Abstract
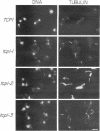
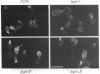
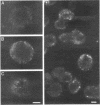
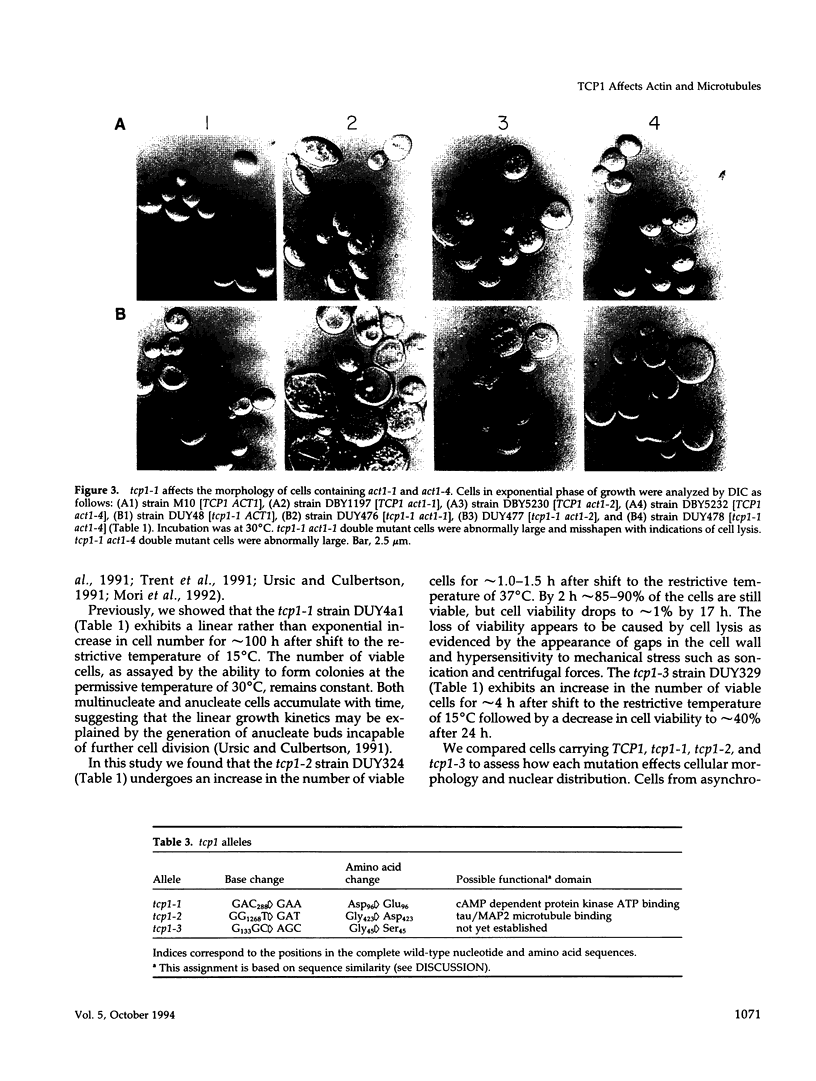
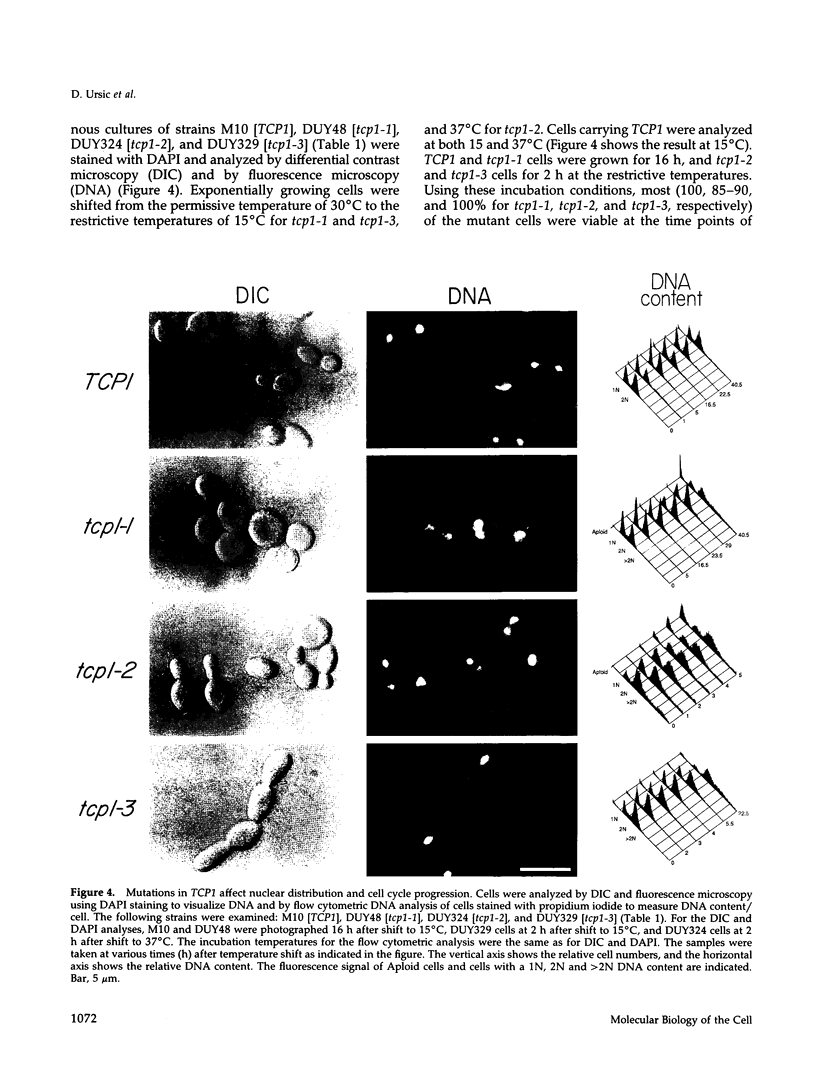
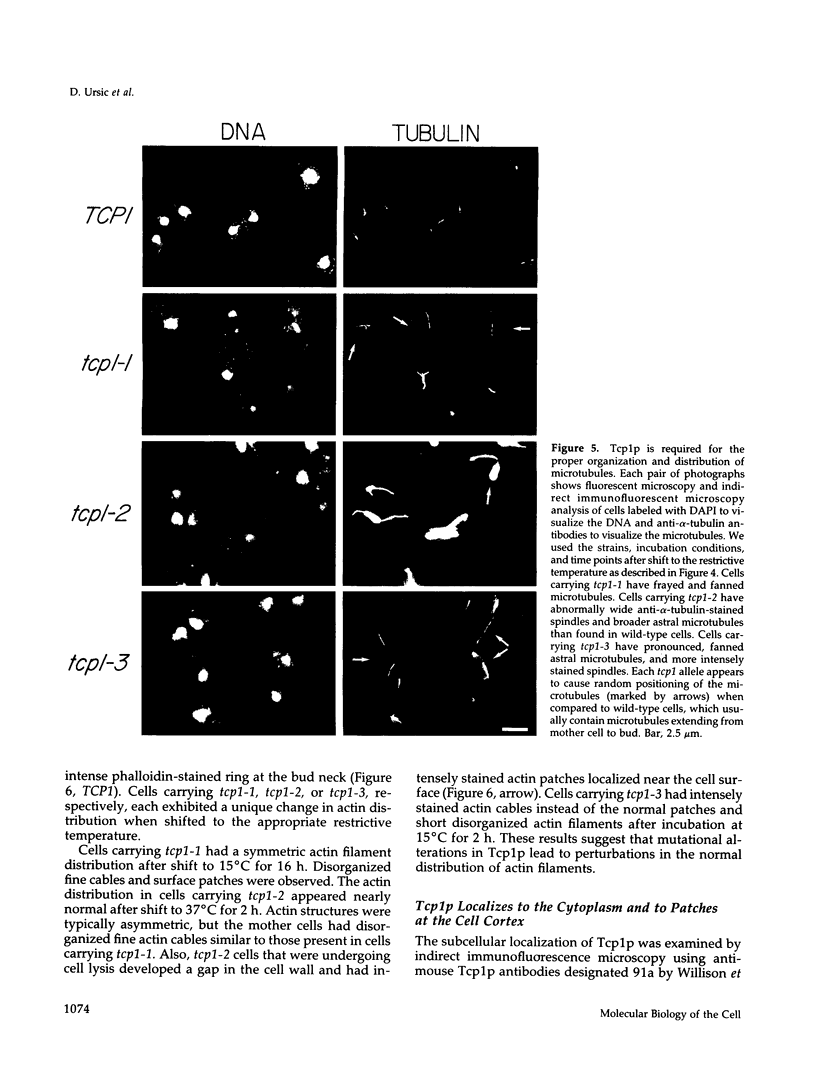
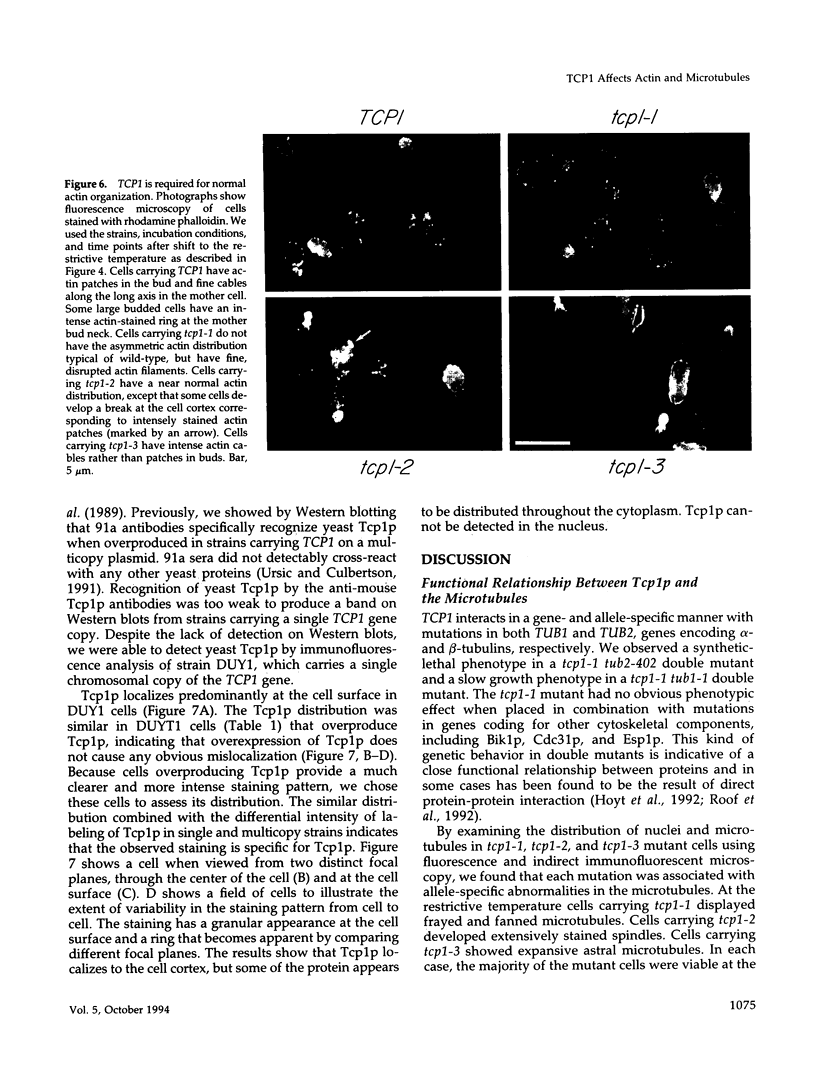
Previously, we showed that the yeast Saccharomyces cerevisiae cold-sensitive mutation tcp1-1 confers growth arrest concomitant with cytoskeletal disorganization and disruption of microtubule-mediated processes. We have identified two new recessive mutations, tcp1-2 and tcp1-3, that confer heat- and cold-sensitive growth. Cells carrying tcp1 alleles were analyzed after exposure to the appropriate restrictive temperatures by cell viability tests, differential contrast microscopy, fluorescent, and immunofluorescent microscopy of DNA, tubulin, and actin and by determining the DNA content per cell. All three mutations conferred unique phenotypes indicative of cytoskeletal dysfunction. A causal relationship between loss of Tcp1p function and the development of cytoskeletal abnormalities was established by double mutant analyses. Novel phenotypes indicative of allele-specific genetic interactions were observed when tcp1-1 was combined in the same strain with tub1-1, tub2-402, act1-1, and act1-4, but not with other tubulin or actin mutations or with mutations in other genes affecting the cytoskeleton. Also, overproduction of wild-type Tcp1p partially suppressed growth defects conferred by act1-1 and act1-4. Furthermore, Tcp1p was localized to the cytoplasm and the cell cortex. Based on our results, we propose that Tcp1p is required for normal development and function of actin and microtubules either through direct or indirect interaction with the major cytoskeletal components.
Full text
PDF





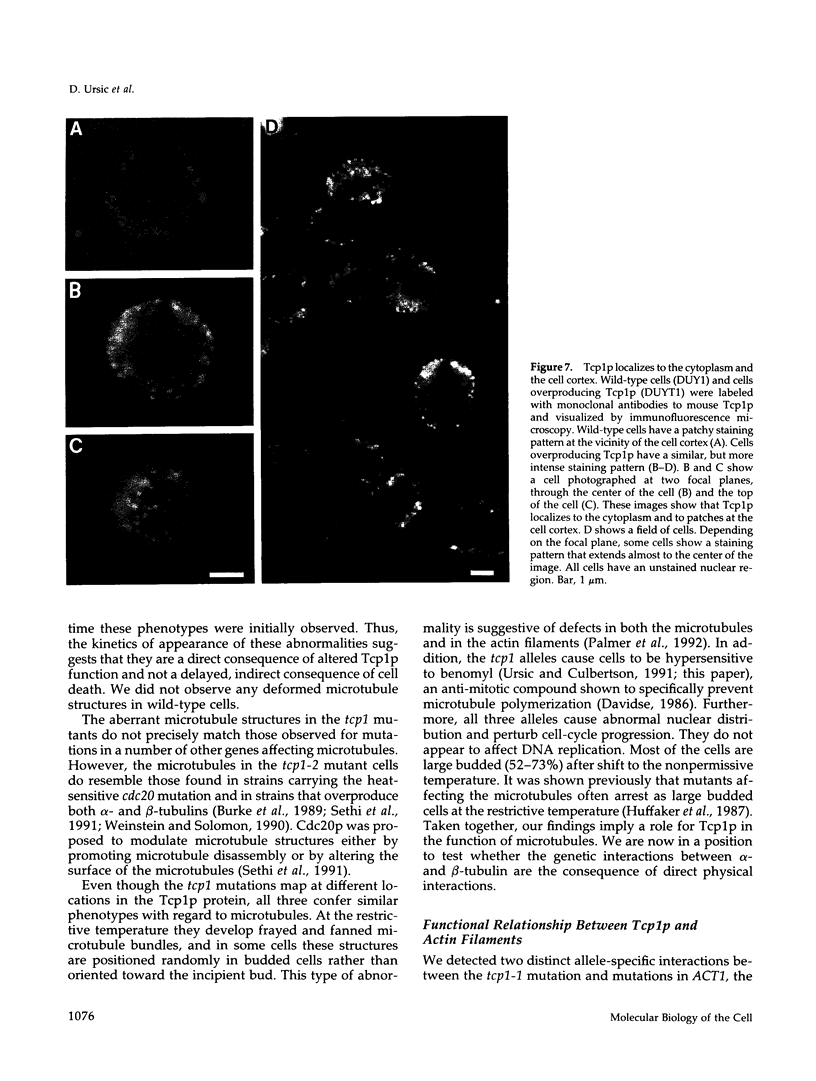




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 1991;194:729–731. doi: 10.1016/0076-6879(91)94054-g. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Gupta R. S. Cloning of a Chinese hamster protein homologous to the mouse t-complex protein TCP-1: structural similarity to the ubiquitous 'chaperonin' family of heat-shock proteins. Biochim Biophys Acta. 1990 Oct 23;1087(2):253–255. doi: 10.1016/0167-4781(90)90214-m. [DOI] [PubMed] [Google Scholar]
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Baum P., Furlong C., Byers B. Yeast gene required for spindle pole body duplication: homology of its product with Ca2+-binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5512–5516. doi: 10.1073/pnas.83.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin V., Styles C. A., Fink G. R. BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J Cell Biol. 1990 Dec;111(6 Pt 1):2573–2586. doi: 10.1083/jcb.111.6.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Burke D., Gasdaska P., Hartwell L. Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1049–1059. doi: 10.1128/mcb.9.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Gambill B. D., Nelson R. J. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993 Jun;57(2):402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T. M., Shortle D. Null alleles of SAC7 suppress temperature-sensitive actin mutations in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2308–2314. doi: 10.1128/mcb.10.5.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. Molecular chaperones: the plant connection. Science. 1990 Nov 16;250(4983):954–959. doi: 10.1126/science.250.4983.954. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Erdjument-Bromage H., Wall J. S., Tempst P., Hartl F. U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992 Dec;11(13):4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga M., Berthet-Colominas C., Yaremchuk A. D., Tukalo M. A., Cusack S. Refined crystal structure of the seryl-tRNA synthetase from Thermus thermophilus at 2.5 A resolution. J Mol Biol. 1993 Nov 5;234(1):222–233. doi: 10.1006/jmbi.1993.1576. [DOI] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G. H., Cowan N. J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992 Jun 12;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gao Y., Vainberg I. E., Chow R. L., Cowan N. J. Two cofactors and cytoplasmic chaperonin are required for the folding of alpha- and beta-tubulin. Mol Cell Biol. 1993 Apr;13(4):2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Sequence and structural homology between a mouse T-complex protein TCP-1 and the 'chaperonin' family of bacterial (GroEL, 60-65 kDa heat shock antigen) and eukaryotic proteins. Biochem Int. 1990;20(4):833–841. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Willison K. R. Protein folding in the cell: functions of two families of molecular chaperone, hsp 60 and TF55-TCP1. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):313–326. doi: 10.1098/rstb.1993.0030. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., He L., Loo K. K., Saunders W. S. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992 Jul;118(1):109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter K. J., Eipel H. E. Flow cytometric determinations of cellular substances in algae, bacteria, moulds and yeasts. Antonie Van Leeuwenhoek. 1978;44(3-4):269–282. doi: 10.1007/BF00394305. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., Lossky M., Beggs J. D. Cloning of the RNA8 gene of Saccharomyces cerevisiae, detection of the RNA8 protein, and demonstration that it is essential for nuclear pre-mRNA splicing. Mol Cell Biol. 1988 Mar;8(3):1067–1075. doi: 10.1128/mcb.8.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap B. K., Walian P. J., Gehring K. Structural architecture of an outer membrane channel as determined by electron crystallography. Nature. 1991 Mar 14;350(6314):167–170. doi: 10.1038/350167a0. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991 May;113(3):539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese P., Mackenzie A., Gibbs A. Nucleotide sequence of the genome of an Australian isolate of turnip yellow mosaic tymovirus. Virology. 1989 Oct;172(2):536–546. doi: 10.1016/0042-6822(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Wang D. H., Cowan N. J. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988 Nov 11;242(4880):936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Hynes G. M., Zheng D., Saibil H., Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992 Jul 16;358(6383):249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Miklos D., Caplan S., Mertens D., Hynes G., Pitluk Z., Kashi Y., Harrison-Lavoie K., Stevenson S., Brown C., Barrell B. Primary structure and function of a second essential member of the heterooligomeric TCP1 chaperonin complex of yeast, TCP1 beta. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2743–2747. doi: 10.1073/pnas.91.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Murata K., Kubota H., Yamamoto A., Matsushiro A., Morita T. Cloning of a cDNA encoding the Tcp-1 (t complex polypeptide 1) homologue of Arabidopsis thaliana. Gene. 1992 Dec 15;122(2):381–382. doi: 10.1016/0378-1119(92)90231-d. [DOI] [PubMed] [Google Scholar]
- Morita T., Kubota H., Gachelin G., Nozaki M., Matsushiro A. Cloning of cDNA encoding rat TCP-1. Biochim Biophys Acta. 1991 Dec 2;1129(1):96–99. doi: 10.1016/0167-4781(91)90219-c. [DOI] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. E., Sullivan D. S., Huffaker T., Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 1992 Nov;119(3):583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Obar R. A., Vallee R. B. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature. 1989 Nov 30;342(6249):569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- Roof D. M., Meluh P. B., Rose M. D. Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol. 1992 Jul;118(1):95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Fink G. R. KAR1, a gene required for function of both intranuclear and extranuclear microtubules in yeast. Cell. 1987 Mar 27;48(6):1047–1060. doi: 10.1016/0092-8674(87)90712-4. [DOI] [PubMed] [Google Scholar]
- Samuels M., Ezzell R. M., Cardozo T. J., Critchley D. R., Coll J. L., Adamson E. D. Expression of chicken vinculin complements the adhesion-defective phenotype of a mutant mouse F9 embryonal carcinoma cell. J Cell Biol. 1993 May;121(4):909–921. doi: 10.1083/jcb.121.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Sethi N., Monteagudo M. C., Koshland D., Hogan E., Burke D. J. The CDC20 gene product of Saccharomyces cerevisiae, a beta-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991 Nov;11(11):5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. M. A structural gene (Tcp-1) within the mouse t complex is separable from effects on tail length and lethality but may be associated with effects on spermatogenesis. Genet Res. 1981 Oct;38(2):115–123. doi: 10.1017/s0016672300020474. [DOI] [PubMed] [Google Scholar]
- Silver L. M., Artzt K., Bennett D. A major testicular cell protein specified by a mouse T/t complex gene. Cell. 1979 Jun;17(2):275–284. doi: 10.1016/0092-8674(79)90153-3. [DOI] [PubMed] [Google Scholar]
- Silver L. M., Remis D. Five of the nine genetically defined regions of mouse t haplotypes are involved in transmission ratio distortion. Genet Res. 1987 Feb;49(1):51–56. doi: 10.1017/s0016672300026720. [DOI] [PubMed] [Google Scholar]
- Silver L. M., White M. A gene product of the mouse t complex with chemical properties of a cell surface-associated component of the extracellular matrix. Dev Biol. 1982 Jun;91(2):423–430. doi: 10.1016/0012-1606(82)90048-3. [DOI] [PubMed] [Google Scholar]
- Stearns T., Botstein D. Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics. 1988 Jun;119(2):249–260. doi: 10.1093/genetics/119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht H., Farr G. W., Sternlicht M. L., Driscoll J. K., Willison K., Yaffe M. B. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus M. A., Warrick H. M., Spudich J. A. Multiple actin-based motor genes in Dictyostelium. Cell Regul. 1989 Nov;1(1):55–63. doi: 10.1091/mbc.1.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. D., Nimmesgern E., Wall J. S., Hartl F. U., Horwich A. L. A molecular chaperone from a thermophilic archaebacterium is related to the eukaryotic protein t-complex polypeptide-1. Nature. 1991 Dec 12;354(6353):490–493. doi: 10.1038/354490a0. [DOI] [PubMed] [Google Scholar]
- Ursic D., Culbertson M. R. Is yeast TCP1 a chaperonin? Nature. 1992 Apr 2;356(6368):392–392. doi: 10.1038/356392a0. [DOI] [PubMed] [Google Scholar]
- Ursic D., Culbertson M. R. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol Cell Biol. 1991 May;11(5):2629–2640. doi: 10.1128/mcb.11.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic D., Ganetzky B. A Drosophila melanogaster gene encodes a protein homologous to the mouse t complex polypeptide 1. Gene. 1988 Sep 7;68(2):267–274. doi: 10.1016/0378-1119(88)90029-7. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Vancompernolle K. Structural relationships of actin-binding proteins. Curr Opin Cell Biol. 1992 Feb;4(1):36–42. doi: 10.1016/0955-0674(92)90056-i. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Gatenby A. A., Lorimer G. H. Purified chaperonin 60 (groEL) interacts with the nonnative states of a multitude of Escherichia coli proteins. Protein Sci. 1992 Mar;1(3):363–369. doi: 10.1002/pro.5560010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelsch S. G. In praise of complexity. Genetics. 1989 Aug;122(4):721–725. doi: 10.1093/genetics/122.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R., Sherman L. A. Chaperones classified. Nature. 1992 Oct 8;359(6395):485–486. doi: 10.1038/359485b0. [DOI] [PubMed] [Google Scholar]
- Weinstein B., Solomon F. Phenotypic consequences of tubulin overproduction in Saccharomyces cerevisiae: differences between alpha-tubulin and beta-tubulin. Mol Cell Biol. 1990 Oct;10(10):5295–5304. doi: 10.1128/mcb.10.10.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G., Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992 Oct;132(2):337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison K. R., Dudley K., Potter J. Molecular cloning and sequence analysis of a haploid expressed gene encoding t complex polypeptide 1. Cell. 1986 Mar 14;44(5):727–738. doi: 10.1016/0092-8674(86)90839-1. [DOI] [PubMed] [Google Scholar]
- Willison K., Kelly A., Dudley K., Goodfellow P., Spurr N., Groves V., Gorman P., Sheer D., Trowsdale J. The human homologue of the mouse t-complex gene, TCP1, is located on chromosome 6 but is not near the HLA region. EMBO J. 1987 Jul;6(7):1967–1974. doi: 10.1002/j.1460-2075.1987.tb02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison K., Lewis V., Zuckerman K. S., Cordell J., Dean C., Miller K., Lyon M. F., Marsh M. The t complex polypeptide 1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell. 1989 May 19;57(4):621–632. doi: 10.1016/0092-8674(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Miklos D., Horwich A. L., Sternlicht M. L., Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992 Jul 16;358(6383):245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- de Vetten N. C., Lu G., Feri R. J. A maize protein associated with the G-box binding complex has homology to brain regulatory proteins. Plant Cell. 1992 Oct;4(10):1295–1307. doi: 10.1105/tpc.4.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]