Abstract
In this study, we examined the effects of size, shape, and surface chemistry of gold nanostructures on their uptake (including both adsorption and internalization) by SK-BR-3 breast cancer cells. We used both spherical and cubic Au nanostructures (nanospheres and nanocages, respectively) of two different sizes, and their surface was modified with poly(ethylene glycol) (PEG), antibody anti-HER2, or poly(allyamine hydrochloride) (PAA). Our results showed that the size of the Au nanostructures influenced their uptake by the cells in a similar way regardless of the surface chemistry, while the shape dependency could vary depending on the surface functional group. In addition, the cells preferred to take up the Au nanostructures covered by different surface groups in the following order: PAA>> anti-HER2> PEG. The fraction of Au nanostructures attached to the cell surface was also dependent on the aforementioned parameters.
Keywords: Cellular uptake, gold nanostructure, adsorption, internalization, surface chemistry
Gold-based nanostructures have received increasing interest in biomedical applications owing to their unique optical and photothermal properties.[1] These properties arise from a phenomenon known as localized surface plasmon resonance (LSPR), which typically includes both scattering and absorption components.[1,2] By properly tuning the scattering and absorption cross sections, Au nanostructures can be applied as either contrast agents for optical imaging and thus cancer diagnosis[3–7] or as therapeutic agents for photothermal destruction and thus cancer treatment.[8–10] In addition, rapid conversion of the absorbed light into heat has opened the door to new applications such as controlled release.[11] All of these applications will benefit from an effective way to deliver the Au nanostructure onto and/or into the cancer cells.
Several strategies have been proposed to facilitate the uptake of Au nanostructures by targeted cells, thereby enhancing their efficacy in various biomedical applications. Examples include the variation in size and shape of the nanostructures,[12] as well as surface modification with targeting ligands such as antibodies,[9] and other chemical or biological moieties.[10, 12, 13] Together with these efforts, fundamental studies of the interaction between Au nanostructures and targeted cells have also been undertaken from different angles. For example, Chan and co-workers have observed that the cellular uptake of antibody-modified Au nanospheres strongly depended on the size of the nanospheres.[14] They have also shown that more Au nanospheres were delivered into cells than Au nanorods.[15] Other studies suggested that Au nanostructures having different surface charges will result in different cellular uptakes.[16] However, to date, it is still not clear whether the size of Au nanostructures will influence their uptake by cells regardless of the surface functional group. In addition, in previous studies regarding the shape effect, the surface of the Au nanostructures was not fully characterized, and a comparison between spherical and other shapes (e.g., cubic) has not been conducted. Also, there is essentially no report on the effects of these parameters on the surface adsorption versus internalization by cells. This information is practically important because the location of Au nanostructures relative to a cell membrane can influence the behavior of the cell and possibly the efficacy of a photothermal treatment.
In this study, we have investigated the effects of size, shape, and surface chemistry of Au nanostructures on their adsorption and internalization by SK-BR-3 breast cancer cells. Specifically, we synthesized Au nanospheres (spherical shape) and Au nanocages (cubic shape) of two different sizes, and then studied their uptake by SK-BR-3 cells. The surface of these Au nanostructures were also modified with poly(ethylene glycol) (PEG), anti-HER2, and poly(allyamine hydrochloride) (PAA) to investigate their impact on cellular uptake. Typically, these ligands have been explored for targeting cancer cells or tissues and increasing the delivery efficiency of genes into cells.[17] In addition, we further differentiated the Au nanostructures attached to the cell surface from those being internalized into the cells by using an etching system based on iodine (I2) and potassium iodide (KI) that can selectively dissolve the Au nanostructures attached to the cell surface.[16a] Combined together, this research has allowed us to achieve a deeper understanding of the cellular uptake process of nanostructured materials.
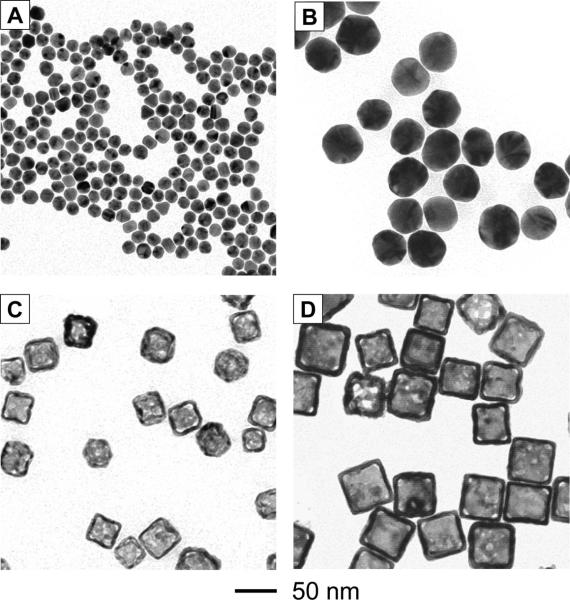
Figure 1 shows transmission electron microscopy (TEM) images of the Au nanospheres and nanocages, each with two different sizes. We synthesized the 15-nm Au nanospheres by reducing chloroauric acid (HAuCl4) with sodium citrate while we obtained the 45-nm Au nanospheres from a commercial source. The Au nanocages were prepared by titrating Ag nanocubes with HAuCl4.[5b] The 33-nm and 55-nm Au nanocages were prepared from Ag nanocubes of 30 and 50 nm in edge length, respectively.
Figure 1.
TEM images of (A) 15-nm Au nanospheres, (B) 45-nm Au nanospheres, (C) 33-nm Au nanocages, and (D) 55-nm Au nanocages.
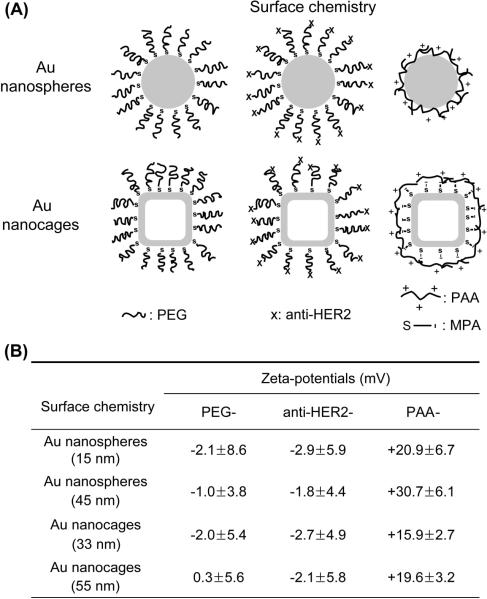
The as-prepared Au nanostructures were capped by different chemical species on the surface such as citrate and poly(vinyl pyrrolidone) (PVP). To properly compare the effect(s) of size, shape, and surface chemistry between the different Au nanostructures, we modified their surface with the same functional group, as illustrated in Figure 2A. For PEG modification, methoxy-terminated PEG-thiol (mPEG-SH, Mw≈5,000) was added to a dispersion of the Au nanostructures, followed by incubation for 12 h and removal of the unreacted PEG-thiol. Antibody anti-HER2 was selected as the targeting ligand for this study because the SK-BR-3 cells are known to overexpress HER2 receptors. The surface modification involved two steps. Succinimidyl propionyl PEG disulfide (Mw≈5,000) was first grafted to the surface of the Au nanostructures, followed by the addition of anti-HER2. The succinimidyl group on the Au nanostructures then reacted with the amine group of the antibody. We employed electrostatic assembly to coat the surface of the Au nanostructures with PAA. For Au nanospheres, we added PAA solution to the aqueous dispersion of citrate-stabilized nanospheres to induce the deposition of a PAA layer on their surface.[16a] For Au nanocages, we first displaced the PVP on the surface with mercaptopropionic acid (MPA), followed by the introduction of PAA. As shown in Figure 2B, both the PEG- and anti-HER2-modified Au nanostructures were essentially neutral or slightly negative while the PAA-coated nanostructures had positive charges on the surface. These results confirm that the surface of the Au nanostructures had been derivatized with the desired functional groups. For the PAA-coated Au nanostructures, there were some differences in terms of surface charge density, but we found that these differences had a negligible effect on the explanation about the influence of size and shape on the cellular uptake for the Au nanostructures.
Figure 2.
(A) Schematic showing Au nanostructures whose surface was modified with different functional groups. (B) Summary of zeta-potentials for the Au nanostructures whose surface were modified with different chemical species. Abbreviation: PEG = poly(ethylene glycol); PAA = poly(allyamine hydrochloride); MPA = mercaptopropionic acid.
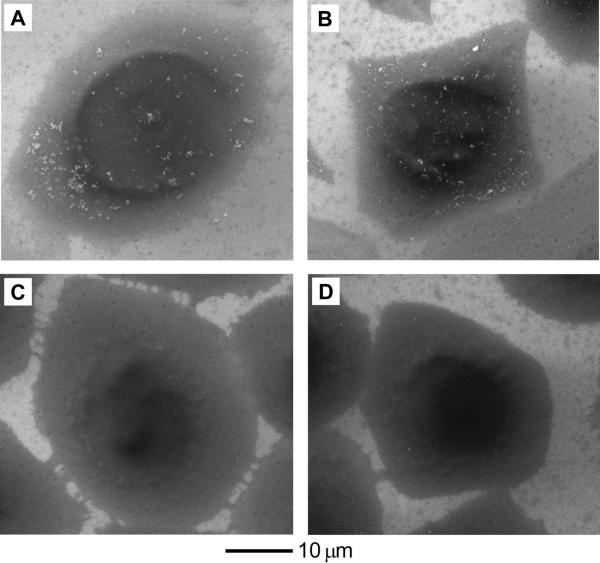
We next conducted cell uptake studies with these Au nanostructures and SK-BR-3 cells. We incubated culture media containing 0.02 nM (particle concentration) of Au nanostructures with SK-BR-3 at 37 °C for 24 h, and the number of nanostructures up-taken by the cells was then quantified using inductively coupled plasma mass spectroscopy (ICP-MS). At this low particle concentration, all types of the Au nanostructures were found to be not significantly toxic to the cells (see Figure S1, where 90% of the cells remained viable relative to the untreated control). We also differentiated the Au nanostructures attached to the cell surface from those being internalized into the cells by treating the samples with an etchant based on I2/KI after incubation with the Au nanostructures. In a recent study, we demonstrated that I2/KI could selectively dissolve Au nanospheres attached to the cell surface, leaving behind those inside the cells untouched.[16a] In addition, this etching method could maintain over 90% viability for the SK-BR-3 cells when the etching was conducted at a concentration of 0.34 mM for I2 and for 5 min. To further demonstrate the feasibility of this etchant, we compared scanning electron microscopy (SEM) images between SK-BR-3 cells after incubation with PAA-coated Au nanostructures (Figure 3, A and B) and those incubated with identical nanostructures and then treated with the etching solution for 5 min (Figure 3, C and D). Before etching, we clearly observe the presence of Au nanospheres and Au nanocages (white specks), respectively, deposited on the cell surface. After etching, these Au nanostructures were no longer visible. These results suggest that the etching could be used to distinguish the Au nanostructures attached to the cell surface from those being internalized into the cells.
Figure 3.
(A, B) SEM images of SK-BR-3 cells after incubation with the PAA-coated (A) 45-nm Au nanospheres and (B) 33-nm Au nanocages at 37 °C for 24 h, respectively. (C, D) SEM images of SK-BR-3 cells after incubation with the PAA-coated (C) 45-nm Au nanospheres and (D) 33-nm Au nanocages at 37 °C for 24 h, respectively, followed by etching with 0.34 mM I2 for 5 min. The particle concentration of Au nanostructures in the culture medium was 0.02 nM.
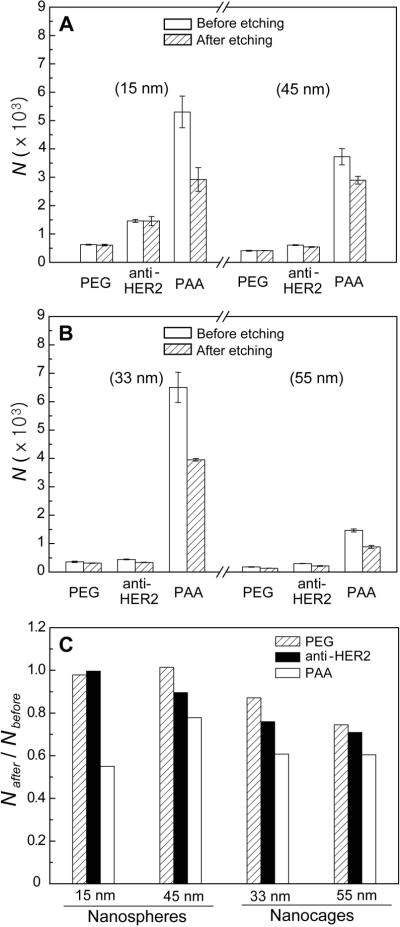
Figure 4, A and B, plots the number of Au nanospheres and nanocages up-taken per SK-BR-3 cell. We also quantified the number of Au nanostructures being internalized per cell after removal of the surface-bound nanostructures with the I2/KI etchant. First, let us take a look at the size effect. The data clearly shows that the cells had a higher uptake for smaller Au nanostructures (including both nanospheres and nanocages) than the larger ones, regardless of the functional group on the surface. For the nanospheres, the cell had 1.5–2.4 times higher uptake for the 15-nm than for the 45-nm samples. For the nanocages, the 33-nm sample showed 1.5–4.4 times higher uptake than the 45-nm sample. This result is in contrast with the previous report by Chan and co-workers.[14] They reported that 40–50 nm was an optimal size range for maximizing the uptake of antibody-modified Au nanospheres. Another publication reported that large silica and iron oxide nanoparticles were taken up by cells more than small ones when the surface was modified with a dye and dextran, respectively.[18] These authors, however, only compared the uptake in terms of mass, not number of nanoparticles. When their results are converted to the number of particles, the smaller ones were taken up more than the larger ones. In addition, a recent study of Au nanoparticles by Xu et al. also supported our results.[19] It is hard to explain the difference between our results and those by Chan and co-workers. In their studies, they grafted antibodies to Au nanospheres by directly attaching antibodies to the surface of Au nanospheres. In our work, we attached the anti-HER2 to the end of PEG chains that had been grafted to Au nanostructures to prevent denaturation of the antibody. Therefore, it might be possible that the different preparation methods have resulted in different densities and structures for the antibodies used in these two studies, which may account for the discrepancy between the results.
Figure 4.
(A) Uptake of surface-modified Au nanospheres by SK-BR-3 cells after incubation at 37 °C for 24 h, followed by etching with 0.34 mM I2 for 5 min. N represents the number of uptaken Au nanostructures per cell. (B) Uptake of surface-modified Au nanocages by SK-BR-3 cells under the same conditions as for nanospheres. (C) Ratio of the numbers of Au nanostructures after etching (Nafter) to that before etching (Nbefore) for the surface-modified Au nanostructures. The number of samples we tested was 6 for each data point.
With regard to the effect of shape, the SK-BR-3 cells seem to prefer spherical particles over cubic particles when the surface was modified with PEG or anti-HER2. This is supported by the observation that the 45-nm Au nanospheres (PEG: 410 per cell; anti-HER2: 605 per cell) showed a slightly higher uptake than both the 33-nm Au nanocages (PEG: 358 per cell; anti-HER2: 442 per cell) and 55-nm Au nanocages (PEG: 179 per cell; anti-HER2: 300 per cell). However, when the surface of the Au nanostructures was coated with PAA, the 33-nm Au nanocages showed a higher uptake by the cells (6,500 per cell) than both the 15-nm Au nanospheres (5,300 per cell) and the 45-nm Au nanospheres (3,700 per cell). These results suggest that the effect of shape is susceptible to the specific functional group on the nanostructure's surface.
As for the dependence on surface chemistry, the uptake of various Au nanostructures by the SK-BR-3 cells decreased in the following order: PAA >> anti-HER2 > PEG. This trend was across the board for all Au nanostructures with different sizes and shapes. It is worth pointing out that the Au nanostructures functionalized with anti-HER2 showed a less uptake by the cells than the PAA-coated Au nanostructures. We previously reported that the rate constant (0.011 min−1) for internalization of PAA-coated Au nanospheres was comparable to that (0.012 – 0.033 min−1) of anti-HER2-tagged liposomes.[16a, 20] For this reason, we had expected that the anti-HER2-modified Au nanostructures should be taken up by the cells at a level comparable to the PAA-coated ones. The observed difference can be explained as follows. Liposomes are known to be soft in terms of structure, which can increase the uptake of drugs into cells via liposome-cell membrane fusion and endocytosis of liposome by the cell.[21] In contrast, the Au nanostructures are very rigid in terms of structure, whose uptake is thought to be only dependent on the receptor-mediated endocytosis. Therefore, although liposome and Au nanostructures are modified with the same chemical group, their uptake by the cells can also be influenced by other physical and/or chemical properties such as structural rigidity. The PAA-coated Au nanostructures generally show very high affinities to the cell membrane as many of them could be readily attached to the cell membrane via electrostatic interactions. In this case, the fluidity and permeability of the cell membrane can only be changed back to normal by getting rid of the attached Au nanostructures through internalization.[22–27] In addition, the nanostructures could also be delivered into cells via an ATP-independent process by generating a hole or disruption in the cell membrane.[28] These arguments are sufficient to explain the highest uptake of the PAA-coated Au nanostructures by the cells.
Through selective etching, we found that the fraction of Au nanostructures on the cell surface also increased in the order of PAA > anti-HER2 ≥ PEG (Figure 4C). In general, delivery of nanoparticles into a cell involved their adsorption onto the cell surface, followed by internalization. Since the rate of adsorption was slower than that of internalization, adsorption is the rate-limiting step that determines the amount of internalized Au nanostructures.[16a] The PEG-modified Au nanostructures could hardly adhere to the cell membrane so that their adsorption rate by the cells was the lowest among the Au nanostructures with three different surface groups. In this case, they were readily internalized as soon as they arrived at the cell membrane, remaining very few on the cell surface due to their low adhesion to the cell membrane. However, it is interesting to note that there were more PEG-modified nanocages (especially for the 55-nm sample) on the cell surface than the PEG-modified nanospheres. This result indicates that the shape of the nanostructures could impair the internalization process. We also found that the receptor-mediated endocytosis had some dependence on the shape of the nanostructures. While the anti-HER2-modified nanospheres internalized by cells represent >90% of the total uptake, only 70–80% of the anti-HER2-modified nanocages were internalized. In addition, the fraction of Au nanostructures on the cell surface increased with increasing the size. Meanwhile, when the Au nanostructures were highly adhesive to the cell membrane (e.g., PAA-coated sample), a considerable amount of the Au nanostructures could remain on the cell surface. In this case, the fraction of Au nanostructures on the cell surface was not much influenced by the geometric shape.
In conclusion, we have examined the effects of size, shape, and surface chemistry of the Au nanostructures on their uptake (including both adsorption and internalization) by SK-BR-3 cells. In some cases, we found that the uptake of Au nanostructures by the cells was influenced by size and shape of the Au nanostructures, but the dependency on these parameters (especially for shape) could be varied when the surface was covered by different functional groups. The fraction of Au nanostructures attached to the cell surface was also influenced by these parameters. This study is fundamentally helpful in understanding the delivery mechanisms of nanoparticle-based carriers commonly used in biomedical applications such as delivery of drugs, gene, or contrast agents for biomedical imaging and therapy.
Experimental Section
Preparation of Au nanostructures
The 15-nm Au nanospheres were prepared by adding 1 mL of 0.5 wt% sodium citrate to 19 mL of 0.2 mM HAuCl4 (Aldrich) aqueous solution at 100 °C. The 45-nm Au nanospheres were purchased from Ted Pella Inc. (Redding, CA). A brief description of the synthesis of Au nanocages is as follows. Silver nitrate was reduced in the presence of PVP with a sulfide-mediated synthesis to form 30-nm and 50-nm Ag nanocubes, respectively. We then converted these Ag nanocubes into Au nanocages by adding 9 mL of 0.2 mM HAuCl4 aqueous solution dropwise to the Ag nanocubes dispersed in 1 mg/mL PVP aqueous solution at 100 °C.
Surface modification of Au nanostructures
The PEG-modified Au nanospheres were prepared by adding 0.5 mL of 1 mM mPEG-SH (Laysan Bio Inc., Arab, AL) to 1 mL of the as-prepared or as-obtained Au nanospheres at room temperature. After 12 h, the PEG-modified Au nanospheres were separated by centrifugation and redispersed in deionized water. For the anti-HER2-modified Au nanospheres, we first removed excess stabilizer (e.g., citrate ions) and gold precursors in the as-prepared Au nanospheres dispersions by dialysis. We then added 0.5 mL of 1 mM succinimidyl propionyl PEG disulfide (Laysan Bio Inc.) to 1 mL of the dispersion of Au nanospheres. After 8 h at room temperature, the nanospheres were centrifuged and redispersed in phosphate buffered saline (PBS, pH 7.4), which was followed by addition of 40 μL anti-HER2 (Invitrogen) solution. After storing for 12 h at 7 °C, the solid was recovered by centrifugation and redispersed in PBS. The experimental procedures for the PEG- and anti-HER2-covered Au nanocages were described in previous publications.[9b,c] For positively-charged Au nanospheres, we followed the same procedure described in ref. 15a. For PAA-coated Au nanocages, we first mixed 100 μL of 0.1 mM mercaptopropionic acid (MPA, Aldrich) with 1 mL of the Au nanocages at room temperature and waited for 24 h. After purification by centrifugation, we added 10 μL of 0.1 wt% PAA to 1 mL of the dispersion of Au nanocages at room temperature. After 24 h, the dispersion was purified again by centrifugation and additional 10 μL of PAA was added to completely cover the Au nanocages with PAA. The excess PAA was removed by centrifugation. In the present work, we only used samples without any aggregation or precipitation for the Au nanostructures in the culture medium for 24 h. This can be easily confirmed from the color of the suspension or more clearly from the UV-Vis spectra. If there is aggregation, the surface plasmon resonance peak associated with the Au nanostructures will be red-shifted. The sign of surface charges on these nanostructures was measured using 90 Plus Particle Size Analyzer (Brookhaven Instrument Corp., Holtsville, NY) which is equipped with a zeta-potential analyzer.
Cell culture
Human breast cancer cells (SK-BR-3, ATCC HTB-30™) were cultured in McCoy's 5a Medium Modified (ATCC Cat. No. 30-2007), supplemented with 10% fetal bovine serum (FBS, Invitrogen), and 1% antibiotics (containing penicillin and streptomycin, Invitrogen). The medium was changed every other day, and the culture was incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Preparation of ICP-MS samples
We first treated the culture medium containing the Au nanostructures (0.02 nM in particle concentration) to the cells (80% confluence) cultured in a 6-well plate. After 24 h, we removed culture medium, washing with PBS three times. The cells were then treated with 1.4 mL of trypsin solution (2%) at 37 °C and the trypsinized cell dispersion was further sonicated in a hot water bath (60 °C) to completely disrupt the cell membranes. Finally, the Au nanostructures were dissolved by successfully adding 0.3 mL hydrochloric acid and 0.1 mL nitric acid to the solution. The concentration of Au, determined by ICP-MS, was converted to the number of Au nanostructures per cell. The number of cells in each well was counted by hematocytometer. For each data point, we obtained the average and standard deviation by testing 6 samples (two separate trials, with 3 parallel experiments in each trial).
Etching of Au nanostructures attached to the cell surface
The etching solution was prepared by mixing I2 (Aldrich) and KI (Aldrich) in deionized water with a molar ratio of 1:6. After incubation of Au nanostructures with the cells, the sample was washed with PBS three times, and 1 mL of the I2/KI aqueous solution (0.34 mM of I2) was added to each well. After etching at room temperature for 5 min, the solution was removed, the culture plate was washed with 2 mL of deionized water, and 1.4 mL trypsin solution (2%) was added. The remaining steps are the same as those described in the previous section.
SEM imaging of SK-BR-3 cells
After the cells had been incubated with Au nanostructures and washed with PBS three times, we fixed the cells with cold methanol (−20 °C) for 10 min. We then washed the cells with tris buffered saline three times and finally with deionized water once. After drying the cells at 7 °C, we took SEM images from these cells. The acceleration voltage was 5 kV and we did not coat the cell surface with any conductive material. The cells treated with I2/KI were processed using the same procedure.
Supplementary Material
Acknowledgments
This work was supported by a 2006 NIH Pioneer Award (5DP1 OD000798). E.C.C. was also partially supported by a postdoctoral fellowship from the Korea Research Foundation (KRF-2007-357-D00070) funded by the Korean Government. Part of the research was conducted at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the NSF under award no. ECS-0335765. NRF is part of School of Engineering and Applied Science at Washington University in St. Louis.
Footnotes
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
References
- [1].a) Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Acc. Chem. Res. 2008;41:1578. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]; b) Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y. Acc. Chem. Res. 2008;41:1587. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xia Y, Halas NJ. MRS Bull. 2005;30:338. [Google Scholar]
- [3].El-Sayed MA. Acc. Chem. Res. 2001;34:257. doi: 10.1021/ar960016n. [DOI] [PubMed] [Google Scholar]
- [4].a) Mohamed MB, Volkov V, Link S, El-Sayed MA. Chem. Phys. Lett. 2000;317:517. [Google Scholar]; b) Hu M, Chen J, Li Z-Y, Au L, Hartland GV, Li X, Marquez M, Xia Y. Chem. Soc. Rev. 2006;35:1084. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- [5].a) Métraux GS, Cao YC, Jin R, Mirkin CA. Nano Lett. 2003;3:519. [Google Scholar]; b) Skrabalak SE, Au L, Li X, Xia Y. Nat. Prot. 2007;2:2182. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]; c) Siekkinen AR, McClellan JM, Chen J, Xia Y. Chem. Phys. Lett. 2006;432:491. doi: 10.1016/j.cplett.2006.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Zagaynova EV, Shirmanova MV, Kirillin MY, Khlebtsov BN, Orlova AG, Balalaeva IV, Sirotkina MA, Bugrova ML, Agrba PD, Kamensky VA. Phys. Med. Biol. 2008;53:4995. doi: 10.1088/0031-9155/53/18/010. [DOI] [PubMed] [Google Scholar]; b) Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li XD, Xia Y. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]; c) Alric C, Taleb J, Duc GL, Mandon C, Billotey C, Meur-Herland AL, Brochard T, Vocanson F, Janier M, Perriat P, Roux S, Tillement O. J. Am. Chem. Soc. 2008;130:5908. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- [7].a) Kim K, Huang S-W, Ashkenazi S, O'Donnell M, Agarwal A, Kotov NA, Denny MF, Kaplan MJ. Appl. Phys. Lett. 2007;90:223901. [Google Scholar]; b) Agarwal A, Huang S-W, O'Donnell M, Day KC, Day M, Kotov M, Ashkenazi S. J. Appl. Phys. 2007;102:064701. [Google Scholar]; c) Yang X, Skrabalak SE, Li Z-Y, Xia Y, Wang LV. Nano Lett. 2007;7:3798. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]; d) Song KH, Kim C, Cobley CM, Xia Y, Wang LV. Nano Lett. 2009;9:183. doi: 10.1021/nl802746w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Adler DC, Huang S-W, Huber R, Fujimoto JG. Opt. Exp. 2008;16:4376. doi: 10.1364/oe.16.004376. [DOI] [PubMed] [Google Scholar]; b) van Dijk MA, Tchebotareva AL, Orrit M, Lippitz M, Berciaud S, Lasne D, Cognetc L, Lounisc B. Phys. Chem. Chem. Phys. 2006;8:3486. doi: 10.1039/b606090k. [DOI] [PubMed] [Google Scholar]
- [9].a) Li JL, Day D, Gu M. Adv. Mater. 2008;20:3866. [Google Scholar]; b) Au L, Zheng D, Zhou F, Li Z-Y, Li X, Xia Y. ACS Nano. 2008;2:1645. doi: 10.1021/nn800370j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li Z-Y, Zhang H, Xia Y, Li X. Nano Lett. 2007;7:1318. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Adv. Mater. 2008;20:3832. [Google Scholar]
- [11].Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song K, Schwartz AG, Wang LV, Xia Y. Nat. Mater. 2009 doi: 10.1038/nmat2564. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sonavane G, Tomoda K, Makino K. Coll. Sur. B. 2008;66:274. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- [13].a) Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv. Drug Del. Rev. 2008;60:1307. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]; b) Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]; c) Ghosh PS, Kim C-K, Han G, Forbes NS, Rotello VM. ACS Nano. 2008;2:2213. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Chithrani BD, Chan WCW. Nano Lett. 2007;7:1542. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]; b) Jiang W, Kim BYS, Rutka JT, Chan WCW. Nat. Nanotech. 2008;3:145. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- [15].Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- [16].a) Cho EC, Xie J, Wurm PA, Xia Y. Nano Lett. 2009;9:1080. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]; b) Hauck TS, Ghazani AA, Chan WCW. Small. 2008;4:153. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- [17].Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit J-P. Biomaterials. 2008;29:3477. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- [18].a) Lu F, Wu S-H, H. Y, Mou C-Y. Small. 2009;5:1408. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]; b) Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, Poremba C, Ebert W, Heindel W, Bremer C. Radiology. 2005;235:155. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- [19].Xu C, Tung GA, Sun S. Chem. Mater. 2008;20:4167. doi: 10.1021/cm8008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kirpotin D, Park JW, Hong K, Zalipsky S, Li W-L, Carter P, Benz CC, Papahadjopoulos D. Biochemistry. 1997;36:66. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- [21].Duzgunes N, Nir S. Adv. Drug Del. Rev. 1999;40:3. doi: 10.1016/s0169-409x(99)00037-x. [DOI] [PubMed] [Google Scholar]
- [22].Larsen B. Mol. Cell. Biochem. 1977;15:117. doi: 10.1007/BF01793333. [DOI] [PubMed] [Google Scholar]
- [23].Skutelsky E, Danon D. J. Cell Biol. 1976;71:232. doi: 10.1083/jcb.71.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mutsaers SE, Papadimitriou JM. J. Luek. Biol. 1988;44:17. doi: 10.1002/jlb.44.1.17. [DOI] [PubMed] [Google Scholar]
- [25].Farquhar MG. J. Cell Biol. 1978;78:R35. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seiler MW, Venkatachalam MA, Cotran RS. Science. 1975;189:390. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- [27].Quinton PM, Philpott CW. J. Cell Biol. 1973;56:787. doi: 10.1083/jcb.56.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Leroueil PR, Hong S, Mecke A, Baker JR, Jr., Orr BG, Holl MMB. Acc. Chem. Res. 2007;40:335. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F. Nat. Mater. 2008;7:588. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen J, Hessler JA, Putchakayala K, Panama BK, Khan DP, Hong S, Mullen DG, DiMaggio SC, Som A, Tew GN, Lopatin AN, Baker JR, Jr., Holl MMB, Orr BG. J. Phys. Chem. B. 2009;113:11179. doi: 10.1021/jp9033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.