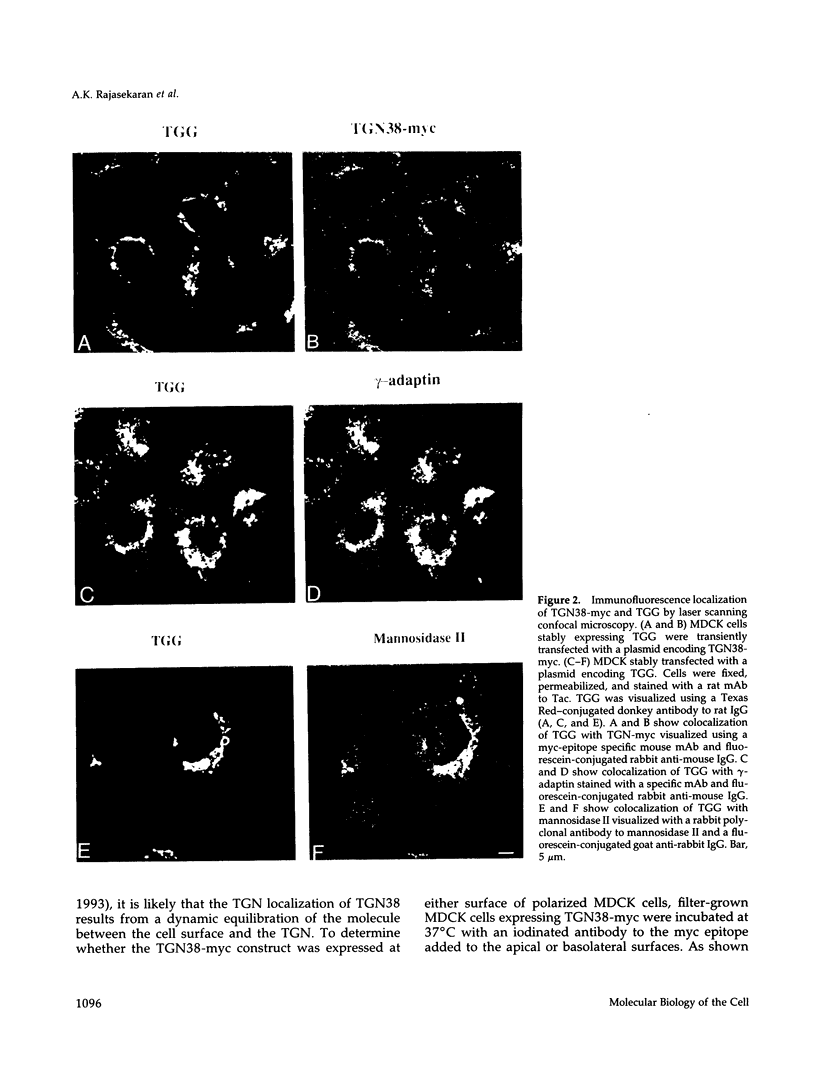
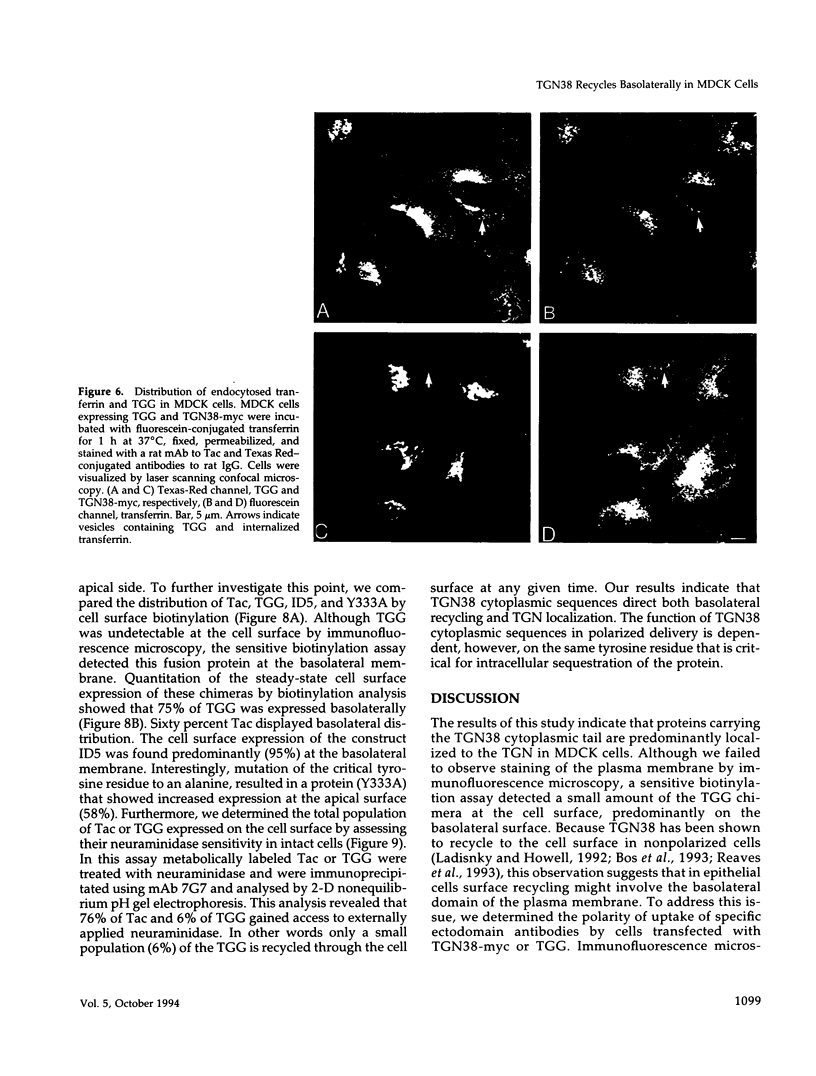
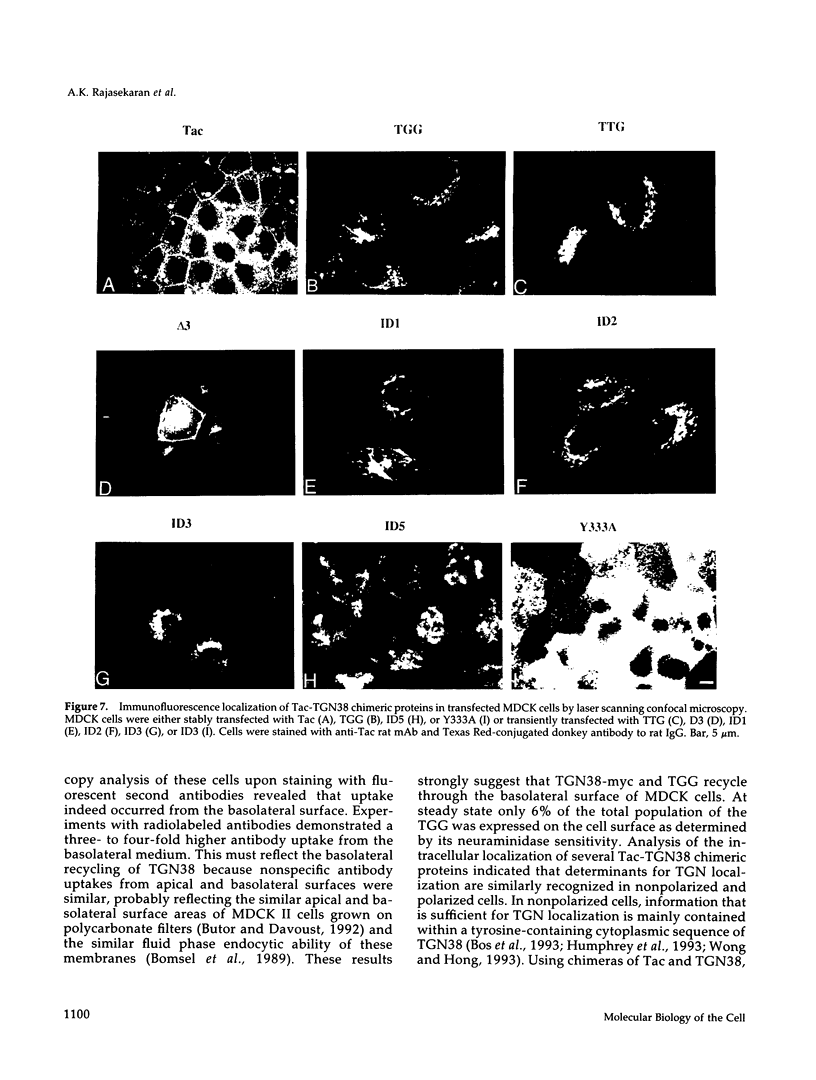
Abstract
Sorting of newly synthesized plasma membrane proteins to the apical or basolateral surface domains of polarized cells is currently thought to take place within the trans-Golgi network (TGN). To explore the relationship between protein localization to the TGN and sorting to the plasma membrane in polarized epithelial cells, we have expressed constructs encoding the TGN marker, TGN38, in Madin-Darby canine kidney (MDCK) cells. We report that TGN38 is predominantly localized to the TGN of these cells and recycles via the basolateral membrane. Analyses of the distribution of Tac-TGN38 chimeric proteins in MDCK cells suggest that the cytoplasmic domain of TGN38 has information leading to both TGN localization and cycling through the basolateral surface. Mutations of the cytoplasmic domain that disrupt TGN localization also lead to nonpolarized delivery of the chimeric proteins to both surface domains. These results demonstrate an apparent equivalence of basolateral and TGN localization determinants and support an evolutionary relationship between TGN and plasma membrane sorting processes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomsel M., Prydz K., Parton R. G., Gruenberg J., Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K., Wraight C., Stanley K. K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993 May;12(5):2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butor C., Davoust J. Apical to basolateral surface area ratio and polarity of MDCK cells grown on different supports. Exp Cell Res. 1992 Nov;203(1):115–127. doi: 10.1016/0014-4827(92)90046-b. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Colley K. J., Lee E. U., Paulson J. C. The signal anchor and stem regions of the beta-galactoside alpha 2,6-sialyltransferase may each act to localize the enzyme to the Golgi apparatus. J Biol Chem. 1992 Apr 15;267(11):7784–7793. [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hsu V. W., Yuan L. C., Nuchtern J. G., Lippincott-Schwartz J., Hammerling G. J., Klausner R. D. A recycling pathway between the endoplasmic reticulum and the Golgi apparatus for retention of unassembled MHC class I molecules. Nature. 1991 Aug 1;352(6334):441–444. doi: 10.1038/352441a0. [DOI] [PubMed] [Google Scholar]
- Humphrey J. S., Peters P. J., Yuan L. C., Bonifacino J. S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993 Mar;120(5):1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Harter C., Matter K., Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991 Sep 6;66(5):907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Tooze S. A. Biosynthetic protein transport in the secretory pathway. Curr Opin Cell Biol. 1989 Aug;1(4):648–654. doi: 10.1016/0955-0674(89)90029-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992 Oct;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Ladinsky M. S., Howell K. E. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur J Cell Biol. 1992 Oct;59(1):92–105. [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Patzak A., Patil N., Chao M., Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991 Nov;115(3):607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Brake B., Banting G., Howell K. E., Braghetta P., Stanley K. K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J. 1990 Aug 15;270(1):97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E. Golgi retention signals: do membranes hold the key? Trends Cell Biol. 1991 Dec;1(6):141–144. doi: 10.1016/0962-8924(91)90001-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews P. M., Martinie J. B., Fambrough D. M. The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J Cell Biol. 1992 Sep;118(5):1027–1040. doi: 10.1083/jcb.118.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992 Nov 27;71(5):741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- McGraw T. E., Dunn K. W., Maxfield F. R. Isolation of a temperature-sensitive variant Chinese hamster ovary cell line with a morphologically altered endocytic recycling compartment. J Cell Physiol. 1993 Jun;155(3):579–594. doi: 10.1002/jcp.1041550316. [DOI] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991 Dec;10(12):3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991 Aug;3(4):585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Prill V., Lehmann L., von Figura K., Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993 May;12(5):2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992 Jan;116(1):85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Horn M., Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993 Jan;4(1):93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. Polarized assembly of enveloped viruses from cultured epithelial cells. Methods Enzymol. 1983;98:486–501. doi: 10.1016/0076-6879(83)98176-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985 Summer;4(2):91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991 Aug;3(4):634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Targeting and processing of glycophorins in murine erythroleukemia cells: use of brefeldin A as a perturbant of intracellular traffic. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6992–6996. doi: 10.1073/pnas.86.18.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Harford J. B., Svetlik P. B., Leonard W. L., Depper J. M., Waldmann T. A., Greene W. C., Klausner R. D. Only high-affinity receptors for interleukin 2 mediate internalization of ligand. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1463–1466. doi: 10.1073/pnas.83.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. H., Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993 Oct 25;268(30):22853–22862. [PubMed] [Google Scholar]
- van 't Hof W., van Meer G. Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J Cell Biol. 1990 Sep;111(3):977–986. doi: 10.1083/jcb.111.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., van 't Hof W. Epithelial sphingolipid sorting is insensitive to reorganization of the Golgi by nocodazole, but is abolished by monensin in MDCK cells and by brefeldin A in Caco-2 cells. J Cell Sci. 1993 Mar;104(Pt 3):833–842. doi: 10.1242/jcs.104.3.833. [DOI] [PubMed] [Google Scholar]