Abstract
α8β1 integrin is highly expressed in cells with contractile function, such as mesangial cells of the kidneys and vascular smooth muscle cells (VSMCs). Although it promotes migration of neural crest cells and breast cancer cells, recent studies suggest that α8 integrin has a negative regulatory role in VSMC migration. In this Review, the question of why α8β1 integrin plays a dual role in cell migration is raised and discussed. It seems that cells require optimum contractility and balanced tensile forces for migration. α8β1 integrin promotes migration of cells that are initially in a less than optimal contractile state (e.g., neural cells) and reduces the migration of cells known as contractile cells. α8β1 integrin can be called “Tensegrin” as it fits perfectly into the tensegrity model (tensional integrity) and seems to play a prominent role in the integration of the tensile forces.
Key words: integrin, migration, adhesion, mesenchymal cell, epithelial cell, vascular smooth muscle cell
Introduction
Integrin cell adhesion receptors serve as integrators of the cell's exterior and interior, after which property they are named. Each integrin has its own signaling properties.1 α8β1 integrin is one of the latest integrins to be discovered. The only partner of α8 integrin is the β1-subunit. In contrast to α5β1, whose only known ligand is fibronectin, α8β1 can bind to several matrix components, including fibronectin,2 osteopontin,3 vitronectin, tenascin-C,4–6 tenascin-W7 and nephronectin.8
α8β1 integrin is highly expressed during kidney and lung development and α8-deficient mice display abnormal renal development suggesting that α8β1 integrin plays a critical role in organogenesis.9,10 Using FISH and genomic database analysis, Ekwa-Ekoka et al. have shown that α8 gene maps to chromosome 10p13 and consists of >200 kbp organized into 30 exons.11
α8β1 integrin, is intensely expressed in vascular smooth muscle cells (VSMCs), visceral smooth muscle cells, kidney mesangial cells, liver stellate cells and lung interstitial cells.4 In the adult lung, α8 integrin is expressed in contractile interstitial cells, including alveolar myofibroblasts, lipid-containing fibroblasts and pericytes.12 It seems that α8β1 integrin is expressed in cells with contractile properties. Existent evidence indicates a link between α8β1 integrin expression and cardiac, lung, kidney and liver fibrosis.12–14 When we look at the pathological conditions in which α8 expression is increased, we see that there is one property in common. In these fibrotic organs, tensile forces are increased.
Using gain and loss of function strategies, we demonstrated that α8 integrin functions to retard vascular smooth muscle cell (VSMC) migration.15,16 These observations have become controversial, as some reports have implicated α8 integrin as a positive regulator of motility in different cell types. Although data about α8 integrin's role in differentiated epithelial cells is sparse, what is worthy of attention is that α8 integrin is upregulated during the migration of neural and breast cancer cells.
In neuronal cells, α8 integrin is found to promote cell attachment, spreading and neurite outgrowth.2 In addition, α8 integrin promotes breast cancer cell migration.7 It is interesting that α8 integrin can positively and negatively control migration in different contexts. Therefore, it seems that the role of α8β1 integrin is depending on the cell types.
Although it is speculative, whether α8 integrin can promote or inhibit migration may depend on the initial or differentiated state of the cells. In cells, which are differentiated for contractile function, including mesangial cells of kidney and VSMCs, reduced α8 integrin expression heightens migration, whereas in cells which are not initially contractile (e.g., neural cells), α8 integrin upregulation may promote migration.
In this work, we reviewed literature regarding α8β1 integrin's role in different cell types with a stronger focus on VSMC function. Lessons from α8β1 integrin function in VSMCs may shed light on its dual role in different situations.
α8β1 Integrin Promotes Cell Migration
α8β1 integrin was first identified in the chick embryo nervous system.17
Zhang et al. have shown that α8 integrin is upregulated during development of the chicken optic tectum.18 α8 integrin promotes the migration of immature neurons during this process. It is noteworthy that neurite outgrowth is also driven by tension19,20 and application of tensional forces through the ECM directly promotes axon elongation.21
Another condition with which α8 integrin upregulation is associated is heightened migratory activity in human tumors,22 especially in more malignant tumors.7 Mammary tumors have high exogenous tension compared to normal mammary glands.23 The gradient in exogenous tension is high in tumors and low in surrounding tissues. Interestingly, α8 integrin is increased in the more invasive and migratory regions of tumors.7
α8 Integrin as a Differentiation Marker and a Negative Regulator of Migration
The main function of VSMCs is contraction to maintain vascular tone. Thus, VSMCs are differentiated for contractile function. After vascular injury, VSMCs are modulated from the contractile to the less contractile phenotype, which is a prerequisite for their migratory activity. Then, VSMCs migrate from the tunica media toward the intima, resulting in neointima formation.
After balloon injury concomitant with loss of the contractile phenotype, α8β1 integrin is downregulated in the tunica media.15 α8 integrin gene silencing evokes the downregulation of VSMC contractile markers and the upregulation of de-differentiation markers.24 Moreover, α8 integrin gene silencing results in the VSMC changes from spindle to polygonal shape and stress fiber fragmentation into short bundles that are moved to the perinuclear region.24 These changes are characteristics of the de-differentiation state of VSMC and leads to the heightened VSMC migration,15 while α8 integrin overexpression in de-differentiated VSMCs attenuates migratory activity.16 Interestingly, it has been reported that α8β1 integrin is upregulated during the differentiation of mesenchymal cells from the kidney and lung.9,25 Therefore, α8β1 integrin seems to be upregulated during the differentiation of cells with contractile abilities and can serve as a differentiation marker of these cells.
Taken together, α8 integrin in all these conditions has a positive relationship with contractile state. Therefore, the question is: why does α8 integrin promote migration in cancer, while inhibiting it in VSMCs and mesangial cells? The answer resides in the concept that cells require adjustment for optimal adhesion and contractility to migrate.
Migration and Contractility
Cell movement is a complicated process involving dissolution of the cell's contacts with other cells and the extracellular matrix (ECM), the formation of lamellipodia and new contacts with the environment and the contraction of actin filaments in the trailing edge, eliciting movement of the cell body.7 On an optimally-stiff surface, cells form adhesions and assemble actin structures that are sufficient to permit attachment and generate enough tractional force for movement, yet not so adhesive or contractile as to inhibit the release of adhesions at the trailing edge necessary to translocate the cell body.26
Because their primary function is contraction, VSMCs are in a highly contractile state. In this phenotype, the tensile forces exerted at the connecting point between the cell-ECM result in the development of focal adhesions and the assembly of parallel actin stress fibers.27 The consequence of this feature of cell-ECM interaction is excessive adhesiveness and contractility accompanied by reduced migratory activity.27
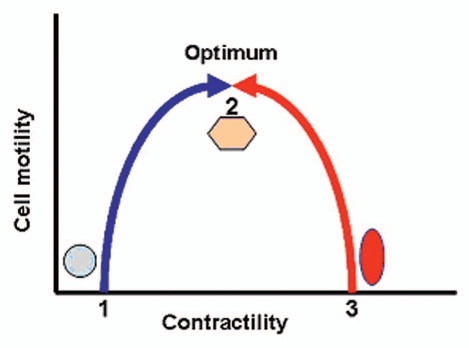
As mentioned earlier, cells require optimum adhesiveness and contractility to migrate. It has been shown that integrins may contribute to an increase in migration if adhesion is initially less than optimal.28 However, if cells are initially in an optimal adhesion state,28 integrins may decrease migration. It also appears that cells require optimum contractility to migrate. If cells are initially in a prestress situation and contractile mode (e.g., VSMCs and mesangial cells), the increase in contractility reduces migration. On the other hand, in cells, which are not initially contractile in nature (e.g., neuronal cells and ductal epithelial cells of breast) increase in contractility promotes migration (Fig. 1). In this context, α8 integrin may provide tensile forces required for optimal contractility and adhesiveness.
Figure 1.
Contractility and migration. The blue line shows that the cells, which are not initially in the contractile state (1), increase their contractility and approach the optimum level (2) in order to reach their maximum migratory ability. However, contractile cells (3) have maximum migration when their contractility is reduced (red line).
Therefore, the initial state of the cells and the balance between tensile forces outside and inside the cells can account for the different modes of α8 integrin action in different cell types.
Tensegrity and Integrins
In an attempt to explain the balance between internal and external forces, Donald Ingber introduced the tensional integrity model, which proposes that the whole cell is a prestressed structure.29 In this model, tensional forces are balanced by forces that resist compression. He explains how individual filaments can have dual functions and, therefore, exert either tension or compression in different structural contexts. The efficiency of mechanical coupling between these forces depends on the type of molecular adhesion complex that forms on the cell surface. We know that tension in the environment surrounding the cell is distributed by integrins. Integrins regulate cellular tension by triggering actin cytoskeleton organization.30 The tensegrity model holds that changes in the balance of mechanical forces across integrins can provide additional signaling to regulate cell function.31 By showing that with the same growth factors and integrin signaling different outcomes could result, depending on whether the cell is spread or round,29 Ingber emphasized the situation in cells before applying tensile forces. However, the question that can be raised is whether this tensegrity role is a general role attributed to all integrins or restricted to a small group of integrins?
To address this question I will discuss the role of different integrins in VSMC, which is a cell type with a prominent contractile function.
Integrin or Integrins?
Each integrin αβ combination has its own signaling properties1 and different functions. It is likely that different integrins recruit different signaling molecules and differentially control cell signaling and cellular tension.32 Hence, we should avoid using the term “integrin” in general, which sounds as if all members of the group act synonymously. For instance, the pattern of integrin expression changes during VSMC phenotype modulation from contractile to non-contractile state. Some of the integrins that are poorly expressed in contractile VSMCs, especially α2β1, α5β1 and αvβ3, become more prominent in non-contractile state33 and mediate VSMC migration.34–37
α2β1 integrin, a collagen receptor, has been implicated in platelet-derived growth factor (PDGF)-induced VSMC migration.34 It has been reported that stress fiber disassembly by fibroblast growth factor may promote the differential utilization of α2β1 integrin for VSMC motility.38 Bix et al. have demonstrated that the interaction between α2β1 integrin and endorepellin triggers a unique signaling pathway that leads to the disassembly of focal adhesions and stress fibers.39 α5β1 and α6β1 are poorly expressed in contractile VSMCs. However, they are upregulated in phenotype-modulated VSMCs and promote migration.35,36 αvβ3 is one of the most-studied integrins. αvβ3 and αvβ5 integrins both mediate VSMC migration.37 Moreover, endothelin1, which is known to enhance contractility, inhibits av expression.40 Therefore, it appears that αv integrin is downregulated in conditions of increased contractile forces. On the other hand, some other integrins are more related to the contractile state of VSMCs including α8β1,25 α1β1,41 and α7β1 integrins.42 α8β1 integrin is one of the integrins that is intensely expressed in VSMCs. It has been reported that α1 integrin is also another integrin involved in contraction.43 Downregulation of some other integrins, including α7,42 and α1,33 has been shown to be associated with the VSMC noncontractile phenotype. Therefore, it is plausible to divide integrins into two groups: contractile and less contractile integrins. However, there is always a balance between the expressions of different integrins. In our work, we observed that when α8 integrin is knocked down, α1 integrin is downregulated, whereas integrins which are poorly expressed in differentiated VSMCs, including α2, α5 and αv, are upregulated. Moreover, concomitant with loss of the VSMC-differentiated phenotype after several passages, α8β1 integrin is downregulated while αvβ3 is upregulated.24 It has been demonstrated that αvβ3/β5 and α5β1 integrins were found to be elevated on the lumen side of the neointima44 and their expression was lower on the medial side, while our data disclosed that α8β1 integrin was expressed and distributed more on the medial side of the neointima. Interestingly, the lack of α1 integrin is accompanied by an increase in αv and α5 integrins.45 Therefore, it appears that the integrins involved in contractile function counterbalance other integrins.
Taken together, it seems that among different members of the integrin superfamily, α8 integrin's role is prominent in the inducing of tensile forces and its expression is always accompanied with an increase in contractility.
To further highlight the unique properties of α8 integrin, it should be noted that α8β1 integrin binds to the RGD site in ECM proteins through mechanisms that are distinct and separate from α5 and αv integrins.4 The cytoplasmic domain sequence of α8 integrin is distinct from all other known α-subunit cytoplasmic domains, including αv and α5. αv and α5 along with αIIb are the α-subunits most closely related to α8 (42–43% amino acid identity).4
α8 Integrin and Contractile Ability in VSMC
In vitro studies have confirmed that α8β1 integrin is upregulated in the VSMC contractile state while downregulated during phenotype modulation.15 It has been shown that transforming growth factor-beta (TGFβ) can revert the phenotype of less contractile VSMCs to the contractile phenotype.46 However, in the presence of siRNA-α8 integrin, TGFβ stimulation fails to induce VSMC re-differentiation.24 Moreover, TGFβ-induced myofibroblastic features are impaired in α8 knocked down fibroblasts.47 On the other hand, in VSMCs that exhibit a less contractile phenotype, high-passage cells, α8 integrin overexpression elicits the restoration of contractile phenotype characteristics.16 Therefore, α8 integrin seems to exert a prominent role on both sides of VSMC phenotypic transition.
Moreover, α8 integrin as well as SM α-actin are upregulated in the neointima during constrictive remodeling concomitant with the late lumen loss.47
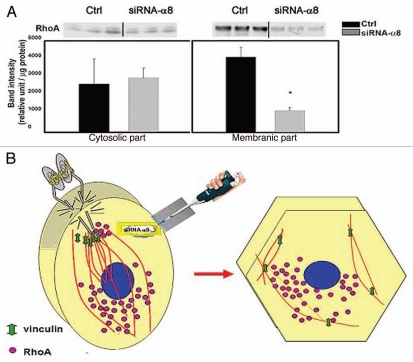
It seems likely that α8 integrin downregulation can shut down the mechanisms responsible for the VSMC contractile phenotype. It is well-documented that RhoA activity is critical for controlling the VSMC contractile phenotype,48 the assembly of actin stress fibers and focal adhesions.49 To be fully functional, RhoA needs to be anchored to the cell membrane. However, the membrane-associated molecules with which RhoA interacts remain uncharacterized.50 Interestingly, there is an interaction between α8 integrin and RhoA in VSMCs and α8 integrin gene silencing leads to reduced membrane-anchored RhoA, a hallmark of RhoA activity51 (Fig. 2A).
Figure 2.
Reduced membrane-associated RhoA after α8 integrin gene silencing. (A) Western blotting analysis showed that α8 gene silencing decreased membrane-associated RhoA (right diagram and blots), while cytosolic RhoA was not significantly changed (left diagram and blots). siRNA-luciferase served as the control for siRNA-α8. Adapted from ref. 51. (B) Schematic presentation of VSMC phenotype modulation after α8 gene silencing. α8 gene silencing leads to the disassembly of actin stress fibers, and dislocation of RhoA from focal adhesion sites. Actin fibers are shown as red lines and RhoA as purple dots. Left part is before applying siRNA-a8 and right part is after applying siRNA.
Tension-Dependent Growth and α8 Integrin
Another example of a biological process, in which tensile forces are increased, is proliferation. Proliferation requires augmented tension and contractility.52 Although the cellular mechanism involved is not clear, Rho proteins may play an important role in tension-dependent growth control,53 as they regulate cytoskeletal contractility and G1 progression.54,55 It has been suggested that expression of the proliferation genes is associated with the expression of contractile marker genes.56 The relationship between enhanced VSMC contractility and accelerated proliferation also seems to be reasonable according to studies by Ingber et al.57 who demonstrated that the cell's ability to respond to surrounding mitogens is enhanced by increased contractility.
Interestingly, α8 integrin is upregulated in proliferating VSMCs and its gene silencing reduces DNA synthesis,58 disassembly of actin stress fibers and dislocation of vinculin from focal adhesion sites (Fig. 2B). Moreover, siRNA-α8 leads to the reduced membrane associated-RhoA (Fig. 2A). It appears that after α8 gene silencing, RhoA cannot be anchored to the plasma membrane, thus, it leads to the disassembly of focal adhesions and stress fibers as well as the shape alteration, which are all characteristics of phenotype-modulated VSMCs. Although it seems that α8 integrin and RhoA may be closely intertwined, our knowledge of α8 integrin signaling is insufficient; hence, further clarification is fundamentally important.
Lessons from Parallel Universes
Cell types with contractile function share many characteristics with VSMCs and could be considered as parallel universes. Patterns of expression and function of α8 integrin in these cells and also in pathological conditions where α8 is upregulated could further elucidate its tensional role.
α8 integrin is expressed in mesangial cells of the glomerulus.13 Stellate cells of the liver, lung alveolar myofibroblasts, and lung interstitial cells are other cell types with intense α8 integrin expression in which contraction is a common feature. It has been shown that α8-deficient mice have a defect in sensory hair cells of the inner ear.59 The role of tension in the morphogenesis and function of these cells has also been reported.60 When we look at the pathological conditions in which α8 expression is increased, for example, after injury in models of pulmonary and hepatic fibrosis, in carotid constrictive remodeling after angioplasty or glomerulonephritis13,47,61 there is one property in common. In these fibrotic organs, tensile forces are increased. The other avenues where a significant role for α8 is observed are in development, in which tension is a central factor,62 especially in the later stages of morphogenesis. In these situations, cytoskeletal tension rises within nearby cells.63 Expectedly, α8 integrin is a marker for lung mesenchymal cells, starting early in development, and plays a role in branching morphogenesis.25 α8 integrin is critically important for epithelio-mesenchymal interactions during kidney morphogenesis.9 In a recent study, Benjamin et al.64 have verified a role for α8 integrin in lung development using α8-null mice. By using in vivo and in vitro studies they demonstrated that α8 integrin-null fetal lung mesenchymal cells fail to form stable adhesions and have increased migration. They suggested a critical role for α8 integrin in lung morphogenesis by regulating mesenchymal cell adhesion and migration.
Altogether, α8 integrin expression in the pathological and physiological conditions in which tensile forces required led us to propose an important tensile role for this integrin.
Conclusion
α8β1 integrin seems to play a critical role in regulating cell migration. α8β1 integrin reduces the migration of cells known as contractile cells. However, it promotes the migration of breast tumor cells as well as neural cells, which are initially in a less than optimal contractile state. As mentioned earlier, cells require optimum contractility to migrate. If cells are initially in a contractile mode (e.g., VSMCs and mesangial cells) the increase in contractility by α8 integrin reduces migration. On the other hand, in cells that are not initially contractile in nature (e.g., neuronal cells and ductal epithelial cells of breast) α8 integrin may provide tensile forces required for optimal contractility and migration. Therefore, α8β1 integrin is upregulated when tension and contractility are required and its effect on cell migration may depend on the overall contractile nature of the cells and their environment.
α8 integrin properties seems to be different from those of other integrins. To the best of our knowledge, α8 integrin is the only integrin that has been reported to inhibit VSMC migration. Does α8 integrin induce conformational changes in the β1-subunit that are different from those of the other α-subunit partners of β1 integrin? The uniqueness of α8 signaling compared to other integrin subunits is an exciting issue that needs to be investigated.
One of the easiest ways to elucidate α8 integrin role in cell migration is to silence it in the different cell types (Mesangial cells, as an example of contractile cell and neural cell, as an example of non-contractile cell) and see how migration occurs. My speculation is that α8 integrin downregulation might promote migration in mesangial cells, while reducing migration in neural cells. To verify if the effect of α8 is due to the increase of tensile forces we can pretreat cells with RhoA activators and inhibitors.
Pretreatment of mesengial cells with Rho-kinase inhibitor Y-27632 can reduce tensile forces.65 Then, we can overexpress α8 integrin and examine whether it can increase tensile forces leading to the reduced migration or not. On the other hand, we can increase tensile forces in neural cells by activating actin fibers assembly or by RhoA activators and examine the migratory activity. After this step, siRNA-α8 can be applied to verify if reducing α8 inhibits migratory activity.
Another important experiment would be to verify if the tensile effect of α8 integrin is unique to α8 or applies to other contractile integrins (e.g., α1, α7) as well. The suggested experiment examines whether overexpression of al integrin in VSMCs can overcome the reduced tensile forces due to α8 integrin gene-silencing.
Doubtless, further studies, especially in vivo experiments, are necessary to unveil some elements of α8β1 integrin's mode of action.
Finally, α8 integrin fits perfectly into the tensegrity model (tensional integrity). Integrins serve as integrators of the cell's exterior and interior and α8 integrin seems to play a more prominent role in the integration of tensile forces. Therefore, inspired by the tensegrity model of Donald Ingber, I coin the name “Tensegrin” for α8β1 integrin. I hope that future investigations may shed light on its mysterious role in cell biology.
Acknowledgements
Hereby, I acknowledge the comments of Dr. Alan Hall. I also thank Dr. Gaetan Thibault for everything he provided during the years of my research on α8 integrin.
Abbreviations
- VSMCs
vascular smooth muscle cells
- SM-α-actin
smooth muscle α-actin
- ECM
extracellular matrix
- PDGF
platelet-derived growth factor
- TGFβ
transforming growth factor-beta
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/12403
References
- 1.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 2.Muller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha8beta1 promotes attachment, cell spreading and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Bio Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha8beta1 functions as a receptor for tenascin, fibronectin and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- 5.Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, et al. The integrin receptor alpha8beta1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denda S, Muller U, Crossin Kl, Erickson HP, Reichardt LF. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin alpha8beta1 receptor interactions with tenascin: murine alpha8beta1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry. 1998;37:5464–5474. doi: 10.1021/bi9727489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNFalpha induced expression in vitro. Oncogene. 2005;24:1525–1532. doi: 10.1038/sj.onc.1208342. [DOI] [PubMed] [Google Scholar]
- 8.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, et al. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner TE, Frevert CW, Herzog EL, Schnapp LM. Expression of the integrin subunit alpha8 in murine lung development. J Histochem Cytochem. 2003;51:1307–1315. doi: 10.1177/002215540305101008. [DOI] [PubMed] [Google Scholar]
- 11.Ekwa-Ekoka C, Diaz GA, Carlson C, Hasegawa T, Samudrala R, Lim KC, et al. Genomic organization and sequence variation of the human integrin subunit alpha8 gene (ITGA8) Matrix Biol. 2004;23:487–496. doi: 10.1016/j.matbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Levine D, Rockey DC, Milner TA, Breuss JM, Fallon JT, Schnapp LM. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am J Pathol. 2000;156:1927–1935. doi: 10.1016/s0002-9440(10)65066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartner A, Schocklmann H, Prols F, Muller U, Sterzel RB. Alpha8 integrin in glomerular mesangial cells and in experimental glomerulonephritis. Kidney Int. 1999;56:1468–1480. doi: 10.1046/j.1523-1755.1999.00662.x. [DOI] [PubMed] [Google Scholar]
- 14.Thibault G, Lacombe MJ, Schnapp LM, Lacasse A, Bouzeghrane F, Lapalme G. Upregulation of alpha(8) beta(1)-integrin in cardiac fibroblast by angiotensin II and transforming growth factor-beta1. Am J Physiol Cell Physiol. 2001;281:1457–1467. doi: 10.1152/ajpcell.2001.281.5.C1457. [DOI] [PubMed] [Google Scholar]
- 15.Zargham R, Thibault G. alpha8beta1 Integrin expression in the rat carotid artery: involvement in smooth muscle cell migration and neointima formation. Cardiovasc Res. 2005;65:813–822. doi: 10.1016/j.cardiores.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Zargham R, Touyz RM, Thibault G. alpha8 Integrin overexpression in de-differentiated vascular smooth muscle cells attenuates migratory activity and restores the characteristics of the differentiated phenotype. Atherosclerosis. 2007;195:303–312. doi: 10.1016/j.atherosclerosis.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin alpha8 subunit: a new integrin beta1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Galileo DS. Retroviral transfer of antisense integrin alpha6 or alpha8 sequences results in laminar redistribution or clonal cell death in developing brain. J Neurosci. 1998;18:6928–6938. doi: 10.1523/JNEUROSCI.18-17-06928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chada S, Lamoureux P, Buxbaum RE, Heidemann SR. Cytomechanics of neurite outgrowth from chick brain neurons. J Cell Sci. 1997;110:1179–1186. doi: 10.1242/jcs.110.10.1179. [DOI] [PubMed] [Google Scholar]
- 20.Condron BG, Zinn K. Regulated neurite tension as a mechanism for determination of neuronal arbor geometries in vivo. Curr Biol. 1997;7:813–816. doi: 10.1016/s0960-9822(06)00343-5. [DOI] [PubMed] [Google Scholar]
- 21.Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, et al. Integrin gene expression profiles of human hepatocellular carcinoma. World J Gastroenterol. 2002;8:631–637. doi: 10.3748/wjg.v8.i4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 24.Zargham R, Thibault G. Alpha8 integrin expression is required for maintenance of the smooth muscle cell differentiated phenotype. Cardiovasc Res. 2006;71:170–178. doi: 10.1016/j.cardiores.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Wagner TE, Frevert CW, Herzog EL, Schnapp LM. Expression of the integrin subunit alpha8 in murine lung development. J Histochem Cytoche. 2003;51:1307–1315. doi: 10.1177/002215540305101008. [DOI] [PubMed] [Google Scholar]
- 26.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 27.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 28.Worth NF, Rolfe BE, Song J, Campbell GR. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskeleton. 2001;49:130–145. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 29.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 30.Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70:547–560. doi: 10.1046/j.1432-0436.2002.700908.x. [DOI] [PubMed] [Google Scholar]
- 31.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 32.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 33.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 34.Skinner MP, Raines E, Ross R. Dynamic expression of alpha1beta1 and alpha2beta1 integrin receptors by human vascular smooth muscle cells. Alpha2beta1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 35.Pickering JG, Chow LH, Li S, Rogers KA, Rocnik EF, Zhong R, et al. alpha5beta1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. Am J Pathol. 2000;156:453–465. doi: 10.1016/s0002-9440(10)64750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6) beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 37.Kappert K, Blaschke F, Meehan WP, Kawano H, Grill M, Fleck E, et al. Integrins alphavbeta3 and alphavbeta5 mediate VSMC migration and are elevated during neointima formation in the rat aorta. Basic Res Cardiol. 2001;96:42–49. doi: 10.1007/s003950170076. [DOI] [PubMed] [Google Scholar]
- 38.Fera E, O'Neil C, Lee W, Li S, Pickering JG. Fibroblast growth factor-2 and remodeled type I collagen control membrane protrusion in human vascular smooth muscle cells: biphasic activation of Rac1. J Biol Chem. 2004;279:35573–35582. doi: 10.1074/jbc.M400711200. [DOI] [PubMed] [Google Scholar]
- 39.Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doi M, Shichiri M, Yoshida M, Marumo F, Hirata Y. Suppression of integrin alpha(v) expression by endothelin-1 in vascular smooth muscle cells. Hypertens Res. 2000;23:643–649. doi: 10.1291/hypres.23.643. [DOI] [PubMed] [Google Scholar]
- 41.Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem. 1999;190:105–118. [PubMed] [Google Scholar]
- 42.Yao CC, Breuss J, Pytela R, Kramer RH. Functional expression of the alpha7 integrin receptor in differentiated smooth muscle cells. J Cell Sci. 1997;110:1477–1487. doi: 10.1242/jcs.110.13.1477. [DOI] [PubMed] [Google Scholar]
- 43.Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem. 1997;272:30911–30917. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 44.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplaa C, et al. Vitronectin is upregulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002;3:952–962. doi: 10.1016/s0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 45.Louis H, Kakou A, Regnault V, Labat C, Bressenot A, Gao-Li J, et al. Role of alpha1beta1 integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. Am J Physiol Heart Circ Physiol. 2007;293:2597–2604. doi: 10.1152/ajpheart.00299.2007. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Sinha S, Owens G. A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278:48004–48011. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- 47.Zargham R, Pepin J, Thibault G. alpha8beta1 Integrin is upregulated in the neointima concomitant with late luminal loss after balloon injury. Cardiovasc Patho. 2007;16:212–220. doi: 10.1016/j.carpath.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Manabe I, Owens GK. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J Clin Invest. 2001;107:823–834. doi: 10.1172/JCI11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 50.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 51.Zargham R, Wamhoff BR, Thibault G. RNA interference targeting alpha8 integrin attenuates smooth muscle cell growth. FEBS Lett. 2007;581:939–943. doi: 10.1016/j.febslet.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 52.Moore KA, Huang S, Kong Y, Sunday ME, Ingber DE. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J Surg Res. 2002;104:95–100. doi: 10.1006/jsre.2002.6418. [DOI] [PubMed] [Google Scholar]
- 53.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 54.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 55.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 56.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, et al. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 57.Ingber DE. Integrins, tensegrity and mechanotransduction. Gravi Space Biol Bull. 1997;10:49–55. [PubMed] [Google Scholar]
- 58.Zargham R, Wamhoff BR, Thibault G. RNA interference targeting alpha8 integrin attenuates smooth muscle cell growth. FEBS Lett. 2007;581:939–943. doi: 10.1016/j.febslet.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 59.Littlewood Evans A, Muller U. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nat Genet. 2000;24:424–428. doi: 10.1038/74286. [DOI] [PubMed] [Google Scholar]
- 60.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 61.Levine D, Rockey DC, Milner TA, Breuss JM, Fallon JT, Schnapp LM. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am J Pathol. 2000;156:1927–1935. doi: 10.1016/s0002-9440(10)65066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 63.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 64.Benjamin JT, Gaston DC, Halloran BA, Schnapp LM, Zent R, Prince LS. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol. 2009;335:407–417. doi: 10.1016/j.ydbio.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber DS, Webb RC. Enhanced relaxation to the rho-kinase inhibitor Y-27632 in mesenteric arteries from mineralocorticoid hypertensive rats. Pharmacology. 2001;63:129–133. doi: 10.1159/000056123. [DOI] [PubMed] [Google Scholar]