Abstract
Objective To examine whether treatment of periodontal disease with scaling and root planing during pregnancy is associated with a reduction in the preterm birth rate.
Design Systematic review and meta-analysis of randomised controlled trials.
Data sources Cochrane Central Trials Registry, ISI Web of Science, Medline, and reference lists of relevant studies to July 2010; hand searches in key journals.
Study selection Randomised controlled trials including pregnant women with documented periodontal disease randomised to either treatment with scaling and root planing or no treatment.
Data extraction Data were extracted by two independent investigators, and a consensus was reached with the involvement a third. Methodological quality of the studies was assessed with the Cochrane’s risk of bias tool, and trials were considered either high or low quality. The primary outcome was preterm birth (<37 weeks). Secondary outcomes were low birthweight infants (<2500 g), spontaneous abortions/stillbirths, and overall adverse pregnancy outcome (preterm birth <37 weeks and spontaneous abortions/stillbirths).
Results 11 trials (with 6558 women) were included. Five trials were considered to be of high methodological quality (low risk of bias), whereas the rest were low quality (high or unclear risk of bias). Results among low and high quality trials were consistently diverse; low quality trials supported a beneficial effect of treatment, and high quality trials provided clear evidence that no such effect exists. Among high quality studies, treatment had no significant effect on the overall rate of preterm birth (odds ratio 1.15, 95% confidence interval 0.95 to 1.40; P=0.15). Furthermore, treatment did not reduce the rate of low birthweight infants (odds ratio 1.07, 0.85 to 1.36; P=0.55), spontaneous abortions/stillbirths (0.79, 0.51 to 1.22; P=0.28), or overall adverse pregnancy outcome (preterm births <37 weeks and spontaneous abortions/stillbirths) (1.09, 0.91 to 1.30; P=0.34).
Conclusion Treatment of periodontal disease with scaling and root planing cannot be considered to be an efficient way of reducing the incidence of preterm birth. Women may be advised to have periodical dental examinations during pregnancy to test their dental status and may have treatment for periodontal disease. However, they should be told that such treatment during pregnancy is unlikely to reduce the risk of preterm birth or low birthweight infants.
Introduction
Periodontitis is a relatively common clinical condition, which occurs in more than 30% of people in some populations1; it has a prevalence of between 5% and 20% in pregnant women.2 Treatment in pregnancy is safe and easily applicable and involves scaling and root planing.3 An association between periodontal disease and preterm birth has engendered much interest. Despite advances in obstetric care, preterm birth continues to be the leading cause of perinatal morbidity and mortality. This suggestion has led many investigators to seek evidence in this field. Since 1996, when a relation of periodontal disease with preterm birth was proposed,4 many observational studies have been carried out. Although the pathophysiological mechanism remains unclear, several studies support the hypothesis that periodontal disease is associated with preterm labour and other conditions complicating pregnancy, such as pre-eclampsia and fetal growth restriction.5 This association has also been reported by most of the 17 observational studies up to 2005 that were included in a meta-analysis published by Vergnes,6 which concluded that pregnant patients with periodontal disease have a 2.8-fold increased risk of preterm birth.
Researchers have suggested that periodontal disease causes the release of pathogens or inflammatory products such as cytokines, which then affect embryonic tissue or amniotic fluid through haematogenous transport.7 Taking into account the fact that treatment of periodontal disease is simple and safe during pregnancy,3 many studies have examined whether active management of periodontal disease has a potential beneficial effect on outcomes of pregnancy. In 2006 several of the US insurers offered their clients scaling and root planing during pregnancy at no extra cost,8 saying that they expected that spending more on preventive dental care would yield big savings in the medical treatment of costly chronic illnesses. Although the assumption that treatment of periodontitis would have a positive result on reducing the incidence of preterm births seems logical, broad investigation is essential to reach valid conclusions. In the past, treatment of conditions correlated with preterm birth, such as vaginitis, failed to alter the incidence of preterm birth.9 10 11
Between 2003 and 2008 seven prospective randomised studies were published, and most of them showed a decrease in preterm birth and low birthweight infants among women with periodontal disease when the disease was treated, with scaling and root planning, compared with no treatment. In contrast, one of the largest studies showed no difference.12 Whereas pooled results of the early trials supported the hypothesis that applying treatment in patients with periodontal disease may reduce the risk of preterm birth and low birthweight infants,13 the low methodological quality of most of them prevented us from prompt implementation of the findings. Today, after the publication of more recent, well designed randomised trials, a more comprehensive meta-analysis is essential to provide solid guidelines for the treatment of periodontal disease during pregnancy.
Methods
Search strategy and eligibility criteria
Two independent investigators (DM and AV) searched the Cochrane Central Trials Registry, ISI Web of Science, and Medline without language restriction up to July 2010 by using the search algorithm “(periodontal disease OR periodontitis OR gingivitis) AND (preterm labor OR preterm birth OR premature rupture of membranes OR low birthweight OR PTB OR PROM OR LBW).” They compared the results and reached a consensus on the eligibility of the trials with the involvement of a third investigator (IPP). In addition, we reviewed the references of all eligible trials, did cross searches in Medline by using the names of the investigators who were lead authors on at least one eligible trial, and hand searched the last two year volumes of two key dentistry journals (Journal of Periodontology and Journal of Clinical Periodontology).
All randomised controlled trials that allocated pregnant women to receive treatment with scaling and root planing versus no treatment or prophylaxis were eligible for inclusion. We considered trials to be eligible if they included patients with documented periodontal disease (periodontitis or gingivitis), as defined by the International Workshop for a Classification of Periodontal Diseases and Conditions.14 All trials were eligible regardless of the depth and the severity of periodontal disease. We adopted a further classification of the severity of periodontal disease based on the conclusions of the working group by the Centers for Disease Control and Prevention and the American Association of Periodontology in 2003.15 We defined moderate and severe periodontitis, according to this classification, in terms of probing depth and clinical attachment loss to enhance case definitions and provide distinct categories.
For trials that, according to their protocol, had included arms in which patients received concomitant treatment (such as antibiotics), we focused on the subgroups of eligible patients. We excluded from the analysis randomised trials that included patients with threatened preterm delivery who received tocolytic agents, non-randomised trials, and pseudo-randomised trials.
Data extraction and assessment of methodological quality
Two independent investigators (AZ and IPP) were involved in the data extraction. A third investigator (NPP) examined the results, and a consensus was reached. We extracted the following data from each arm of the eligible trials: authors’ names, journal and year of publication, country of origin, enrolment years, gestational age at enrolment, gestational age at completion of treatment, number of patients randomised and eligible, number of live births, and patients’ inclusion criteria. In addition, we recorded the methodological quality of the trials by using Cochrane’s risk of bias tool.16 Two independent investigators (NPP and DM) assessed the methodological quality of the trials, and consensus was reached.
The primary outcome was the rate of preterm births, defined as the number of preterm births before 37 weeks of gestation (spontaneous or indicated) among all successful pregnancies (all randomised pregnancies except patients lost to follow-up and pregnancies that led to spontaneous abortions or stillbirths). Secondary outcomes included the rate of low birthweight infants, defined as the number of infants under 2500 g among all successful pregnancies; the rate of spontaneous abortions/stillbirths, defined as the number of spontaneous abortions or stillbirths among all patients randomised except those lost to follow-up; and the overall rate of adverse outcomes of pregnancy, defined as the number of preterm births (<37 weeks) and the number of spontaneous abortions and stillbirths, in an intention to treat analysis among all randomised patients. Finally, other secondary outcomes included the rate of spontaneous preterm births, rate of preterm birth before 35 weeks of gestation, and rate of very low birthweight infants (<1500 g).
Data analysis
We constructed two by two tables and calculated the odds ratio for each primary study to estimate the relative risk of preterm birth, low birthweight infants, and spontaneous abortion/stillbirth among the treatment group compared with the control group. To test the homogeneity of the estimates of odds ratios among eligible studies, we used the χ2 test with a level of significance of 0.1, and we further quantified the degree of heterogeneity by using the I2 test. We synthesised data across studies by using the fixed effects (Mantel-Haenszel) model whenever no statistical heterogeneity was apparent,17 or by using the random effects model (DerSimonian and Laird). Whenever studies reported zero events in both arms (treatment and no treatment), we excluded these trials from the final analysis.
We included all trials in the final analysis. Because of the increased heterogeneity seen after we pooled the data from all the trials, we did separate meta-analyses including only high quality or low quality trials. We considered high quality trials to be those that had a low risk of bias as was assessed by Cochrane’s risk of bias tool. We detected the possibility of publication bias visually by using contour funnel plots and tested for small study effect bias with Harbord’s modified test.
We used Review Manager (RevMan) version 5 statistical software to analyse data.16 All P values were two tailed with a level of significance of <0.05. We used Stata SE 10.0 to do contour enhanced funnel plots and Harbord’s test for small study effect bias.
Results
Characteristics of eligible trials
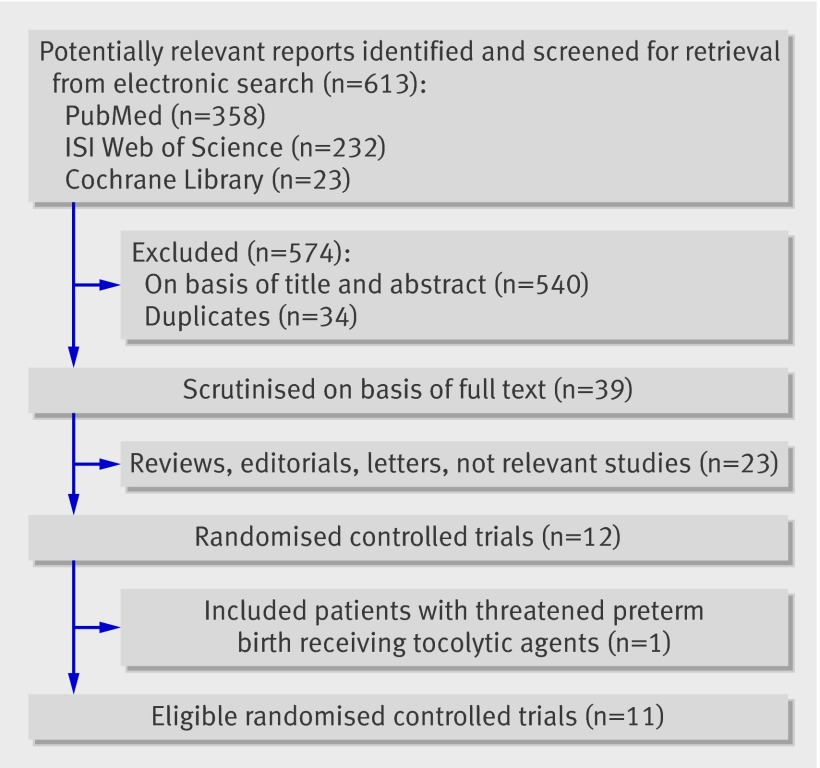
We retrieved 613 reports through searches in the Cochrane Central Trials Registry, ISI Web of Science, and Medline. We did not identify any additional trials through additional searches. We retrieved 12 randomised controlled trials. However, we considered one randomised trial to be ineligible because randomised patients had been admitted to hospital for threatened preterm birth and received tocolytic agents.18 Another recent trial did not clarify whether all patients in the intervention arm received treatment with scaling and root planing19; however, personal contact with the primary investigators of the trial clarified that all patients had received such treatment. Finally, we considered 11 trials to be eligible. These trials included 6558 patients—3438 allocated to periodontal disease treatment and 3120 allocated to no treatment or placebo (fig 1). The table shows the baseline characteristics of eligible trials.
Fig 1 Flow chart of selection of trials
Table 1.
Baseline characteristics of included trials
| Author, year | Country | Total No of patients | No of patients followed | No of live births | Gestational age at enrolment (weeks) | Gestational age at completion of treatment (weeks) | Definition of periodontal disease | Reclassification of severity of disease inclusion criteria according to CDC AAP 2003* |
|---|---|---|---|---|---|---|---|---|
| Lopez, 200221 | Chile | 400 | 372 | 358 | 9-21 | 28 | ≥4 teeth with ≥1 sites with PD≥4 mm and CAL≥3 mm | Mild periodontitis |
| Jeffcoat, 200320† | USA | 246 | 246 | 246 | 21-25 | NA | ≥3 sites with CAL≥3 mm | Mild periodontitis |
| Lopez, 200522 | Chile | 870 | 856 | 845 | <22 | 28 | BOP≥25% of sites and no sites with CAL>2 mm | Gingivitis |
| Michalowicz, 200612† | USA | 823 | 812 | 793 | 13-17 | Until delivery when necessary | 4 or more teeth with PD≥4 mm and CAL≥2 mm and BOP>35% of sites | Mild periodontitis |
| Offenbacher, 200625 | USA | 74 | 67 | 67 | <22 | NA | ≥2 sites with PD≥5 mm and CAL 1-2 mm at ≥1 sites with PD≥5 mm | Moderate periodontitis |
| Sadatmansuri, 200626 | Iran | 30 | 30 | 30 | 13-20 | 30 | ≥4 teeth with ≥1 sites with PD≥4 mm and CAL≥3 mm | Mild periodontitis |
| Tarranum, 200724 | India | 220 | 192 | 188 | 9-21 | 28 | CAL≥2 mm at ≥50% of examined sites | Mild periodontitis |
| Offenbacher, 20095† | USA | 1806 | 1760 | 1761 | <24 | NA | ≥3 periodontal sites with CAL≥3 | Mild periodontitis |
| Newnham, 200923† | Australia | 1087 | 1080 | 1073 | 12-20 | 28 | ≥12 probing sites with PD≥4 | Mild periodontitis |
| Macones, 20101† | USA | 756 | 713 | 720 | 6-20 | NA | CAL≥3 mm on ≥3 teeth | Mild periodontitis |
| Oliveira, 201019 | Brazil | 246 | 239 | 233 | 12-20 | 30-32 | ≥1 sites with PD≥4 mm and CAL≥3 mm | Mild periodontitis |
BOP=bleeding on probing site; CAL=clinical attachment loss; NA=no data available; PD=probing depth.
*Mild periodontitis refers to category neither moderate nor severe periodontitis according to Centres for Disease Control and Prevention and American Association of Periodontology 2003 criteria; gingivitis is not included in clinical case definitions by CDC AAP 2003 but is in accordance with term proposed by International Workshop for a Classification of Periodontal Diseases and Conditions in 1999.
†Trials considered to be of high methodological quality.
Methodological quality of trials
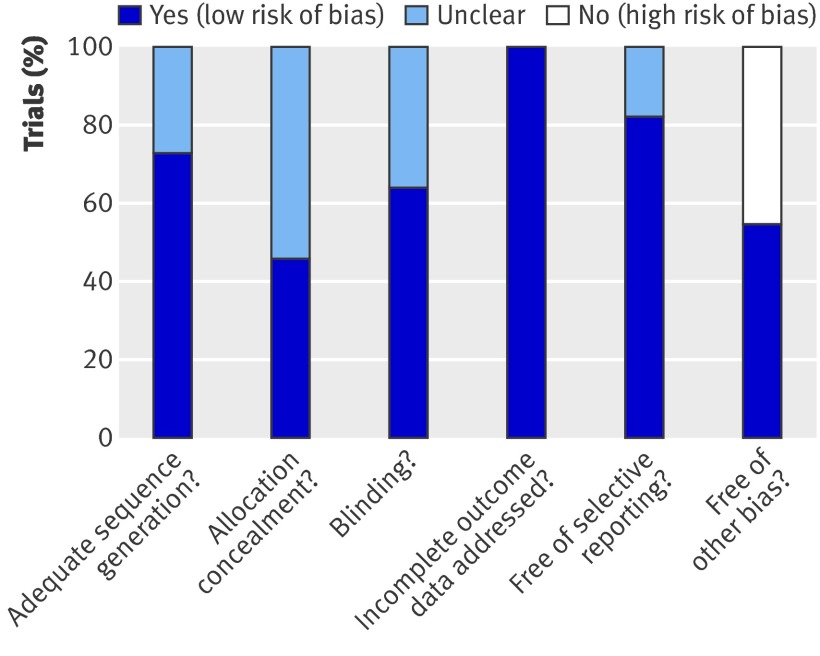
We used Cochrane’s risk of bias tool to assess the methodological quality of the trials (fig 2). Among eligible trials, eight reported an adequate randomisation mode,1 5 12 20 21 22 23 24 four used an adequate mode of allocation concealment,1 5 12 20 23, and six used blinding.1 5 12 20 23 25 Overall, we considered five trials to be of high methodological quality,1 5 12 20 23 with a low risk of bias. One study had an unclear risk of bias, whereas the five remaining studies had a high risk of bias.
Fig 2 Risk of bias among included trials
Primary outcome—preterm birth (<37 weeks)
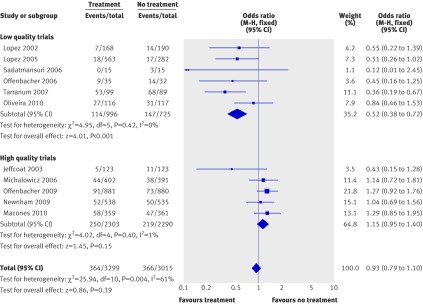
Preterm births were reported in all of the eligible trials. Overall, 364 preterm births were reported in women receiving treatment compared with 366 in patients who received no treatment. Meta-analysis of the overall preterm birth rate indicated no difference when we used either the random effects model (odds ratio 0.79, 95% confidence interval 0.58 to 1.06; P=0.12) or the fixed effects model (0.93, 0.79 to 1.10; P=0.39). Subgroup analysis according to the methodological quality of randomised studies showed diverse results. Whereas low quality trials supported a significant beneficial effect of treatment with scaling and root planing on the rate of preterm birth (odds ratio 0.52, 0.38 to 0.72; P<0.0001), no such effect was apparent among high quality studies (1.15, 0.95 to 1.40; P=0.15). Heterogeneity was present when we pooled all the trials together but not for the separate analyses of high quality or low quality trials (fig 3).
Fig 3 Meta-analysis plot for preterm birth <37 weeks of gestation. M-H=Mantel-Haenszel model
Secondary outcomes
Low birthweight infants (<2500 g)
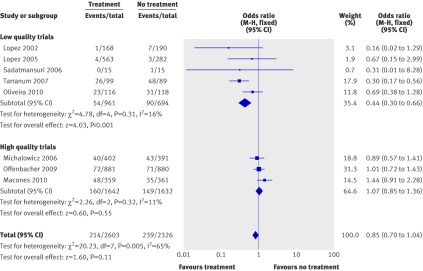
The number of low birthweight infants was reported in eight of the eligible trials. Meta-analysis found no significant difference between compared arms (odds ratios 0.85, 0.70 to 1.04 (P=0.11) with fixed effects model and 0.74, 0.49 to 1.12 (P=0.16) with random effects model). Low quality and high quality trials again differed in the effect of treatment (odds ratios 0.44, 0.30 to 0.66 (P<0.0001) for low quality trials and 1.07, 0.85 to 1.36 (P=0.55) for high quality trials), whereas heterogeneity was significant when we included all the trials (Q=20.23, P=0.005, I2=65%) but not for either high quality or low quality studies separately (fig 4).
Fig 4 Meta-analysis plot for low birthweight infants (<2500 g). M-H=Mantel-Haenszel model
Spontaneous abortion/stillbirth
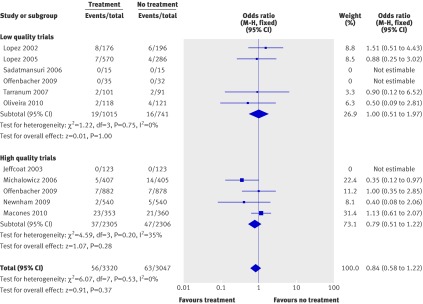
All trials reported data for spontaneous abortions or stillbirths. Three trials reported no outcomes in both treatment and control arms,20 25 26 so we excluded these from the final analysis. The pooled odds ratio for the rate of spontaneous abortion/stillbirth was 0.84 (0.58 to 1.22; P=0.37), suggesting that no significant difference exists. Results remained non-significant when we pooled the data separately for high quality trials (odds ratio 0.79, 0.51 to 1.22; P=0.28) or low quality trials (1.00, 0.51 to 1.97; P=1.00) (fig 5). No significant heterogeneity existed.
Fig 5 Meta-analysis plot for spontaneous abortions/stillbirths. M-H=Mantel-Haenszel model
Overall adverse pregnancy outcomes
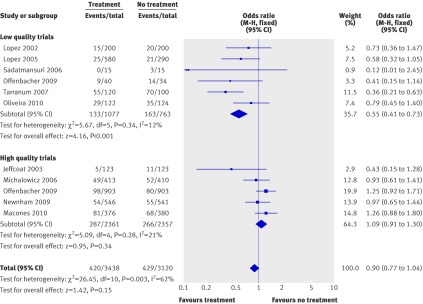
Data for the overall adverse pregnancy outcome (preterm birth <37 weeks and spontaneous abortions/stillbirths) were available for all of the trials. We calculated the odds ratio for this outcome in an intention to treat analysis among all randomised patients. Statistically significant heterogeneity was present (Q=26.45, P=0.003, I2= 62%). We found no significant difference when we used either the random effects model (odds ratio 0.78, 0.59 to 1.02; P=0.07) or the fixed effects model (0.90, 0.77 to 1.04; P=0.15). Low quality studies again tended to consistently overestimate the treatment effect. When we did separate analyses according to the methodological quality of the trials, low quality trials showed a strong significant effect of treatment (odds ratio 0.55, 0.41 to 0.73; P<0.0001), whereas high quality studies showed that treatment had no effect on the observed outcome (odds ratio 1.09, 0.91 to 1.30; P=0.34) (fig 6). We found no heterogeneity between either high quality or low quality trials.
Fig 6 Meta-analysis plot for overall adverse pregnancy outcome (preterm birth <37 weeks of gestation and spontaneous abortions/stillbirths). M-H=Mantel-Haenszel model
Other secondary outcomes
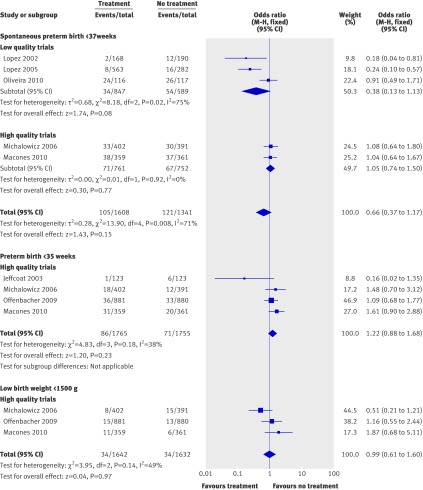
Other secondary outcomes were reported in fewer than half of the trials (fig 7), and results should be interpreted with caution. Treatment with scaling and root planing did not significantly improve the rate of spontaneous preterm birth (<37 weeks) (odds ratio 0.66, 0.37 to 1.17; P=0.12), preterm birth <35 weeks (1.22, 0.88 to 1.68; P=0.23), or very low birthweight infants (<1500 g) (0.99, 0.61 to 1.60; P=0.97) (fig 7).
Fig 7 Meta-analysis plots for other secondary outcomes: spontaneous preterm birth <37 weeks of gestation (top); preterm birth <35 weeks of gestation (middle); very low birthweight infants (<1500 g) (bottom). M-H=Mantel-Haenszel model
Publication bias
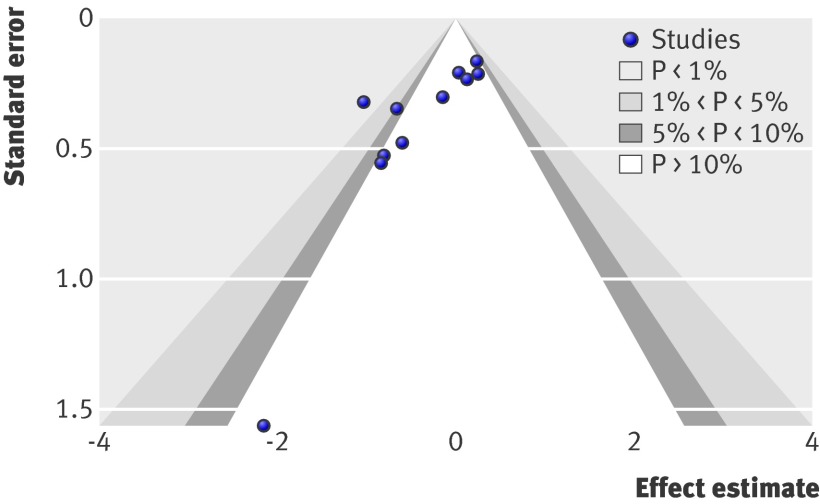
We assessed the presence of publication bias by using the contour enhanced funnel plots.27 The funnel plot was asymmetrical (fig 8); less precise (smaller) studies reported higher odds ratios than did larger studies (Harbord’s test, P=0.002). Trials perceived to be missing were in parts where no statistical significance existed, suggesting the presence of publication bias (fig 8)
Fig 8 Contour enhanced funnel plot for estimation of publication bias
Discussion
This meta-analysis shows that treatment of periodontitis with scaling and root planing in pregnant women has no significant effect on the incidence of preterm birth. Furthermore, treatment does not seem to have a significant effect on the incidence of low birthweight infants or spontaneous abortions/stillbirths or on the overall rate of adverse outcomes of pregnancy (preterm births and spontaneous abortions/stillbirths).
Comparison with other studies
The results of this meta-analysis are in contrast to those of a previous meta-analysis published in 2009.13 A potential reason for this discrepancy may be the fact that the trials included in the earlier study had considerable methodological shortcomings. Today, after the publication of three new, well designed, large randomised trials,1 5 23 treatment of periodontal disease during pregnancy does not seem to offer any clear benefit for the reduction of preterm births or low birthweight infants and therefore should not be routinely recommended in pregnant women as a measure for prevention of preterm birth.
Another reason for the discrepancy between this meta-analysis and the previous one may be the potential threat of publication bias. Considerable evidence from many clinical domains indicates that trials with “negative” results, especially small ones, may have difficulty getting published or may be published with considerable delays compared with trials that find significant benefits for the tested interventions28 29; the odds of publication are approximately four times greater if the results are statistically significant.30 Thus, early trials that did not favour treatment of periodontal with scaling and root planing during pregnancy may have been left unpublished, resulting in the accumulation of small trials with significant results in favour of treatment. According to the funnel plot constructed, publication bias is highly likely to exist. Furthermore, the application of Harbord’s modified test for detecting small study effect bias showed that small studies were more likely to provide inflated outcomes in favour of the treatment arm. Despite the fact that recent evidence suggests that in most meta-analyses the application of funnel plot asymmetry tests to detect publication bias is inappropriate or not meaningful, especially when few trials are included in the meta-analysis,31 we applied the contour enhanced funnel plots, which seem to be more convincing in detecting publication bias.27
Strengths and limitations of study
The most important strength of this study is the large sample size of patients included among high quality trials. Despite the fact that less than half of the trials were of high methodological quality, they cumulatively included more than 4500 patients, with a 65% weight in the overall estimate of the pooled study effect. This constitutes a large sample size, sufficiently powered to exclude at least a 2.5% reduction in the incidence of preterm birth after treatment with scaling and root planing, given that the preterm birth rate in the United States is around 12%.32
Another advantage of this meta-analysis is that we separately analysed our results according to the methodological quality of the studies. The most interesting observation is the completely different results obtained when we pooled the data separately from low quality and high quality trials for most of the primary and secondary outcomes. Strong statistical significance was achieved when we included only low quality trials, and this significance completely disappeared when we considered only high quality trials. One should be cautious when interpreting the data from low quality randomised trials and possibly reconsider the adoption of outcomes retrieved from such trials. Considering that only two of the early trials published up to 2008 reported key methodological parameters for randomised trials,12 20 the discrepancy between the results of our previous analysis and this one should be undeniably attributed to the suboptimal quality of the early trials. The subgroups analyses we did for all the outcomes clearly support this hypothesis, as low quality trials showed a strong significant effect of treatment whereas high quality trials reported no difference in any of the outcomes tested.
Poor reporting of the methodological characteristics of randomised trials has been previously described.33 Although a substantial improvement in key aspects of trials’ methods has been seen over the past years, the quality of reporting remains well below an acceptable level.34 The most important aspect related to the suboptimal methodological quality of randomised trials is the substantial threat to the validity of the results and conclusions obtained. Reporting of methodological quality parameters is directly correlated with the provision of outcomes that favour the experimental arm.35 36 Previous reports have shown an inverse relation between the methodological quality score and the efficacy of preventive strategies,37 whereas the quality of reports of randomised trials seems to affect estimates of the efficacy of interventions reported in meta-analyses.38 Thus, taking into account the fact that studies of low methodological quality in which the estimate of quality is incorporated into the meta-analyses can alter the interpretation of the benefit of the intervention,38 this meta-analysis has a major strength in that it separately considered the effect of treatment among high quality and low quality trials. This is accordance with a large meta-epidemiological study, which clearly recommends that systematic reviewers should present meta-analyses restricted to trials at low risk of bias for each outcome, either as the primary analysis or in conjunction with less restrictive analyses.39 Furthermore, the adoption of Cochrane’s risk of bias tool to assess the quality of the randomised trials included in our review is another advantage, given that it is a validated tool developed to overcome some of the shortcomings of existing quality assessment instruments.40
Our meta-analysis has certain limitations. Firstly, the definition of periodontal disease differed among the trials included. A recent position paper commissioned by the European Association of Dental Public Health showed a distinct lack of consensus and uniformity in the definition of periodontitis in epidemiological studies.41 Furthermore, a review showed that the significance of the association between periodontal disease and pregnancy outcomes may be determined by the definition or measurement of periodontal disease used.42 This discrepancy in the definition of periodontal disease is also present in the trials of treatment of periodontal disease during pregnancy. However, we re-evaluated the severity of periodontal disease among patients included within the trials, on the basis on the classification proposed by the Centres for Disease Control and Prevention and the American Association of Periodontology in 2003.15 On the basis of trials’ inclusion criteria, the five high quality trials had comparable inclusion criteria as regards the severity of disease (table). Consequently, differences in the definition of the disease are unlikely to have contributed to the lack of effect of treatment.
Secondly, one of the trials included in the main analysis enrolled only patients with gingivitis who were treated with scaling.22 These patients might have had considerable improvement in their disease with treatment, as they were women with less severe periodontal disease and the effect of treatment on the rate of preterm birth may have been greater. However, this study had important methodological shortcomings and had a significantly higher percentage of patients with a previous history of preterm birth in the treatment arm, so we did not include it in the analysis of high quality studies. Furthermore, considering the positive results of the trial, exclusion from the main analysis would have not affected our results.
Finally, according to our tests, an increased likelihood of publication bias exists in our analysis. However, given that publication bias usually involves trials with “negative” results,29 any unpublished trial would be highly unlikely to have favoured the treatment arm. Furthermore, taking into account the quality of the five highest quality trials included here and their weight in the overall analysis, the effect of any unpublished or missed trial on the overall effect of treatment would probably be detrimental.
Conclusions and policy implications
In an attempt to explain the lack of a significant effect of treatment of periodontal disease during pregnancy on perinatal outcomes, we could speculate that treatment with scaling and root planing does not alter the sequence of events leading from regional inflammation due to periodontitis to systemic inflammation and onset of preterm birth. This lack of treatment effect may be related to the diverse systemic response after scaling and root planing on different stages of periodontal disease. For example, in non-pregnant patients with documented periodontal disease, non-surgical treatment results in different local and systemic inflammatory responses.43 Furthermore, in more severe cases, treatment may lead to higher levels of circulatory inflammatory cytokines.7 44 A subgroup analysis in our previous report showed that treatment was significantly more effective when applied in patients with less severe disease. However, our updated meta-analysis now includes five high quality trials, among which the definition of periodontal disease does not differ widely. Thus, taking into account the fact that none of the outcomes among these trials favoured treatment with scaling and root planing during pregnancy, such treatment is unlikely to have any beneficial effect on perinatal outcomes.
Some investigators suggest that adjuvant antibiotic treatment could be used in patients with periodontal disease to prevent preterm birth. However, the beneficial effect of antibiotic treatment in reducing the incidence of preterm birth, either among patients with periodontal disease or among those with other infections, is controversial. The only randomised trial that allocated patients to a combination of scaling and root planing and administration of antibiotic failed to support this hypothesis,20 and previous meta-analyses failed to show a significant effect of antibiotics on the incidence of preterm birth when given for the treatment of bacterial vaginosis.9 10 11
Another consideration is that the success of periodontal disease treatment may indeed be a determinant of the effect of scaling and root planing on the overall incidence of preterm birth. Some randomised trials included in our meta-analysis examined periodontal status only before delivery and not after treatment. Consequently, a well designed prospective randomised trial to test whether pregnant patients who have successfully controlled their periodontal disease may eventually have a substantially reduced incidence of preterm birth would be of interest.
Finally, despite the fact that we failed to show any benefit of treatment during pregnancy, we cannot exclude the possibility that women who start treatment and have their periodontal disease controlled early in the first trimester of pregnancy or even before becoming pregnant, may have a substantial improvement in perinatal outcomes. Although we cannot be sure that a causal relation between periodontal disease and preterm birth exists, the likelihood of an association is very high.6 Therefore, pre-pregnancy treatment seems to be a reasonable alternative, and a randomised trial including patients allocated to scaling and root planing or control before pregnancy and examining its effect in a subsequent pregnancy may show significant differences in the future. However, given that many pregnancies are unplanned, such a trial would be very difficult to do and would need a large sample size.
In conclusion, updated evidence does not encourage the use of scaling and root planing as an efficient method of reducing the rate of preterm birth or improving perinatal outcomes. Women may be advised to evaluate their dental status during pregnancy and may have treatment for periodontal disease. However, they should be told that such a treatment during pregnancy is unlikely to reduce the risk of preterm birth or low birthweight infants and therefore should not be considered as routine antenatal care.
What is already known on this topic
Periodontal disease is associated with an increased risk of preterm birth, and a causal relation may exist
Existing reports on the effect of treatment with scaling and root planning on the incidence of preterm birth are conflicting
What this study adds
Treatment of periodontal disease with scaling and root planing during pregnancy does not reduce the risk of preterm birth and should not be routinely recommended as a measure to prevent preterm birth
Randomised trials of low methodological quality tend to overestimate the effect of treatment, whereas high quality trials provide strong evidence that no significant effect of treatment exists
Contributors: NPP, IPP, and DM had the idea for the study. NPP did the analyses. AV, AZ, IPP, DM, and NPP did the literature searches and data extraction and assessed the methodological quality of the studies. NPP, AZ, and IPP wrote the manuscript. EGP and ST contributed to the initial revision of the manuscript. DW and IEM contributed to the critical revision of the manuscript before publication. NPP is the guarantor.
Funding: This research received no specific funding from any funding agency in the public, commercial, or non-profit sectors.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not needed.
Data sharing: No additional data available
Cite this as: BMJ 2010;341:c7017
References
- 1.Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). Am J Obstet Gynecol 2010;202:147,e1-8. [DOI] [PubMed] [Google Scholar]
- 2.Laine MA. Effect of pregnancy on periodontal and dental health. Acta Odontol Scand 2002;60:257-64. [DOI] [PubMed] [Google Scholar]
- 3.Michalowicz BS, DiAngelis AJ, Novak MJ, Buchanan W, Papapanou PN, Mitchell DA, et al. Examining the safety of dental treatment in pregnant women. J Am Dent Assoc 2008;139:685-95. [DOI] [PubMed] [Google Scholar]
- 4.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996;67(suppl 10):1103-13. [DOI] [PubMed] [Google Scholar]
- 5.Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, et al. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol 2009;114:551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol 2007;196:135,e1-7. [DOI] [PubMed] [Google Scholar]
- 7.Offenbacher S, Jared HL, O’Reilly PG, Wells SR, Salvi GE, Lawrence HP, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol 1998;3:233-50. [DOI] [PubMed] [Google Scholar]
- 8.McQueen MP. Health plans expand dental benefits: studies linking gum disease to health problems spur new focus on preventive treatments. Wall Street Journal 2006. Sept 19.
- 9.Simcox R, Sin WT, Seed PT, Briley A, Shennan AH. Prophylactic antibiotics for the prevention of preterm birth in women at risk: a meta-analysis. Aust N Z J Obstet Gynaecol 2007;47:368-77. [DOI] [PubMed] [Google Scholar]
- 10.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2007;1:CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol 2005;105:857-68. [DOI] [PubMed] [Google Scholar]
- 12.Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med 2006;355:1885-94. [DOI] [PubMed] [Google Scholar]
- 13.Polyzos NP, Polyzos IP, Mauri D, Tzioras S, Tsappi M, Cortinovis I, et al. Effect of periodontal disease treatment during pregnancy on preterm birth incidence: a metaanalysis of randomized trials. Am J Obstet Gynecol 2009;200:225-32. [DOI] [PubMed] [Google Scholar]
- 14.1999 International Workshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook, Illinois, October 30-November 2, 1999. Ann Periodontol 1999;4:i, 1-112. [DOI] [PubMed] [Google Scholar]
- 15.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78(suppl 7):1387-99. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S, Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell, 2008.
- 17.Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. 2nd ed. BMJ Books, 2001.
- 18.Radnai M, Pal A, Novak T, Urban E, Eller J, Gorzo I. Benefits of periodontal therapy when preterm birth threatens. J Dent Res 2009;88:280-4. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira AM, de Oliveira PA, Cota LO, Magalhaes CS, Moreira AN, Costa FO. Periodontal therapy and risk for adverse pregnancy outcomes. Clin Oral Invest 2010. May 22 [Epub ahead of print]. [DOI] [PubMed]
- 20.Jeffcoat MK, Hauth JC, Geurs NC, Reddy MS, Cliver SP, Hodgkins PM, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol 2003;74:1214-8. [DOI] [PubMed] [Google Scholar]
- 21.Lopez NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol 2002;73:911-24. [DOI] [PubMed] [Google Scholar]
- 22.Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol 2005;76(suppl 11):2144-53. [DOI] [PubMed] [Google Scholar]
- 23.Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, et al. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstet Gynecol 2009;114:1239-48. [DOI] [PubMed] [Google Scholar]
- 24.Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J Periodontol 2007;78:2095-103. [DOI] [PubMed] [Google Scholar]
- 25.Offenbacher S, Lin D, Strauss R, McKaig R, Irving J, Barros SP, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. J Periodontol 2006;77:2011-24. [DOI] [PubMed] [Google Scholar]
- 26.Sadatmansouri S, Sedighpoor N, Aghaloo M. Effects of periodontal treatment phase I on birth term and birth weight. J Indian Soc Pedod Prev Dent 2006;24:23-6. [DOI] [PubMed] [Google Scholar]
- 27.Moreno SG, Sutton AJ, Turner EH, Abrams KR, Cooper NJ, Palmer TM, et al. Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ 2009;339:b2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867-72. [DOI] [PubMed] [Google Scholar]
- 29.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 1998;279:281-6. [DOI] [PubMed] [Google Scholar]
- 30.Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;1:MR000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercer BM, Goldenberg RL, Meis PJ, Moawad AH, Shellhaas C, Das A, et al. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. Am J Obstet Gynecol 2000;183:738-45. [DOI] [PubMed] [Google Scholar]
- 33.Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365:1159-62. [DOI] [PubMed] [Google Scholar]
- 34.Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ 2010;340:c723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
- 36.Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Nieuwenhoven CA, Buskens E, van Tiel FH, Bonten MJ. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA 2001;286:335-40. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
- 39.Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs Seida J, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;339:b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leroy R, Eaton KA, Savage A. Methodological issues in epidemiological studies of periodontitis—how can it be improved? BMC Oral Health 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol 2008;35:385-97. [DOI] [PubMed] [Google Scholar]
- 43.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006;33:401-7. [DOI] [PubMed] [Google Scholar]
- 44.Lafaurie GI, Mayorga-Fayad I, Torres MF, Castillo DM, Aya MR, Baron A, et al. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J Clin Periodontol 2007;34:873-9. [DOI] [PubMed] [Google Scholar]