second, i.e., intermediate wave, migrate dorsolaterally.30 While the authors suggest that these cells give rise to melanocytes, their fate was not determined experimentally. Our own work confirmed the existence of rare dorsolaterally migrating NCCs in the intermediate wave in mice on embryonic day (E) 9.5 (see Fig. 4 in ref. 26). However, as KIT-expressing melanocyte precursors have not been seen to migrate before E10 in mice,17 these cells are unlikely to give rise to melanocytes. Importantly, the vast majority of NCCs in the intermediate wave of both mouse and chick travel ventrally into the avascular anterior half of the sclerotome and give rise to neuroglial progeny (Fig. 3B). Intermediate wave NCCs that traverse the anterior sclerotome join the early wave to form the sympathetic trunks alongside the dorsal aorta or continue further to the kidney, where they differentiate into adrenal chromaffin cells. The intermediate wave NCCs that settle in the anterior sclerotome differentiate into the sensory neurons and satellite glia that comprise the dorsal root ganglia.
Recent studies by George and colleagues (2007) support the notion that intermediate wave NCCs know their fate before they encounter the sclerotome environment.31 They labeled single neuroepithelial cells in the dorsal chick neural tube at the end of the temporal window in which intermediate NCCs delaminate and found that the labeled cells gave rise to specific subsets of DRG neurons. Specifically, they identified two distinct subgroups of cells that arose from different regions of the dorsal neural tube. While laterally located cells migrated ipsilaterally after delamination to form proprioceptive and mechanoreceptive neurons in the inner core of the DRG, medially located cells migrated contralaterally to form the bulk of the nociceptive neurons on the perimeter of the DRG. The observation that two different NCC derivatives arise from distinct regions of the dorsal neural tube at the same developmental stage lends strong support to the idea that NCCs become specified when still in the neural tube.
The last wave of NCCs travels dorsolaterally between the dermomyotome and epidermis to give rise to melanocytes (Fig. 3C). To determine the mechanism that underlies the switch from a ventral neuroglial path to a dorsolateral melanocyte path, Erickson and colleagues performed heterochronic transplantations in chicks.23,32 Thus, they transplanted melanocyte precursors to younger hosts, in which NCCs had not yet entered the dorsolateral path. Vice versa, they transplanted neuroglial NCCs into older embryos, in which endogenous NCC migration had already switched to the ventrolateral path. In both experiments, the transplanted cells “remembered” the path they were destined to take prior to transplantation. These findings suggest that the choice of migration route is cell-intrinsic and linked to the time of delamination, rather than dictated by the NCC environment, which changes as development proceeds. In the next section, we will discuss recent publications that identify molecular differences between NCCs migrating along the three different paths in chick and mouse.
Neuropilin Signaling Switches NCC Migration from the Early to Intermediate Path
We have recently shown that the switch in NCC migration from the intersomitic to the sclerotome path is controlled by neuropilin 1 (NRP1), a transmembrane receptor for guidance molecules of the class 3 semaphorin (SEMA3) family.26 Thus, the earliest NCCs, which travel in the intersomitic furrow, express little or no NRP1 (Fig. 3A). In contrast, NCCs on the intermediate path express high levels of NRP1, which should make them sensitive to repulsive signals provided by SEMA3A (Fig. 3B). SEMA3A is expressed in the anterior dermomyotome as well as the posterior dermomyotome and sclerotome, so that its expression domains border the intersomitic furrow.26 Thus, SEMA3A may be secreted into the intersomitic space to repel NRP1-expressing NCCs away from this region into the anterior sclerotome. Consistent with this idea, intermediate wave NCCs in Sema3a- and Nrp1-null mutants adopt the early pattern of migration, as they travel in the intersomitic space.26 It is currently unknown if this mechanism of NCC guidance is conserved in the chick, as previous knockdown studies focused on later time points.33
Molecular Mechanisms Implicated in the Switch of NCC Migration from the Ventral to the Dorsolateral Path
Several receptor-ligand pairs have been implicated in controlling access to the dorsolateral path. In the chick, repulsive slits are expressed by the dermomyotome and ROBO receptors by intermediate, but not late, wave NCCs, to prevent neuroglial NCCs from entering the melanocyte path.34,35 In addition, EPH/ephrin plays a dual role in pathway choice in chicks, as ephrinB is expressed by the dermomyotome to force intermediate wave NCCs onto the ventral path, but acts as a chemoattractant for melanocyte precursors to guide them onto a dorsolateral path.36,37 However, perhaps due to genetic redundancy within the large slit/robo and Eph/ephrin families, there is presently no evidence that either signaling pathway is essential for pathway choice in mice.
In the chick, endothelin ligands (EDN) appear to be chemoattractive for the NCC precursors of melanocytes, which express G-protein coupled EDN receptors (EDNR). In this species, the differential expression of EDNR receptors directs NCC migration along the ventral versus dorsolateral path. Whereas EDNRB is expressed in ventral migrating cells, EDNRB2 is expressed in melanocyte precursors to mediate attraction toward EDN3 in the surface ectoderm.36 The chemoattractive signal controlling ventral migration of EDNRB-expressing NCCs has not yet been described. In mice, EDNRB is essential for melanocyte development.27 Yet, it is not yet known if EDN signaling regulates pathway switching in the mouse, as it does in the chick. Arguing against this possibility, mice have no obvious homolog of EDNRB2, and EDNRB is expressed by both ventrally and dorsolaterally migrating NCCs. Unlike chicks, mice appear to employ the tyrosine kinase receptor KIT to regulate NCC entry into the dorsolateral path.38,39 Thus, the KIT ligand, steel factor, is expressed in the dermomyotome at the onset of melanocyte migration40 (Fig. 3C), and loss of either KIT or steel factor impedes entry of NCCs to the dorsolateral path.39,41
Our own work shows that SEMA3A signaling through NRP1 prevents the precocious switch of NCC migration from the ventral to the dorsolateral path in mice.26 Thus, SEMA3A is expressed in the dermomyotome and NRP1 by NCCs in the intermediate wave, and loss of either molecule leads excessive NCC migration on the dorsolateral path. Because SEMA3A expression in the dermomyotome layer is maintained at least until 11.5 dpc (Schwarz Q and Ruhrberg C, unpublished data), NRP1 downregulation may be a prerequisite for melanocyte NCC entry onto the dorsolateral path.
Neuropilins Control NCC Migration within Somites
NCCs that have been diverted from the intersomitic furrow into the sclerotome by SEMA3A/NRP1 signaling are normally channeled into the anterior half of each somite by repulsive signals from the posterior sclerotome and dermomyotome. Studies in chick implicated several different molecular mechanisms in intrasomitic NCC guidance, including semaphorin-mediated repulsion,33,42 EPH/ephrin signaling,43,44 cadherin-mediated cell interactions,45 integrin-mediated extracellular matrix interactions,46–50 and other ECM interactions.51–54 The best characterized of these mechanisms is semaphorin signaling. Thus, mice lacking SEMA3F or its receptor NRP2 contain NCCs in both the anterior and posterior half of each somite, most likely because repulsive SEMA3F signals from the posterior sclerotome repel NRP2-expressing NCCs as they enter the somite.55 SEMA3A is also expressed by the posterior sclerotome to help restrict NCCs to the anterior sclerotome.26,56 Therefore, SEMA3A plays a dual role in guiding intermediate wave NCCs, firstly by diverting them from the intersomitic furrow into the sclerotome and secondly by cooperating with SEMA3F to restrict migration to the anterior half of the sclerotome. Accordingly, the combined activity of both semaphorins induces the characteristic pattern of trunk NCC migration in segmental streams that is a prerequisite for the segmentation of glial and neuronal NCC progeny into the DRGs.56,57
Segregation of the Sensory and Sympathoadrenal NCC Lineages
While NCCs destined for a sympathoadrenal fate migrate through the sclerotome, NCCs forming sensory neurons have to arrest within the sclerotome. Studies in various mouse knockout models have now identified three molecular pathways that contribute to the segregation of these lineages, neuregulin (NRG)-mediated attraction, semaphorin-mediated repulsion and neurogenin (NGN)-induced gene expression (Fig. 3D).
NCCs destined to become sympathetic or adrenal cell types express the receptor tyrosine kinases ERBB2 and ERBB3 and the ERBB ligand NRG1 is present in the environment the NCCs migrate through. In mouse mutants lacking either one of these three molecules, NCCs accumulate near the dorsal neural tube where DRGs assemble, but fail to reach their normal ventral targets, thus causing hypoplasia of the sympathetic ganglia and adrenal medulla.58
Differential neuropilin expression also impacts on the segregation of the sensory and sympathoadrenal lineages. Thus, our analysis of NRP1 and NRP2 expression in mouse embryos raised the possibility that NCCs expressing NRP1, but not NRP2, are destined for the dorsal aorta, whereas NCCs expressing NRP2 and perhaps also NRP1, are destined to arrest in the sclerotome to become DRGs (Fig. 1B).56 In support of this hypothesis, sympathetic NCC migration is affected in Nrp1- but not in Nrp2-null mutant embryos, but loss of both NRP1 and NRP2 is required to severely disrupt DRG assembly.55,58 Because Nrp1-null mutants contain a small contingent of ectopic sensory neurons,26 a small subset of sensory NCCs may also depend on NRP1 for normal positioning.
The basic helix-loop-helix transcription factors of the NGN family are expressed in the NCC precursors of sensory neurons and are essential for sensory differentiation.59–61 Neurogenins are known to stimulate the expression of the neurotrophin receptors TRKA, TRKB and TRKC to help NCCs survive in the dorsal sclerotome.62–64 Whether neurogenins also control the expression of molecules that arrest NCC migration in the dorsal sclerotome is currently unknown. The selective importance in sensory rather than sympathetic NCCs is demonstrated by the complete lack of DRGs, yet normal formation of sympathetic ganglia in mice mutant for Ngn1 and/or Ngn2.59,62–64
Integration of NCC Fate with Migratory Pathway and Environmental Signals
The lineage tracing studies and transplantation studies discussed in previous sections support the hypothesis that the neuroglial and melanocyte NCC lineages are already prespecified when their precursors still reside in the neural tube and before they embark on a migratory path that is appropriate for their fate. According to this model, NCCs should retain their fate potential, even if their migratory pathway is disrupted by an altered expression of or sensitivity to relevant migratory cues. This was first demonstrated for NCCs specified toward a neuroglial fate in mice.26 Thus, neuroglial NCCs that abnormally enter the dorsolateral path in mice lacking SEMA3A or NRP1 still underwent neuroglial differentiation: Firstly, labeling of the ectopic NCCs with the melanocyte marker Trp2 suggested that they do not differentiate into melanocytes (unpublished data, Schwarz Q and Ruhrberg C). Secondly, the ectopic cells express the sensory marker Brn3b when settling in a dorsal position close the DRGs or the sympathetic marker tyrosine hydroxylase when migrating to the ventral half of the embryo.26 By forcing the expression of EDNRB2 in neuroglial precursors of the chick embryo, Krispin and colleagues confirmed that neuroglial NCCs forced to migrate along the dorsolateral pathway differentiated into neurons rather than melanocytes.19
Together, these findings suggest that intermediate wave NCCs have a neuroglial rather than melanocyte fate and that they remember this fate, even if they migrate along ectopic pathways. According to this model, environmental signals are more important for pathway choice than specification along the neuroglial versus melanocyte lineage. Superimposed on this prespecification, environmental conditioning appears to ensure that neuroglial NCCs differentiate into the type of neuron that is appropriate for the anatomical location. Thus, the recent findings of neuroglial NCC development marry the model of NCC prespecification with the concept of environmental conditioning.
Conclusions and Future Work
In this review, we have discussed current knowledge of the molecular and cellular mechanisms that govern trunk NCC fate, highlighting the concepts of mulipotentiality, prespecification and environmental conditioning. It is likely that similar mechanisms control NCC fate in rostral regions of the embryonic body.65 Yet, it has become apparent that the use of different model organisms with their unique experimental advantages precludes us from generalizing the mechanisms of NCC specification. Future work should aim to identify the molecular and cellular mechanisms that are evolutionarily conserved and therefore lie at the heart of NCC diversity. A second challenge for the future will be the identification of markers that identify the multipotent NCCs in an in vivo setting and distinguish them from fate-restricted NCCs. This will be pivotal for us to understand how multipotency and mechanisms of lineage-restriction cooperate to bestow NCCs with their extraordinary ability to navigate the entire embryo and give rise to diverse derivatives only in appropriate locations.
Acknowledgements
The authors thank Laura Gammill and the unnamed reviewers for their constructive feedback in preparing the manuscript. C.R. is funded by the UK Medical Research Council (G0600993) and Q.S. is funded by the National Health and Medical Research Council, Australia (NHMRC 626980).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/13502
References
- 1.Harris ML, Erickson CA. Lineage specification in neural crest cell pathfinding. Dev Dyn. 2007;236:1–19. doi: 10.1002/dvdy.20919. [DOI] [PubMed] [Google Scholar]
- 2.Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev Biol. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin N. The Neural Crest. New York, NY: Cambridge University Press; 1982. [Google Scholar]
- 4.Cohen AM, Konigsberg IR. A clonal approach to the problem of neural crest determination. Dev Biol. 1975;46:262–280. doi: 10.1016/0012-1606(75)90104-9. [DOI] [PubMed] [Google Scholar]
- 5.Sieber-Blum M, Cohen AM. Clonal analysis of quail neural crest cells: they are pluripotent and differentiate in vitro in the absence of noncrest cells. Dev Biol. 1980;80:96–106. doi: 10.1016/0012-1606(80)90501-1. [DOI] [PubMed] [Google Scholar]
- 6.Bronner-Fraser M, Sieber-Blum M, Cohen AM. Clonal analysis of the avian neural crest: migration and maturation of mixed neural crest clones injected into host chicken embryos. J Comp Neurol. 1980;193:423–434. doi: 10.1002/cne.901930209. [DOI] [PubMed] [Google Scholar]
- 7.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 8.Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101:4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bronner-Fraser M, Fraser S. Developmental potential of avian trunk neural crest cells in situ. Neuron. 1989;3:755–766. doi: 10.1016/0896-6273(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 11.Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- 12.Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- 13.Raible DW, Eisen JS. Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development. 1994;120:495–503. doi: 10.1242/dev.120.3.495. [DOI] [PubMed] [Google Scholar]
- 14.Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- 15.Luo R, Gao J, Wehrle-Haller B, Henion PD. Molecular identification of distinct neurogenic and melanogenic neural crest sublineages. Development. 2003;130:321–330. doi: 10.1242/dev.00213. [DOI] [PubMed] [Google Scholar]
- 16.Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- 17.Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–6162. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- 18.Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- 19.Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- 20.Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–1858. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahlstrom JD, Erickson CA. The neural crest epithelial-mesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 23.Erickson CA, Duong TD, Tosney KW. Descriptive and experimental analysis of the dispersion of neural crest cells along the dorsolateral path and their entry into ectoderm in the chick embryo. Dev Biol. 1992;151:251–272. doi: 10.1016/0012-1606(92)90231-5. [DOI] [PubMed] [Google Scholar]
- 24.Thiery JP, Duband JL, Delouvee A. Pathways and mechanisms of avian trunk neural crest cell migration and localization. Dev Biol. 1982;93:324–343. doi: 10.1016/0012-1606(82)90121-x. [DOI] [PubMed] [Google Scholar]
- 25.Spence SG, Poole TJ. Developing blood vessels and associated extracellular matrix as substrates for neural crest migration in Japanese quail, Coturnix coturnix japonica. Int J Dev Biol. 1994;38:85–98. [PubMed] [Google Scholar]
- 26.Schwarz Q, Maden CH, Vieira JM, Ruhrberg C. Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. Proc Natl Acad Sci USA. 2009;106:6164–6169. doi: 10.1073/pnas.0811521106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pla P, Larue L. Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol. 2003;47:315–325. [PubMed] [Google Scholar]
- 28.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 30.Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- 31.George L, Chaverra M, Todd V, Lansford R, Lefcort F. Nociceptive sensory neurons derive from contralaterally migrating, fate-restricted neural crest cells. Nat Neurosci. 2007;10:1287–1293. doi: 10.1038/nn1962. [DOI] [PubMed] [Google Scholar]
- 32.Erickson CA, Goins TL. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development. 1995;121:915–924. doi: 10.1242/dev.121.3.915. [DOI] [PubMed] [Google Scholar]
- 33.Eickholt BJ, Mackenzie SL, Graham A, Walsh FS, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- 34.Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev Biol. 2005;282:411–421. doi: 10.1016/j.ydbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- 36.Harris ML, Hall R, Erickson CA. Directing pathfinding along the dorsolateral path—the role of EDNRB2 and EphB2 in overcoming inhibition. Development. 2008;135:4113–4122. doi: 10.1242/dev.023119. [DOI] [PubMed] [Google Scholar]
- 37.Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- 38.Reedy MV, Johnson RL, Erickson CA. The expression patterns of c-kit and Sl in chicken embryos suggest unexpected roles for these genes in somite and limb development. Gene Expr Patterns. 2003;3:53–58. doi: 10.1016/s1567-133x(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 39.Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 40.Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- 41.Wehrle-Haller B, Meller M, Weston JA. Analysis of melanocyte precursors in Nf1 mutants reveals that MGF/KIT signaling promotes directed cell migration independent of its function in cell survival. Dev Biol. 2001;232:471–483. doi: 10.1006/dbio.2001.0167. [DOI] [PubMed] [Google Scholar]
- 42.Osborne NJ, Begbie J, Chilton JK, Schmidt H, Eickholt BJ. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. [DOI] [PubMed] [Google Scholar]
- 43.Krull CE, Lansford R, Gale NW, Collazo A, Marcelle C, Yancopoulos GD, et al. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr Biol. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- 44.McLennan R, Krull CE. Ephrin-as cooperate with EphA4 to promote trunk neural crest migration. Gene Expr. 2002;10:295–305. doi: 10.3727/000000002783992389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranscht B, Bronner-Fraser M. T-cadherin expression alternates with migrating neural crest cells in the trunk of the avian embryo. Development. 1991;111:15–22. doi: 10.1242/dev.111.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Bronner-Fraser M. Effects of different fragments of the fibronectin molecule on latex bead translocation along neural crest migratory pathways. Dev Biol. 1985;108:131–145. doi: 10.1016/0012-1606(85)90015-6. [DOI] [PubMed] [Google Scholar]
- 47.Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987;104:1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haack H, Hynes RO. Integrin receptors are required for cell survival and proliferation during development of the peripheral glial lineage. Dev Biol. 2001;233:38–55. doi: 10.1006/dbio.2001.0213. [DOI] [PubMed] [Google Scholar]
- 49.Newgreen D, Thiery JP. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- 50.Debby-Brafman A, Burstyn-Cohen T, Klar A, Kalcheim C. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron. 1999;22:475–488. doi: 10.1016/s0896-6273(00)80703-5. [DOI] [PubMed] [Google Scholar]
- 51.Krull CE, Collazo A, Fraser SE, Bronner-Fraser M. Segmental migration of trunk neural crest: time-lapse analysis reveals a role for PNA-binding molecules. Development. 1995;121:3733–3743. doi: 10.1242/dev.121.11.3733. [DOI] [PubMed] [Google Scholar]
- 52.Kubota Y, Morita T, Kusakabe M, Sakakura T, Ito K. Spatial and temporal changes in chondroitin sulfate distribution in the sclerotome play an essential role in the formation of migration patterns of mouse neural crest cells. Dev Dyn. 1999;214:55–65. doi: 10.1002/(SICI)1097-0177(199901)214:1<55::AID-DVDY6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 53.Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, et al. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- 54.Stern CD, Sisodiya SM, Keynes RJ. Interactions between neurites and somite cells: inhibition and stimulation of nerve growth in the chick embryo. J Embryol Exp Morphol. 1986;91:209–226. [PubMed] [Google Scholar]
- 55.Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz Q, Maden CH, Davidson K, Ruhrberg C. Neuropilin-mediated neural crest cell guidance is essential to organise sensory neurons into segmented dorsal root ganglia. Development. 2009;136:1785–1789. doi: 10.1242/dev.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development. 2009;136:1879–1888. doi: 10.1242/dev.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, et al. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 60.Perez SE, Rebelo S, Anderson DJ. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126:1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- 61.Sommer L, Ma Q, Anderson DJ. Neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- 62.ElShamy WM, Ernfors P. A local action of neurotrophin-3 prevents the death of proliferating sensory neuron precursor cells. Neuron. 1996;16:963–972. doi: 10.1016/s0896-6273(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 63.Farinas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaese F, Kolbeck R, Barde YA. Sensory ganglia require neurotrophin-3 early in development. Development. 1994;120:1613–1619. doi: 10.1242/dev.120.6.1613. [DOI] [PubMed] [Google Scholar]
- 65.Sandell LL, Trainor PA. Neural crest cell plasticity. size matters. sAdv Exp Med Biol. 2006;589:78–95. doi: 10.1007/978-0-387-46954-6_5. [DOI] [PubMed] [Google Scholar]


