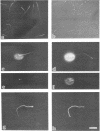
Abstract
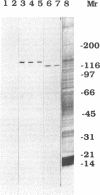
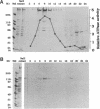
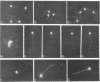
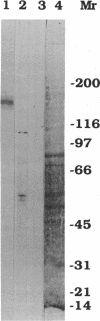
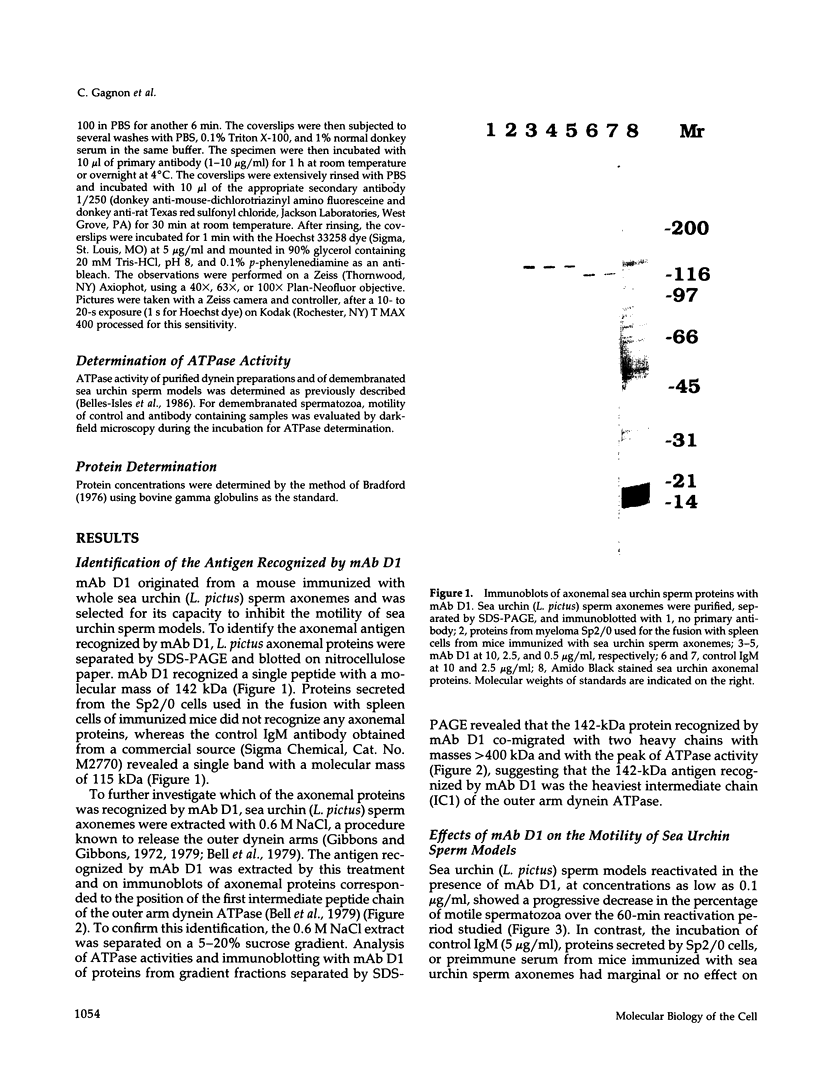
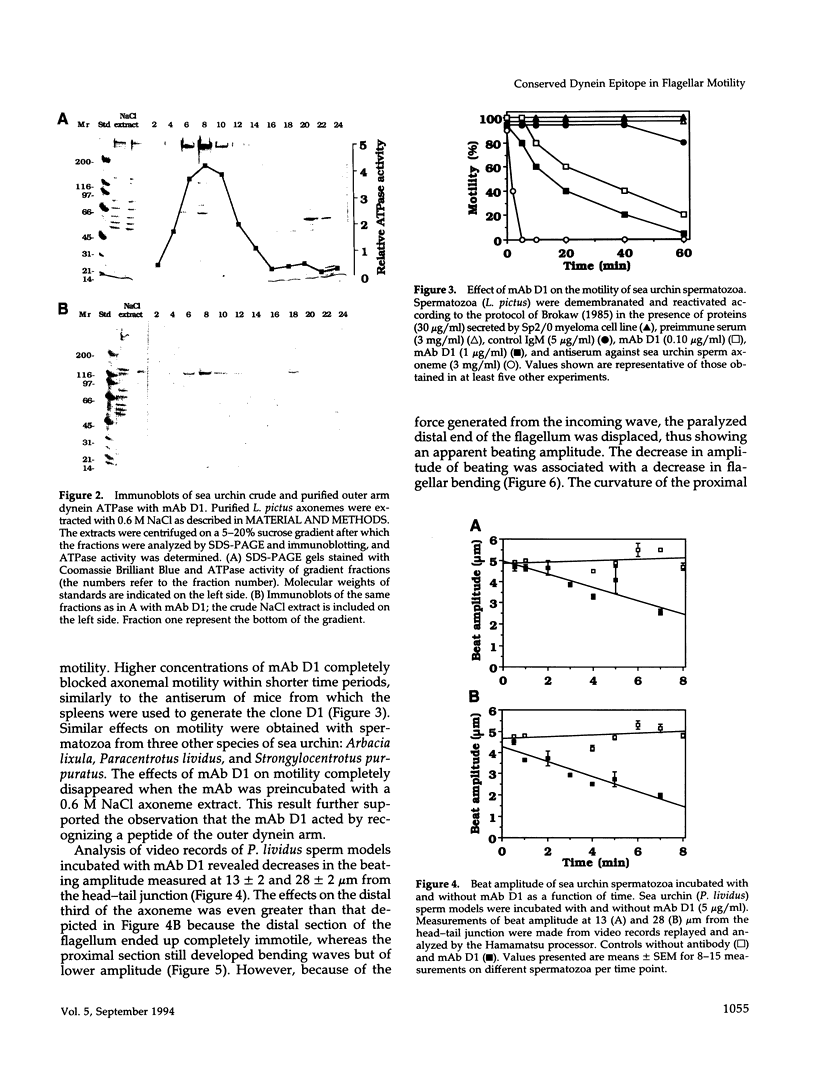
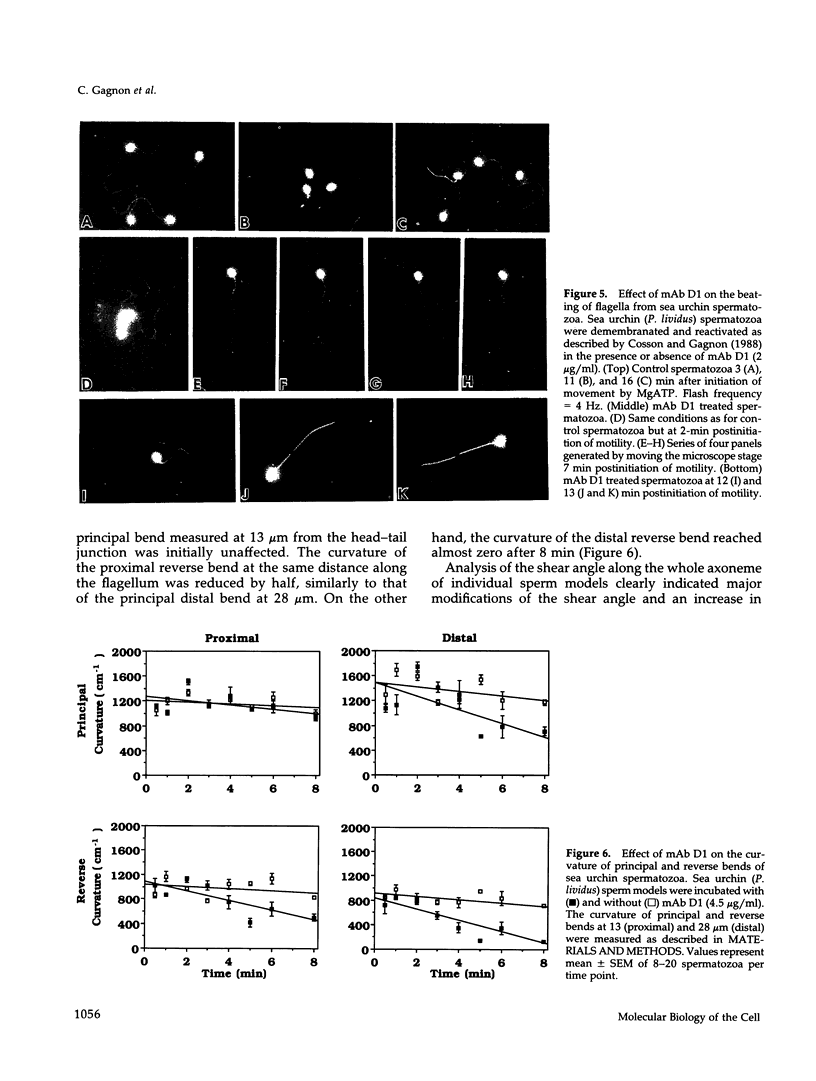
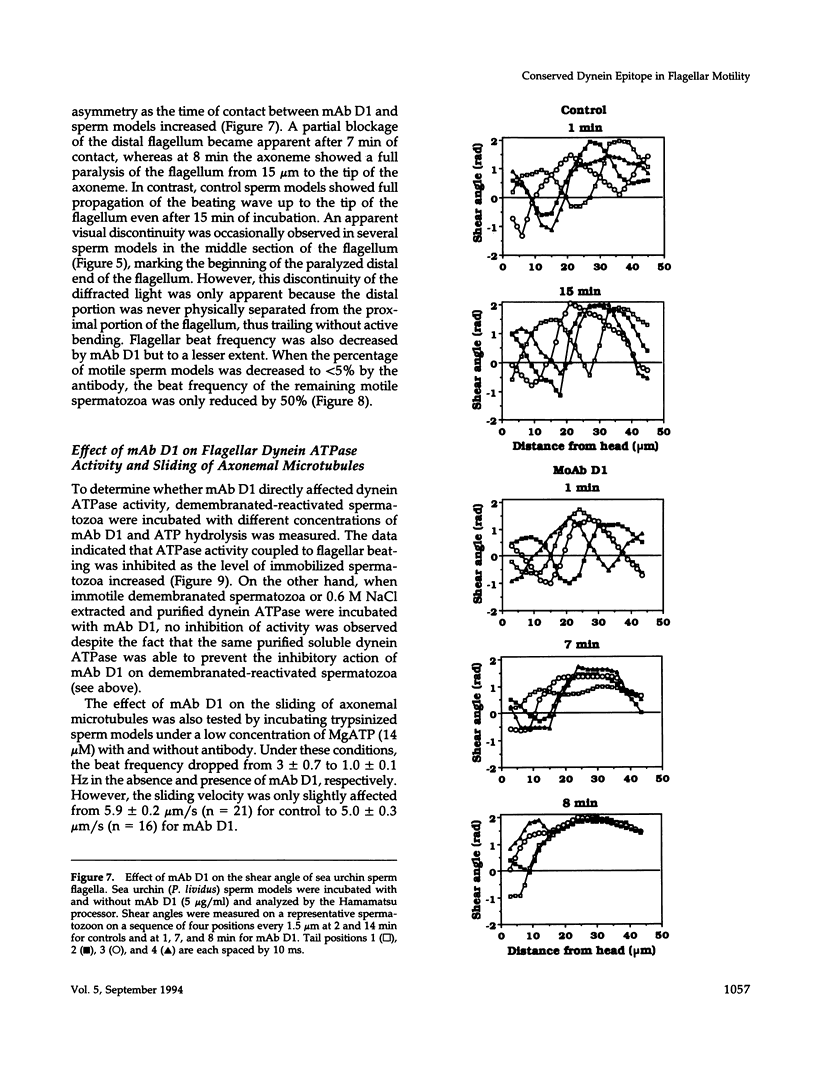
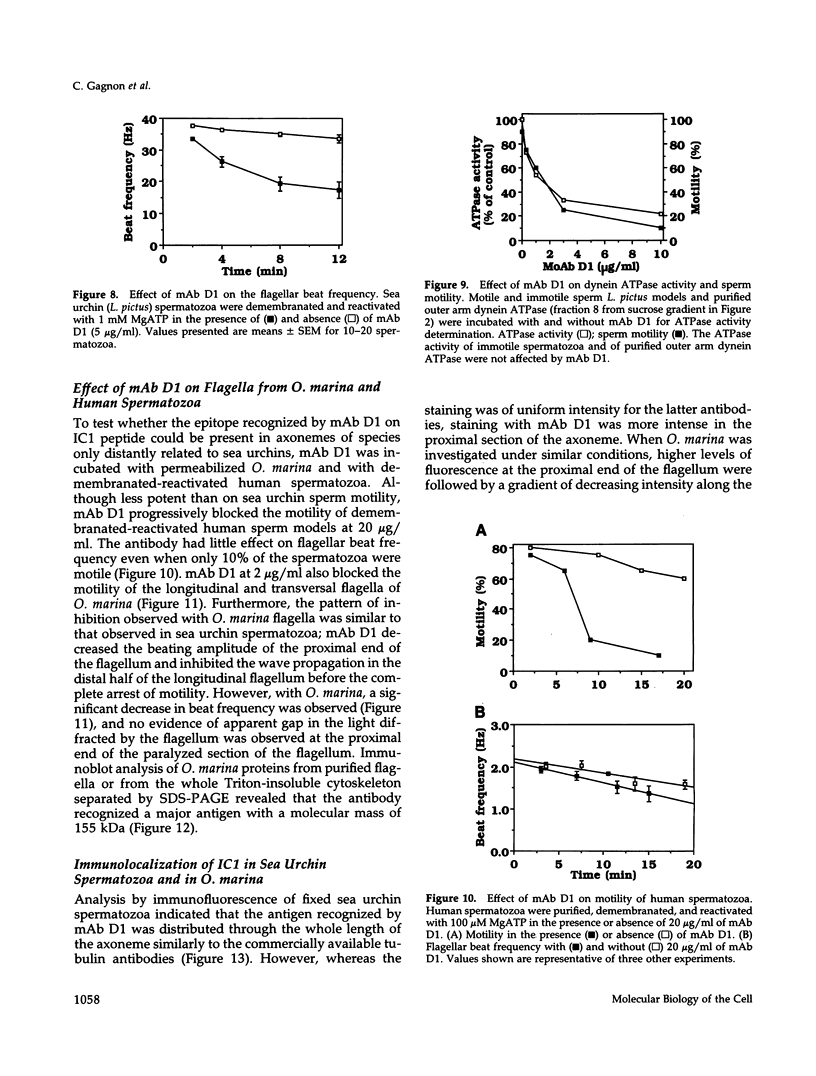
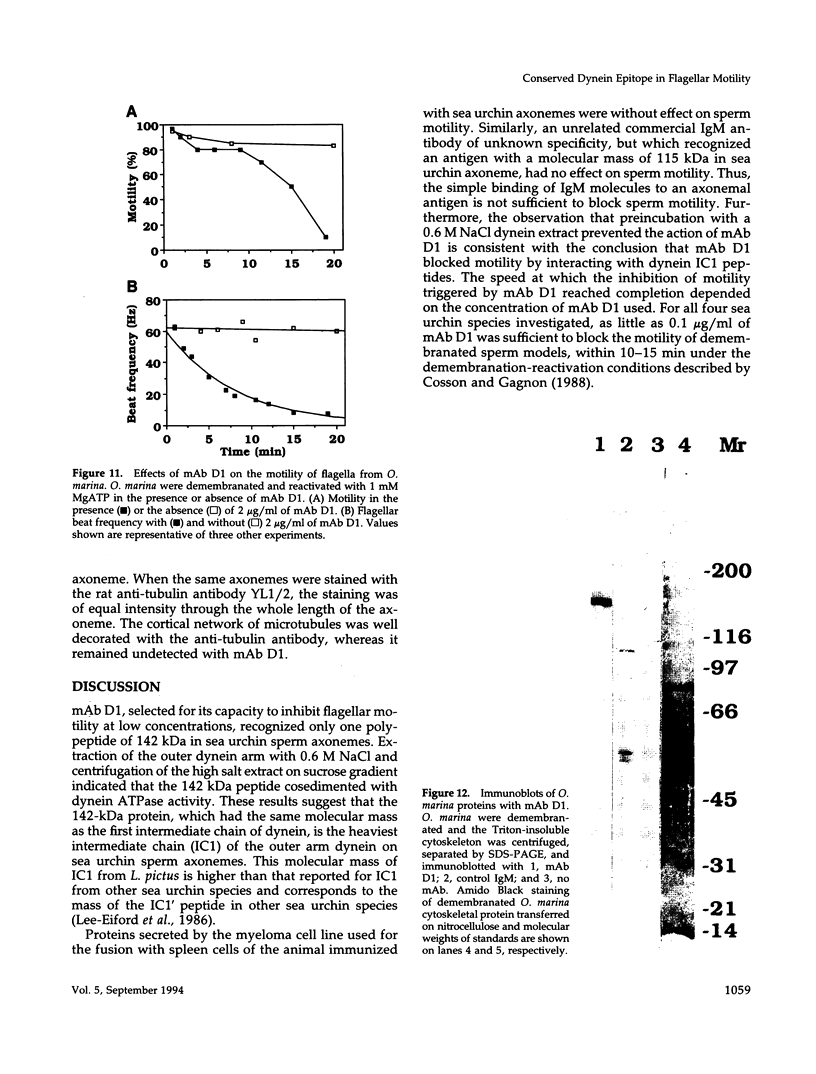
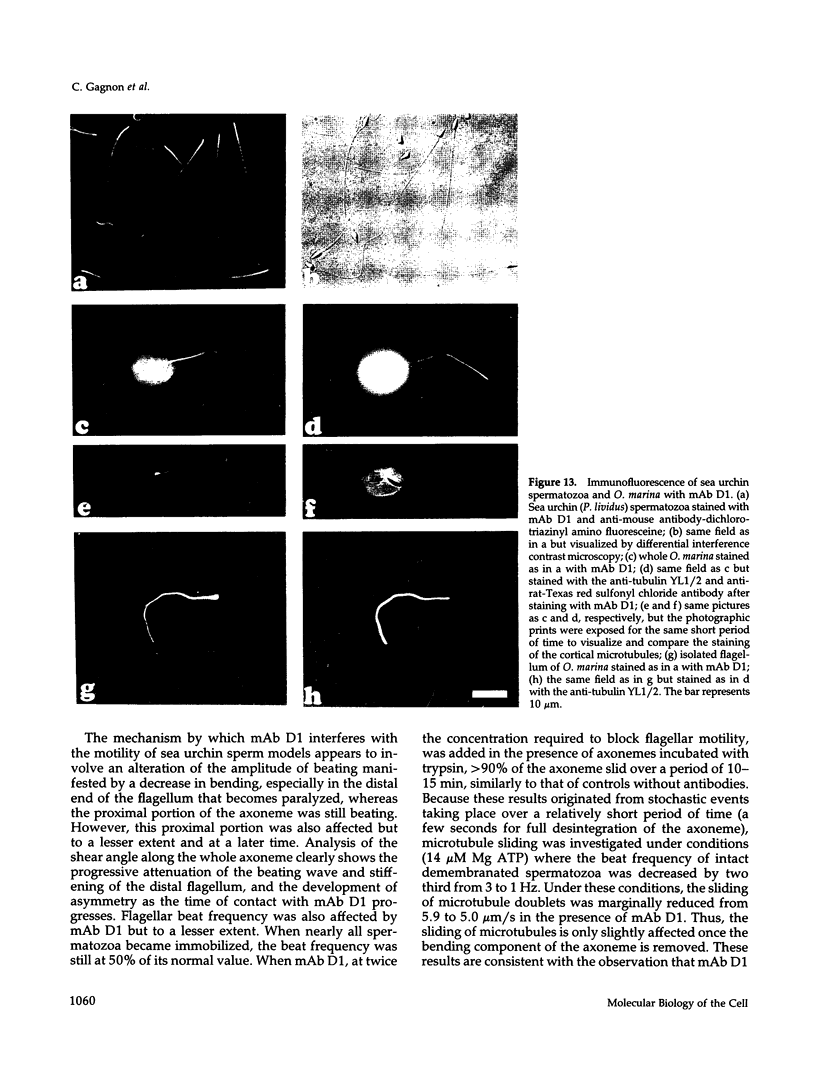
To investigate the role of axonemal components in the mechanics and regulation of flagellar movement, we have generated a series of monoclonal antibodies (mAb) against sea urchin (Lytechinus pictus) sperm axonemal proteins, selected for their ability to inhibit the motility of demembranated sperm models. One of these antibodies, mAb D1, recognizes an antigen of 142 kDa on blots of sea urchin axonemal proteins and of purified outer arm dynein, suggesting that it acts by binding to the heaviest intermediate chain (IC1) of the dynein arm. mAb D1 blocks the motility of demembranated sea urchin spermatozoa by modifying the beating amplitude and shear angle without affecting the ATPase activity of purified dynein or of demembranated immotile spermatozoa. Furthermore, mAb D1 had only a marginal effect on the velocity of sliding microtubules in trypsin-treated axonemes. This antibody was also capable of inhibiting the motility of flagella of Oxyrrhis marina, a primitive dinoflagellate, and those of demembranated human spermatozoa. Localization of the antigen recognized by mAb D1 by immunofluorescence reveals its presence on the axonemes of flagella from sea urchin spermatozoa and O. marina but not on the cortical microtubule network of the dinoflagellate. These results are consistent with a dynamic role for the dynein intermediate chain IC1 in the bending and/or wave propagation of flagellar axonemes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai D. J., Brokaw C. J. Effects of antibodies against tubulin on the movement of reactivated sea urchin sperm flagella. J Cell Biol. 1980 Oct;87(1):114–123. doi: 10.1083/jcb.87.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai D. J., Brokaw C. J., Thompson W. C., Wilson L. Two different monoclonal antibodies to alpha-tubulin inhibit the bending of reactivated sea urchin spermatozoa. Cell Motil. 1982;2(6):599–614. doi: 10.1002/cm.970020608. [DOI] [PubMed] [Google Scholar]
- Bell C. W., Fronk E., Gibbons I. R. Polypeptide subunits of dynein 1 from sea urchin sperm flagella. J Supramol Struct. 1979;11(3):311–317. doi: 10.1002/jss.400110305. [DOI] [PubMed] [Google Scholar]
- Belles-Isles M., Chapeau C., White D., Gagnon C. Isolation and characterization of dynein ATPase from bull spermatozoa. Biochem J. 1986 Dec 15;240(3):863–869. doi: 10.1042/bj2400863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Cyclic AMP-dependent activation of sea urchin and tunicate sperm motility. Ann N Y Acad Sci. 1984;438:132–141. doi: 10.1111/j.1749-6632.1984.tb38282.x. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Future directions for studies of mechanisms for generating flagellar bending waves. J Cell Sci Suppl. 1986;4:103–113. doi: 10.1242/jcs.1986.supplement_4.7. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Microtubule sliding in reduced-amplitude bending waves of Ciona sperm flagella: resolution of metachronous and synchronous sliding components of stable bending waves. Cell Motil Cytoskeleton. 1993;26(2):144–162. doi: 10.1002/cm.970260206. [DOI] [PubMed] [Google Scholar]
- Chapeau C., Gagnon C. Nitrocellulose and polyvinyl coatings prevent sperm adhesion to glass without affecting the motility of intact and demembranated human spermatozoa. J Androl. 1987 Jan-Feb;8(1):34–40. doi: 10.1002/j.1939-4640.1987.tb02416.x. [DOI] [PubMed] [Google Scholar]
- Cosson J., Cachon M., Cachon J., Cosson M. P. Swimming behaviour of the unicellular biflagellate Oxyrrhis marina: in vivo and in vitro movement of the two flagella. Biol Cell. 1988;63(2):117–126. doi: 10.1016/0248-4900(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A. Conformation of the free and antigen-bound IgM antibody molecules. Nature. 1969 Dec 27;224(5226):1307–1309. doi: 10.1038/2241307a0. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gatti J. L., King S. M., Moss A. G., Witman G. B. Outer arm dynein from trout spermatozoa. Purification, polypeptide composition, and enzymatic properties. J Biol Chem. 1989 Jul 5;264(19):11450–11457. [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Functional recombination of dynein 1 with demembranated sea urchin sperm partially extracted with KC1. Biochem Biophys Res Commun. 1976 Nov 8;73(1):1–6. doi: 10.1016/0006-291x(76)90488-5. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Relationship between the latent adenosine triphosphatase state of dynein 1 and its ability to recombine functionally with KCl-extracted sea urchin sperm flagella. J Biol Chem. 1979 Jan 10;254(1):197–201. [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J Cell Sci. 1973 Sep;13(2):337–357. doi: 10.1242/jcs.13.2.337. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Ogawa K., Gibbons I. R. The effect of antidynein 1 serum on the movement of reactivated sea urchin sperm. J Cell Biol. 1976 Dec;71(3):823–831. doi: 10.1083/jcb.71.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. Cilia and flagella of eukaryotes. J Cell Biol. 1981 Dec;91(3 Pt 2):107s–124s. doi: 10.1083/jcb.91.3.107s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R., Gibbons B. H., Mocz G., Asai D. J. Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature. 1991 Aug 15;352(6336):640–643. doi: 10.1038/352640a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- King S. M., Gatti J. L., Moss A. G., Witman G. B. Outer-arm dynein from trout spermatozoa: substructural organization. Cell Motil Cytoskeleton. 1990;16(4):266–278. doi: 10.1002/cm.970160406. [DOI] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991 May 5;266(13):8401–8407. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee-Eiford A., Ow R. A., Gibbons I. R. Specific cleavage of dynein heavy chains by ultraviolet irradiation in the presence of ATP and vanadate. J Biol Chem. 1986 Feb 15;261(5):2337–2342. [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J Cell Biol. 1991 May;113(4):835–842. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Reversion analysis of dynein intermediate chain function. J Cell Sci. 1993 Aug;105(Pt 4):1069–1078. doi: 10.1242/jcs.105.4.1069. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Asai D. J., Brokaw C. J. Properties of an antiserum against native dynein 1 from sea urchin sperm flagella. J Cell Biol. 1977 Apr;73(1):182–192. doi: 10.1083/jcb.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K. Four ATP-binding sites in the midregion of the beta heavy chain of dynein. Nature. 1991 Aug 15;352(6336):643–645. doi: 10.1038/352643a0. [DOI] [PubMed] [Google Scholar]
- Okuno M., Asai D. J., Ogawa K., Brokaw C. J. Effects of antibodies against dynein and tubulin on the stiffness of flagellar axonemes. J Cell Biol. 1981 Dec;91(3 Pt 1):689–694. doi: 10.1083/jcb.91.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter T., King S. M., Witman G. B. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal Biochem. 1987 May 1;162(2):370–377. doi: 10.1016/0003-2697(87)90406-4. [DOI] [PubMed] [Google Scholar]
- Piperno G., Ramanis Z. The proximal portion of Chlamydomonas flagella contains a distinct set of inner dynein arms. J Cell Biol. 1991 Feb;112(4):701–709. doi: 10.1083/jcb.112.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale W. S., Fox L. A. Isolated beta-heavy chain subunit of dynein translocates microtubules in vitro. J Cell Biol. 1988 Nov;107(5):1793–1797. doi: 10.1083/jcb.107.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. F., Sale W. S. Structural and functional reconstitution of inner dynein arms in Chlamydomonas flagellar axonemes. J Cell Biol. 1992 May;117(3):573–581. doi: 10.1083/jcb.117.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B. Axonemal dyneins. Curr Opin Cell Biol. 1992 Feb;4(1):74–79. doi: 10.1016/0955-0674(92)90061-g. [DOI] [PubMed] [Google Scholar]
- Yano Y., Miki-Noumura T. Recovery of sliding ability in arm-depleted flagellar axonemes after recombination with extracted dynein I. J Cell Sci. 1981 Apr;48:223–239. doi: 10.1242/jcs.48.1.223. [DOI] [PubMed] [Google Scholar]
- de Lamirande E., Gagnon C. Effects of protease inhibitors and substrates on motility of mammalian spermatozoa. J Cell Biol. 1986 Apr;102(4):1378–1383. doi: 10.1083/jcb.102.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamirande E., Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 1992 Sep-Oct;13(5):368–378. [PubMed] [Google Scholar]