Abstract
It has long been thought that the same molecules guide both trunk neural crest cells and motor axons as these cell types grow and extend to their target regions in developing embryos. There are common territories that are navigated by these cell types: both cells grow through the rostral portion of the somitic sclerotomes and avoid the caudal half of the sclerotomes. However, these cell types seem to use different molecules to guide them to their target regions. In this Review, I will discuss the common and distinct methods of migration taken by trunk neural crest cells and motor axons as they grow and populate their target regions through chick embryos at the level of the trunk.
Key words: migration, axon, motor neuron, trunk neural crest cells, chick
Introduction
Avian trunk neural crest cells originate in the dorsal neural tube, undergo an epithelial to mesenchymal transition (EMT) and migrate to their target regions where they give rise to a wide range of derivatives including sensory and sympathetic neurons, glia, pigment cells and cells of the adrenal medulla (see Fig. 1).1–3 Trunk neural crest cells have been a well-studied population of migratory cells that can be studied in vitro or in vivo. Evidence that they are guided to their target regions by molecular cues in the environment implicate fibronectin,4–6 laminin,7 integrins,6–10 members of the Eph family11–14 and many other molecules in establishing the avian migratory pattern through rostral, but not caudal, somitic sclerotomes (i.e., talin, vinculin, versican, tenascin). Most of these molecules have inhibitory effects but some of them are growth-promoting.
Figure 1.
Patterns of migration and growth from trunk neural crest cells and motor axons. (A) Trunk neural crest cells originate from the neural plate (NP), and after it undergoes folding, becomes a neural tube (NT). Trunk neural crest cells migrate from the dorsal neural tube and are indicated in gray. (B) Motor neurons form in the ventral neural tube and extend axons to the periphery. (C) To view trunk neural crest cells and motor axons navigating along the body axis, a schematic diagram is shown. Trunk neural crest cells migrate on two pathways to their target regions: (1) a dorsolateral pathway between the ectoderm and somites where these neural crest cells give rise to pigment cells and (2) an ventromedial pathway through the anterior (rostral) sclerotomes but not the posterior (caudal) sclerotomes.
In contrast to trunk neural crest cells, avian motor neurons originate in the ventral neural tube and extend their processes or axons along complicated terrain to specific muscle targets in the axis or the hindlimb (see Fig. 1).
As they mature, motor neurons organize into columns including the medial portion of the medial motor column [MMC(m)] that makes a sharp turn next to the dorsal root ganglia and innervates axial muscles and the lateral motor column (LMC) that projects to and innervates limb muscles (see Fig. 1). Motor axons must find their way to their muscle targets precisely. In describing these cell types, a difference is already visible: trunk neural crest cells migrate as whole cells away from the dorsal neural tube, whereas motor neurons leave their cells bodies in the ventral neural tube and instead grow their processes or axons to target muscles. Avian motor neurons and/or their axons also express Eph family members,15–17 semaphorins and their receptors,18 fibronectin, laminin and integrin receptors.19,20
As trunk neural crest cells leave the dorsal neural tube, they migrate ventromedially and grow through rostral somitic sclerotomes, as well as dorsolaterally, between the ectoderm and somites.1 In a similar manner, motor axons grow through rostral (anterior) somitic sclerotomes.21 Thus, both cell types avoid caudal (posterior) somitic sclerotomes. Therefore, for this portion of their migration or extension, trunk neural crest cells and motor axons travel similar or common pathways.
Results
There is one case where it has been examined in vivo in chick whether trunk neural crest cells and motor axons use the same molecules during their migration.22 Koblar and colleagues were investigating whether members of the Eph family (i.e., Eph receptor tyrosine kinases and their ligands, the ephrins) guided both trunk neural crest cells and motor axons. Using the same fusion proteins that were used previously to disrupt trunk neural crest migration,11 Koblar and colleagues found that although the patterning of trunk neural crest cells was disrupted and trunk neural crest cells were found in the rostral and caudal somitic sclerotomes, they could not alter the patterning of motor axons and they were found instead normally, in rostral somitic sclerotomes (Fig. 2). Importantly, their results suggest that in vivo neural crest cells and motor axons at trunk levels used distinct molecules to guide their growth through rostral somitic sclerotomes to their target regions.
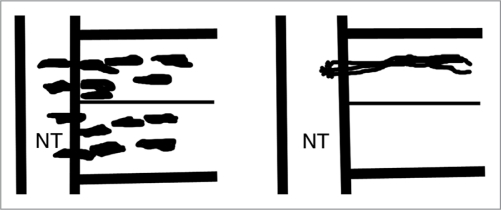
Figure 2.
Schematic diagram showing Koblar et al.22 results. This diagram is magnified to show the rostral and caudal sclerotomes of one somite, with the neural tube (NT) on the left. Applying fusion proteins against ephrins disrupts the patterning of trunk neural crest cell migration (left) but does not alter the patterning of motor axons (right).
The explanation for their findings likely comes from the territory from which the different cell types migrate, the Eph receptors expressed differentially by trunk neural crest and motor neurons/their axons, and which cells they migrate through. In one scenario, trunk neural crest migrate away from the dorsal neural tube. In another scenario, motor neurons extend their axons from the ventral neural tube. There are differences in the expression of Eph receptors by trunk neural crest and motor neurons/their axons.16,22 There may also be differences in the rostral portion of the somitic sclerotomes at the stage these cells migrate through.
Significant evidence indicates that semaphorins affect motor axons.3,23
Semaphorins interact with their receptors neuropilins and plexins and come in two forms: a transmembrane form or soluble form. The expression of semaphorins in avians during trunk neural crest migration has not been reported. Motor axons in mice use semaphorins but in this case, semaphorins have effects on motor axon growth.23 It is not clear whether semaphorins expressed by boundary cap cells (derivatives of neural crest) or motor axons are defective in mouse mutants. It is clear that motor axons grow through the rostral sclerotomes but it is not known that both neural crest cells and motor axons at the same level use the same semaphorins or their receptors to navigate to their target regions in chick or in mouse.
Fibronectin, laminin and their integrin receptors have also been implicated in the migration of trunk neural crest cells in vitro.4–10 The notion is that these factors promote migration of trunk neural crest cells through the rostral sclerotomes. However, mice lacking these factors don't have defects in trunk neural crest migration or motor axon outgrowth, suggesting that other factors or molecules are involved. On one hand, there may be genetic differences between mouse and chick. However, to date, these experiments in vivo have not been performed in chick.
There have been recent studies in cranial neural crest cells that have important implications for motor axon pathfinding. One study shows that in mice, EphB and ephrin-B2 interactions are required for the thymus, a derivative of cranial neural crest cells, to migrate and form in its correct location.24 Apparently, in mice that lack ephrin-B2 in cranial neural crest cells, thymi don't form at their proper location, although they generate T cells and apparently function normally. Do the same receptors and ligands work in chick to provide for the correct migration and formation of the thymi? These experiments must still be done but the expression of Eph family members has been accomplished.25 Importantly, these experiments suggest that Eph family members influence the formation of motor or motor/sensory cranial ganglia. Although some of these experiments have been performed in the chick,26,27 there remains much more to do.
Discussion
Eph family members play a key role in establishing migratory patterns in trunk neural crest cells,11 as well as cranial neural crest cells.24 Disruptions using fusion proteins always result in the migration of trunk neural crest cells into the rostral and caudal somitic sclerotomes. However, the same disruptions do not result in motor axons being mispatterned. Motor axons leave the neural tube normally and navigate to their target muscles. What do the results of these experiments imply? There are different members of the Eph family involved in patterning trunk neural crest cells and motor axons. Our analysis reveals that trunk neural crest cells that migrate ventromedially express EphB receptors and respond to ephrin-B1 in the caudal somitic sclerotomes11 whereas motor neurons from the MMC(m) and their axons express EphA4 and ephrin-As.16
Results thus far demonstrate that some of the semaphorins and their receptors, fibronectin, laminin and their integrin receptors play central roles in trunk neural crest migration and/or motor axon growth. But the experiments have not been done in vivo that allow investigators to proclaim that these molecules work in both processes of trunk neural crest cell migration and motor axon outgrowth through somitic sclerotomes. Mice have been made that lack these factors28–31 but what works in mice may not be the same in chicks, zebrafish, flies or nematodes.
In conclusion, there are a number of reasons to believe that different molecules influence trunk neural crest cells and motor axons as they grow through the rostral somitic sclerotomes in avians. It may be that different combinations of Eph family members also influence cranial neural crest migration and the formation of motor and motor/sensory ganglia25 versus trunk neural crest cells and motor axons that grow through the rostral somitic sclerotomes. I predict that distinct molecules influence these plethora of different cell types, but we will have to wait and see.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/13594
References
- 1.Erickson CA, Reedy MV. Neural crest development: the interplay between morphogenesis and cell differentiation. Curr Top Dev Biol. 1998;40:177–209. doi: 10.1016/s0070-2153(08)60367-1. [DOI] [PubMed] [Google Scholar]
- 2.Knecht AK, Bronner-Fraser Induction of the neural crest: a multigene process. Nat Rev Genetics. 2002;3:453–461. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- 3.Gammill LS, Roffers-Agarwal J. Division of labor during trunk neural crest development. Dev Biol. 2010;344:555–565. doi: 10.1016/j.ydbio.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufour S, Duband JL, Humphries MJ, Obara M, Yamada KM, Thiery JP. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988;7:2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testaz S, Delannet M, Duband J. Adhesion and migration of avian neural crest cells on fibronectin require the cooperating activities of multiple integrins of the (beta)1 and (beta)3 families. J Cell Sci. 1999;112:4715–4728. doi: 10.1242/jcs.112.24.4715. [DOI] [PubMed] [Google Scholar]
- 6.Strachan LR, Condic ML. Neural crest motility on fibronectin is regulated by integrin activation. Exp Cell Res. 2008;314:441–452. doi: 10.1016/j.yexcr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desban N, Duband JL. Avian neural crest cell migration on laminin: interaction of alpha1beta1 integrin with distinct laminin-1 domains mediates different adhesive responses. J Cell Sci. 1997;110:2729–2744. doi: 10.1242/jcs.110.21.2729. [DOI] [PubMed] [Google Scholar]
- 8.Duband JO, Nuckolls GS, Ishihara A, Hasegawa T, Yamada KM, Thiery JP, et al. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988;107:1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- 10.Strachan LR, Condic ML. Neural crest motility and integrin regulation are distinct in cranial and trunk populations. Dev Biol. 2003;259:288–302. doi: 10.1016/s0012-1606(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 11.Krull CE, Lansford R, Gale NW, Collazo A, Marcelle C, Yancopoulos GD, et al. Interactions of Eph-related receptors and ligands confer rostracaudal pattern to trunk neural crest migration. Curr Biol. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- 13.Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- 14.Harris ML, Hall R, Erickson CA. Directing pathfinding along the dorsolateral path-the role of EDNRB2 and EphB2 in overcoming inhibition. Development. 2008;135:4113–4122. doi: 10.1242/dev.023119. [DOI] [PubMed] [Google Scholar]
- 15.Eberhart J, Swartz M, Koblar SA, Pasquale EB, Tanaka H, Krull CE. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev Neurosci. 2000;22:237–250. doi: 10.1159/000017446. [DOI] [PubMed] [Google Scholar]
- 16.Eberhart J, Barr J, O'Connell S, Flagg A, Swartz ME, Cramer K, et al. Ephrin-A5 exerts positive or inhibitory effects on distinct subsets of EphA4-positive neurons. J Neurosci. 2004;24:1070–1078. doi: 10.1523/JNEUROSCI.4719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 18.Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development. 2009;136:1879–1888. doi: 10.1242/dev.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kil SH, Bronner-Fraser M. Expression of the avian alpha 7-integrin in developing nervous system and myotome. Int J Dev Neurosci. 1996;14:181–190. doi: 10.1016/0736-5748(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 20.Agius E, Sagot Y, Duprat AM, Cochard P. Antibodies directed against beta1-integrin subunit and peptides containing the IKAV sequence of laminin perturb neurite outgrowth of peripheral neurons on immature spinal cord substrata. Neuroscience. 1996;71:773–786. doi: 10.1016/0306-4522(95)00447-5. [DOI] [PubMed] [Google Scholar]
- 21.Stern CD, Jaques KF, Lim TM, Fraser SE, Keynes RJ. Segmental lineage restrictions in the chick embryo spinal cord depend on the adjacent somites. Development. 1991;113:239–244. doi: 10.1242/dev.113.1.239. [DOI] [PubMed] [Google Scholar]
- 22.Koblar SA, Krull CE, Pasquale EB, McLennan R, Peale FD, Cerretti DP, et al. Spinal motor axons and neural crest cells use different molecular guides for segmental migration through the rostral half-somite. J Neurobiol. 2000;42:437–447. [PubMed] [Google Scholar]
- 23.Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, et al. Distinct roles for secreted semaphoring signaling in spinal motor axon guidance. Neuron. 2005;48:949–464. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, et al. EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proc Natl Acad Sci USA. 2010;107:13414–13419. doi: 10.1073/pnas.1003747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kury P, Gale N, Connor R, Pasquale E, Guthrie S. Eph receptors and ephrin expression in cranial motor neurons and the branchial arches of chick embryos. Mol Cell Neurosci. 2000;15:123–140. doi: 10.1006/mcne.1999.0812. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Guthrie S, Murakami F. Ephrin A/EphA controls the rostral turning polarity of a lateral commissural tract in chick hindbrain. Development. 2006;133:3837–3846. doi: 10.1242/dev.02564. [DOI] [PubMed] [Google Scholar]
- 27.Prin F, Ng KE, Thaker U, Drescher U. Guthrie S, Ephrin-As play a rhombomere-specific roles in trigeminal motor axons projections in the chick embryo. Dev Biol. 2005;279:402–419. doi: 10.1016/j.ydbio.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- 29.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, et al. Accelerated re-epithelialization in beta3- integrin-deficient mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 31.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, et al. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]