Abstract
Growth factors regulate a diverse array of cellular functions including proliferation, survival and movement, and the ability to do this often involves interactions with the extracellular matrix (ECM) and particularly heparan sulfate proteoglycans (HSPGs). HSPGs have been shown to sequester growth factors and to act as growth factor co-receptors or receptors themselves. Recent studies, however, have revealed a new role for HSPGs in mediating the interactions of growth factors with the ECM. Specifically, heparan sulfate has been shown to modulate fibronectin structure to reveal previously masked growth factor binding sites. In vivo, this mechanism appears to control the guidance of migrating cells during embryonic development as HSPG-modification of fibronectin enables direct platelet derived growth factor-fibronectin interactions necessary for this process. A model based on this observation is discussed here as well as the possibility that other growth factors/morphogens utilize similar mechanisms involving fibronectin or additional ECM proteins.
Key words: heparin, heparan sulfate, fibronectin, cell movement, cell motility, mesoderm, mesendoderm, embryonic development, embryo, Xenopus
The ability of cells to move is critical to normal processes, such as embryonic development and wound healing, and is also a feature of pathological conditions such as cancer metastasis. In normal circumstances, cell movement is tightly regulated and leads to precise cell reorganizations. This is particularly evident during embryonic development where cells need to be in the right place at the right time to give and receive signals, to form tissues and organs and ultimately, to become a functional organism. To achieve this, cells must be able to move in a directed way. This requires that cells sense and move in response to extracellular signals, while coordinating changes in shape with the creation of traction and force and balancing attachment and detachment to the extracellular matrix (ECM) and neighboring cells.
Evidence from a number of systems suggests that growth factors play a significant role in the spatial and temporal control of cell movements. In particular, members of the platelet derived growth factor (PDGF)/vascular endothelial growth factor (VEGF) family act as guidance cues for cells in a variety of processes including the development of both invertebrate and vertebrate embryos (reviewed in ref. 1). How such cues are laid down is an area of intensive investigation; however, a new study from our group suggests that the microenvironment within the ECM, principally the composition of heparan sulfate, modulates direct interactions between PDGF and fibronectin necessary for directed migration of embryonic cells during Xenopus development.2
During the development of all vertebrates, cell movements begin at gastrulation, when changes in the shape, adhesion and motility of cells drive the complex series of tissue rearrangements necessary to establish the basic body plan of the embryo. In Xenopus embryos, some of the first cells to move during gastrulation are the anterior mesendoderm cells. These cells migrate in a characteristic pattern that provides a tractable model to identify the molecular control of directed cell movement. Anterior mesendoderm cells move away from the blastopore lip and towards the animal pole across a fibrillar, fibronectin-rich ECM that covers the ectoderm (see Fig. 1A; reviewed in ref. 3). They migrate as a sheet and are polarized with lamelliform protrusions extending in the direction of movement and the trailing edges underlapping one another like shingles on a roof (reviewed in ref. 3). This mesendoderm movement is guided by PDGF-A signaling.4 Immediately prior to and during Xenopus gastrulation, PDGF-A is expressed in the ectoderm while its receptor, PDGFRα, is expressed in the migrating mesendoderm cells (reviewed in ref. 5). Disruption of PDGF-A signaling by interference with either the receptor or ligand in vivo or in an ex vivo assay disorients the cells and the direction of migration is randomized, although the cells are still able to physically move.4
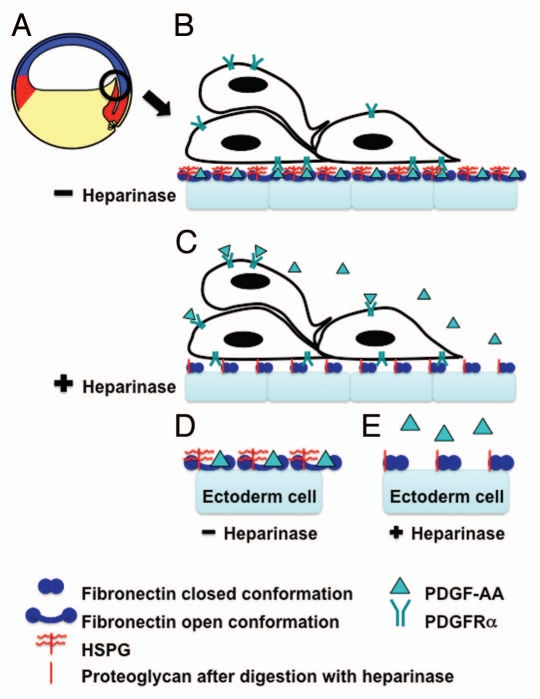
Figure 1.
Heparan sulfate modification of fibronectin enables PDGF-AA-fibronectin interactions necessary for directed cell migration during Xenopus gastrulation. (A) A schematic of a Xenopus embryo immediately after the onset of gastrulation (stage 10+). The prospective mesoderm (red), ectoderm (blue) and endoderm (yellow) are indicated. The black circle indicates migrating anterior mesendoderm cells and the region of tissue shown in (B and C). (B) In vivo the inner surface of the ectoderm (blue blocks) is lined with a fibronectin-rich ECM. This fibronectin is proposed to be in its open conformation (blue dumbbells) due to the presence of heparan sulfate proteoglycans on the surface of these cells (indicated in red). PDGF (green triangle) secreted by the ectoderm cells binds to the C-terminus of the fibronectin. This PDGF is then available for PDGFRα (green fork) binding and activation. The PDGFRα is expressed on the migrating mesendoderm cells. This ligand receptor interaction guides the directed movement of these cells. (C) Heparinase III treatment of these tissues during matrix deposition digests the heparan sulfate side chains leaving the proteoglycan core (red line), which does not alter fibronectin structure leaving it in the closed conformation. PDGF secreted by the ectoderm cells can then diffuse from the site of secretion leaving it available to bind PDGFRαs over the entire surface of the mesendoderm cells and thus, disrupting directed movement. (D and E) A schematic of a “higher magnification” view of one cell in (B and C). A key of the symbols used in (B–D) is shown.
PDGF-A must be associated with the ECM for directed mesendoderm migration. In an ex vivo assay, overexpression of the long form of PDGF-A, which associates with the ECM via a positively charged carboxy-terminal retention motif (reviewed in ref. 6) in the ectoderm cells, disrupts the normal guidance cues causing randomization of mesendoderm movement.4 In contrast, overexpression of the short form of PDGF-A, which diffuses from its site of secretion,6 does not.4 Our recent work now suggests that this growth factor-ECM association represents a direct interaction between PDGF-A and fibronectin.2 Importantly, this interaction requires that the fibronectin is pre-exposed to heparan sulfate proteoglycan (HSPG) before PDGF-A can bind.2 A similar interaction between the related growth factor VEGF and fibronectin is also enhanced by pre-treatment of the fibronectin with heparin or heparan sulfate.7–9 Heparin acts by modifying the structure of fibronectin,8 mediating the transition from the globular form to a stable, more extended conformation,7,8,10–12 which reveals growth factor binding sites.7,8 These data are significant because in an ex vivo directed migration assay, treatment of the native ECM with heparinase III, which degrades heparan sulfate chains, abolishes the guidance cues and results in randomized mesendoderm migration.2 Here we propose a model in which prior to and during Xenopus gastrulation, fibronectin is modified to an extended conformation by endogenous HSPGs as it is being laid down (Fig. 1B and D). PDGF-AA secreted from the ectoderm cells binds to this modified fibronectin and is thus, retained in the ECM (Fig. 1B and D). PDGF-AA then guides the mesendoderm cells by local activation of their cell surface PDGFRαs (Fig. 1B and D). In the absence of HSPG, the fibronectin remains in a more globular form and PDGF-AA binding sites are shielded (Fig. 1C and E). In this case, it is proposed that PDGF-AA diffuses away from the site of secretion resulting in a less localized activation of PDGFRαs and a loss of persistent cell migration (Fig. 1C and E). This idea is supported by data showing that flooding the native ECM with exogenous PDGF-AA prior to performing the ex vivo migration assay, also causes a randomization of directed movement.4 In this case, it seems likely that any gradient or uneven distribution of PDGF-AA is overwhelmed and the guidance cues consequently lost.
In cultured cells, PDGF can also associate with HSPGs via its retention motif.13 However, in the Xenopus ex vivo directed migration assay, heparinase treatment of the ectoderm ECM after its deposition does not alter the direction of mesendoderm migration.2,14 This suggests that in this system, PDGF-AA interacts directly with fibronectin and not with heparan sulfate chains, since heparinase III would have degraded them and caused the release of HS-bound PDGF-AA.13
The type of proteoglycan as well as the specific structural composition of their heparan sulfate chains likely contributes to the modulation of PDGF-fibronectin binding. Both chain length and the chemical composition of heparan sulfate play crucial roles in the binding of VEGF to fibronectin, in which long (>22 saccharides) heparin chains with sulfation on the 6-O and N positions of glucosamine units are required for activity.8 This suggests that a localized control of heparan sulfate biosynthesis through the regulation of the array of enzymes involved might be a critical component to directed cell migration. Interestingly, an analysis of heparan sulfate expression during mouse lung development noted dynamic and discrete localization within the mesenchyme at sites of FGF10-mediated epithelial budding,15 indicating that rapid alterations of heparan sulfate structure may provide a general mechanism for positional control of growth factor activity.
Several HSPGs are expressed in tissue specific patterns during Xenopus gastrulation including Syndecans-1 and -2 (expressed in the ectoderm);16 and Syndecan-4, Glypican-4 and Biglycan (expressed in the ectoderm and mesoderm).16–19 Although the role(s) of these HSPGs in mesendoderm migration are not known, they do affect embryological processes that involve cell rearrangements. For example, Syndecan-2 has recently been shown to regulate the migration of organ primordia and fibrillogenesis in zebrafish embryos.20 During gastrulation, knock-down of either Syndecan-1 or -2 disrupts the native ECM for mesendoderm migration and results in gaps in fibril deposition.21 In addition, Syndecan-4 and Glypican-4 are both essential for convergent extension, another important form of cell movement during gastrulation that involves the coordinated rearrangement of cells.17,18 Similarly, Syndecan-4 is necessary for the directional migration of neural crest cells.22 In these cases, Syndecan-4 and Glypican-4 regulate the planar cell polarity (PCP) pathway, which is also involved in polarized matrix deposition during convergent extension.23 Compellingly, embryos develop with anterior defects when either Syndecan-4 or Glypican-4 is knocked-down, indicating that mesendoderm cell migration is also likely to be affected.17,18
The Slb/Wnt-11 mediated-PCP pathway has been shown in zebrafish embryos to regulate the polarity and directional movement of ingressing mesendoderm cells.24 In Slb/Wnt-11 mutants, however, although these processes are disrupted they are not completely abolished and evidence suggests that PDGF signaling through phosphatidyl inositol 3-kinase (PI3K) is responsible for establishing cell polarity and controlling the velocity of these cells.25 Rho GTPases act downstream in both PCP and PDGF signaling pathways. Recent evidence suggests that RhoA and Rac1 are important for the polarity and protrusive activity of migrating mesendoderm cells and play a role in the guidance of neural crest cells,22,26 but the mechanisms by which these pathways are integrated with signals from other factors that also modulate these processes are still to be determined.
How retention of PDGF-AA in the ECM translates into directional migration still needs to be determined. For example, PDGF-AA may be present in a gradient such that receptors at the front of each cell are exposed to a different level of PDGF than those at the rear. Alternatively, control might involve selective expression of molecules that locally release PDGF from matrix binding sites. Either process might provide a means for uneven activation of PDGFRs and a polarized distribution of downstream signaling components at the leading and lagging edges. Such signaling mechanisms have been identified in other cell types and organisms including leukocytes, fibroblasts and Dictyostelium in which the initial response to a chemoattractant creates cell polarity that is further amplified by feedback (reviewed in ref. 27). For example, in Dictyostelium PI3K and PTEN (phosphatase and tensin homolog) are regulated reciprocally to control the spatial and temporal levels of phosphatidyl inositol-3,4,5-triphosphate (PI(3,4,5)P3). PI(3,4,5)P3 is localized to the leading edge of the cell in response to an extracellular signal, whereas PTEN is downregulated in these regions. Recruitment of proteins that bind preferentially to PI(3,4,5)P3, many of which affect remodeling of the actin cytoskeleton, further enhances this polarity in the direction of the chemoattractant.27 In Xenopus, PDGF signaling does appear to regulate cell polarity since overexpression of PDGF-A in the ectoderm disorients the protrusive activity and abolishes the normal shingle arrangement of the receptor expressing, migrating mesendoderm cells.4 Similarly, in the zebrafish mesendoderm, PDGF signaling induces cell protrusions and polarization.25 In this case, upon PDGF treatment, protein kinase B (PKB)/Akt become localized to the leading edge of the cells in a PI3K dependent manner.25 Such mechanisms require the activation of PDGFR at the leading edge of the cell. In mammalian cells, however, it has been demonstrated that activation of epidermal growth factor receptor (EGFR) can spread laterally in the cell membrane.28 If the same is true for PDGFR, even cells exposed to a gradient or localized source of PDGF-AA may lose spatial information. Recent evidence in Drosophila, however, suggests an alternate mechanism involving receptor endocytosis that ensures that a graded extracellular signal is maintained intracellularly.29
The Drosophila member of the PDGF/VEGF family, PVF1, along with epidermal growth factor, directs the migration of border cells towards the oocyte during oogenesis.30–32 The patterns of expression of ligand and receptor are similar to those during Xenopus gastrulation in that a stationary cell, the oocyte, expresses the ligand, PVF1, while the motile border cells express its receptor, PVR.30 PVR activation is concentrated at the leading edge, and specifically maintained in a localized region of the cell by endocytosis.29 PDGFRβ endocytosis also regulates PDGF-dependent migration of NIH3T3 cells in culture.33 This process involves recruitment of a ternary complex of DOCK4-Grb2-Dynamin2 to the leading edge of the migrating cell, which results in rapid receptor endocytosis, where it is maintained in an active phosphorylation state and leads to polarized signaling. Disruption of the complex, for example by overexpression of DOCK4 ΔC, which cannot bind Grb2, blocks chemotaxis in response to PDGF. Whether a similar mechanism creates spatial signaling during Xenopus gastrulation requires further investigation, but this tantalizing evidence suggests that it is a possibility.
Conclusion
The selective binding of PDGF and VEGF to fibronectin is mediated by the complex glycan, heparan sulfate, such that localized deposition of these critical cell regulatory factors to the ECM may be controlled by dynamic alterations in heparan sulfate structure and expression level. While this process has so far been shown for only PDGF and VEGF, it is possible that other growth factors/morphogens utilize similar mechanisms involving fibronectin or other ECM proteins. Identifying new mechanisms that control growth factor ECM deposition and activity may reveal novel ways to selectively manipulate growth factors for therapeutic purposes.
Acknowledgements
This work was supported in part by NIH grants CA87375 to K.S. and HL056200 and HL088572 to M.A.N.
Abbreviations
- PDGF
platelet-derived growth factor
- PDGFR
PDGF-receptor
- HSPGs
heparan sulfate proteoglycans
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/12427
References
- 1.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EM, Mitsi M, Nugent MA, Symes K. PDGF-A interactions with fibronectin reveal a critical role for heparan sulfate in directed cell migration during Xenopus gastrulation. Proc Natl Acad Sci USA. 2009;106:21683–21688. doi: 10.1073/pnas.0902510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller R. Cell migration during gastrulation. Curr Opin Cell Biol. 2005;17:533–541. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- 5.Ataliotis P, Mercola M. Distribution and functions of platelet-derived growth factors and their receptors during embryogenesis. Int Rev Cytol. 1997;172:95–127. doi: 10.1016/s0074-7696(08)62359-1. [DOI] [PubMed] [Google Scholar]
- 6.Raines EW, Bowen-Pope DF, Ross R. Platelet-derived growth factor. In: Sporn MB, Roberts AB, editors. Peptide growth factors and their receptors. New York: Springer-Verlag; 1991. pp. 173–262. [Google Scholar]
- 7.Mitsi M, Forsten-Williams K, Gopalakrishnan M, Nugent MA. A catalytic role of heparin within the extracellular matrix. J Biol Chem. 2008;283:34796–34807. doi: 10.1074/jbc.M806692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsi M, Hong Z, Costello CE, Nugent MA. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–10328. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- 9.Goerges AL, Nugent MA. pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307–2315. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 10.Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. electron microscopy and sedimentation analysis. J Biol Chem. 1983;258:14539–14544. [PubMed] [Google Scholar]
- 11.Williams EC, Janmey PA, Ferry JD, Mosher DF. Conformational states of fibronectin. Effects of pH, ionic strength and collagen binding. J Biol Chem. 1982;257:14973–14978. [PubMed] [Google Scholar]
- 12.Rocco M, Carson M, Hantgan R, McDonagh J, Hermans J. Dependence of the shape of the plasma fibronectin molecule on solvent composition. Ionic strength and glycerol content. J Biol Chem. 1983;258:14545–14549. [PubMed] [Google Scholar]
- 13.Andersson M, Ostman A, Westermark B, Heldin CH. Characterization of the retention motif in the C-terminal part of the long splice form of platelet-derived growth factor A-chain. J Biol Chem. 1994;269:926–930. [PubMed] [Google Scholar]
- 14.Nagel M, Winklbauer R. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development. 1999;126:1975–1984. doi: 10.1242/dev.126.9.1975. [DOI] [PubMed] [Google Scholar]
- 15.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 16.Teel AL, Yost HJ. Embryonic expression patterns of Xenopus syndecans. Mech Dev. 1996;59:115–127. doi: 10.1016/0925-4773(96)00584-9. [DOI] [PubMed] [Google Scholar]
- 17.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 19.Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrington CB, Yost HJ. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development. 2009;136:3143–3152. doi: 10.1242/dev.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–124. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 22.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 23.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–5384. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero JA, Kilian B, Chan J, Bayliss PE, Heisenberg CP. Phosphoinositide 3-kinase is required for process outgrowth and cell polarization of gastrulating mesendodermal cells. Curr Biol. 2003;13:1279–1289. doi: 10.1016/s0960-9822(03)00505-0. [DOI] [PubMed] [Google Scholar]
- 26.Ren R, Nagel M, Tahinci E, Winklbauer R, Symes K. Migrating anterior mesoderm cells and intercalating trunk mesoderm cells have distinct responses to Rho and Rac during Xenopus gastrulation. Dev Dyn. 2006;235:1090–1099. doi: 10.1002/dvdy.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verveer PJ, Wouters FS, Reynolds AR, Bastiaens PIH. Quantitative imaging of lateral ErbB1 receptor signal propagation in the plasma membrane. Science. 2000;290:1567–1570. doi: 10.1126/science.290.5496.1567. [DOI] [PubMed] [Google Scholar]
- 29.Jekely G, Sung HH, Luque CM, Rorth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Duchek P, Somogyi K, Jekely G, Becarri S, Rørth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 31.Duchek P, Rorth P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 2001;291:131–133. doi: 10.1126/science.291.5501.131. [DOI] [PubMed] [Google Scholar]
- 32.McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 2003;130:3469–3478. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- 33.Kawada K, Upadhyay G, Ferandon S, Janarthanan S, Hall M, Vilardaga JP, et al. Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol Cell Biol. 2009;29:4508–4518. doi: 10.1128/MCB.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]