Abstract
In the title compound, C13H14N2O3, the fused ring system is almost planar (r.m.s. deviation = 0.015 Å). The r.m.s. deviation for all the non-H atoms of the molecule is 0.065Å. In the crystal, N—H⋯O and C—H⋯O hydrogen bonds generate polymeric chains along the b axis containing alternating centrsymmetric R 2 2(8) and R 2 2(20) loops.
Related literature
For the synthesis, see: Taylor et al. (1965 ▶). For the biological activity of benzopyrazines, see: Sona et al. (1998 ▶); Cai et al. (1997 ▶); Toshima et al. (2003 ▶); Benbow et al. (2007 ▶); Sarges et al. (1990 ▶); Smits et al. (2008 ▶); Tandon et al. (2006 ▶).
Experimental
Crystal data
C13H14N2O3
M r = 246.26
Monoclinic,
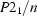
a = 8.3138 (6) Å
b = 13.6868 (8) Å
c = 10.8189 (8) Å
β = 102.002 (3)°
V = 1204.16 (14) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 296 K
0.37 × 0.29 × 0.23 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.965, T max = 0.978
11123 measured reflections
2938 independent reflections
1370 reflections with I > 2σ(I)
R int = 0.060
Refinement
R[F 2 > 2σ(F 2)] = 0.068
wR(F 2) = 0.216
S = 0.96
2938 reflections
167 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.44 e Å−3
Δρmin = −0.30 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810047094/hb5740sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810047094/hb5740Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O1i | 0.96 (6) | 1.87 (6) | 2.827 (3) | 179 (5) |
| C3—H3⋯O3ii | 0.93 | 2.51 | 3.426 (4) | 170 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors are grateful to the HEC (Higher Education Commission of Pakistan) for the financial support of this work.
supplementary crystallographic information
Comment
Benzopyrazine constitute an important class of nitrogen containing heterocyclic compounds. Literature has shown that these compounds possess a vide variety of applications from pharmaceutical to agricultural fields. Several benzopyrazines have been reported as anti-bacterial (Sona et al., 1998), anti-convulsant(Cai et al., 1997), anti-cancer (Toshima et al., 2003), antidiabetic(Benbow et al., 2007), antidepressant(Sarges et al., 1990), antifungal(Tandon et al., 2006), anti-inflammatory (Smits et al., 2008), etc. The present work is based on the synthesis of pyrazines derivatives which may possess enhanced pharmaceutical activities.
The title compound (I) is structurally looks like planer but the dihedral angle between the two fused rings i.e. aromatic ring (C1/C2/C3/C4/C5/C6) and pyrazine ring (C1/C6/N1/N2/C7/C8) is 1.46 (11)%. The planer ester moiety attached to the C8 is oriented at dihedral angle of 5.70 (14)% and 7.13 (13)% with respect to the aomatic and pyrazine rings respectively. The cyclic carboxamide functional group from the pyrazine rings forms dimers through N—H···O type hydrogen bonding interaction which further connects through weak C—H···O type hydrogen bonding interaction to form the polymeric chain along b axes (Fig. 2 Table 1).
Experimental
To the suspension of 3-(3-oxo-3,4-dihydroquinoxalin-2-yl)propanoic acid (Taylor et al., 1965) (5 g, 0.023 mol) in absolute ethanol (100 ml) was added 3N H2SO4 (10 ml) and reaction mixture was refluxed for four hours. Solution was then concentrated under reduced pressure and neutralized with sodium bicarbonate solution to dissolve any unreacted acid. The precipitates were filtered under reduced pressure and washed with excess of water. The resulting ester was then recrystallized in absolute ethanol to yield colourless needles of (I). The product melted at 173-175 C (lit mp 160-162 C). (95% yield).
Refinement
All the C—H H-atoms were positioned with idealized geometry with C—H = 0.93 Å , C—H = 0.96 Å and C—H = 0.97 Å and were refined using a riding model with Uiso(H) = 1.2 Ueq(C) for aromatic and methylene and Uiso(H) = 1.5Ueq(C) for methyl C atoms. The N—H H atom were located in difference map with C—H = 0.96 Å with Uiso(H) = 1.2 Ueq(N). The reflection (011) was omitted during refinement as it was obscured by the beam stop.
Figures
Fig. 1.
The structure of (I) with displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
Unit cell packing diagram showing the hydrogen bonding using dashed lines, the hydrogen atoms not involved in hydrogen bonding have been omitted.
Crystal data
| C13H14N2O3 | F(000) = 520 |
| Mr = 246.26 | Dx = 1.358 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 1697 reflections |
| a = 8.3138 (6) Å | θ = 2.8–24.6° |
| b = 13.6868 (8) Å | µ = 0.10 mm−1 |
| c = 10.8189 (8) Å | T = 296 K |
| β = 102.002 (3)° | Cut needle, colourless |
| V = 1204.16 (14) Å3 | 0.37 × 0.29 × 0.23 mm |
| Z = 4 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 2938 independent reflections |
| Radiation source: fine-focus sealed tube | 1370 reflections with I > 2σ(I) |
| graphite | Rint = 0.060 |
| φ and ω scans | θmax = 28.3°, θmin = 3.5° |
| Absorption correction: multi-scan (SADABS; Bruker, 2007) | h = −11→11 |
| Tmin = 0.965, Tmax = 0.978 | k = −11→18 |
| 11123 measured reflections | l = −14→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.068 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.216 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.96 | w = 1/[σ2(Fo2) + (0.0982P)2 + 0.5331P] where P = (Fo2 + 2Fc2)/3 |
| 2938 reflections | (Δ/σ)max < 0.001 |
| 167 parameters | Δρmax = 0.44 e Å−3 |
| 0 restraints | Δρmin = −0.30 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N2 | 0.5708 (3) | 0.81339 (16) | 0.0220 (2) | 0.0448 (6) | |
| C6 | 0.3369 (3) | 0.70659 (19) | −0.0524 (3) | 0.0427 (7) | |
| C1 | 0.4043 (3) | 0.79969 (19) | −0.0334 (3) | 0.0436 (7) | |
| C8 | 0.6615 (3) | 0.73747 (19) | 0.0532 (3) | 0.0423 (7) | |
| C5 | 0.1719 (4) | 0.6942 (2) | −0.1080 (3) | 0.0534 (8) | |
| H5 | 0.1271 | 0.6318 | −0.1199 | 0.064* | |
| N1 | 0.4382 (3) | 0.62700 (17) | −0.0166 (2) | 0.0478 (7) | |
| O3 | 1.1357 (2) | 0.94521 (16) | 0.1990 (3) | 0.0763 (8) | |
| C7 | 0.5997 (3) | 0.63615 (19) | 0.0353 (3) | 0.0448 (7) | |
| C2 | 0.3048 (3) | 0.8807 (2) | −0.0717 (3) | 0.0518 (8) | |
| H2 | 0.3487 | 0.9433 | −0.0600 | 0.062* | |
| C9 | 0.8402 (3) | 0.7474 (2) | 0.1117 (3) | 0.0485 (8) | |
| H9A | 0.9034 | 0.7137 | 0.0589 | 0.058* | |
| H9B | 0.8613 | 0.7151 | 0.1933 | 0.058* | |
| C10 | 0.9003 (3) | 0.8516 (2) | 0.1298 (3) | 0.0503 (8) | |
| H10A | 0.8863 | 0.8834 | 0.0481 | 0.060* | |
| H10B | 0.8348 | 0.8868 | 0.1797 | 0.060* | |
| O1 | 0.6900 (2) | 0.56381 (14) | 0.0636 (2) | 0.0593 (7) | |
| C11 | 1.0773 (4) | 0.8557 (2) | 0.1947 (3) | 0.0563 (9) | |
| O2 | 1.1589 (3) | 0.78799 (19) | 0.2404 (3) | 0.0934 (10) | |
| C3 | 0.1417 (4) | 0.8677 (2) | −0.1268 (3) | 0.0591 (9) | |
| H3 | 0.0754 | 0.9219 | −0.1518 | 0.071* | |
| C12 | 1.3079 (4) | 0.9554 (3) | 0.2601 (4) | 0.0911 (14) | |
| H12A | 1.3271 | 0.9249 | 0.3428 | 0.109* | |
| H12B | 1.3760 | 0.9226 | 0.2102 | 0.109* | |
| C4 | 0.0752 (4) | 0.7746 (2) | −0.1455 (3) | 0.0577 (9) | |
| H4 | −0.0351 | 0.7667 | −0.1834 | 0.069* | |
| C13 | 1.3500 (6) | 1.0526 (4) | 0.2726 (6) | 0.131 (2) | |
| H13C | 1.3410 | 1.0811 | 0.1904 | 0.196* | |
| H13A | 1.4611 | 1.0587 | 0.3193 | 0.196* | |
| H13B | 1.2773 | 1.0858 | 0.3169 | 0.196* | |
| H1N | 0.394 (6) | 0.562 (5) | −0.033 (5) | 0.157* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N2 | 0.0344 (13) | 0.0382 (13) | 0.0576 (16) | −0.0021 (10) | −0.0004 (11) | 0.0004 (11) |
| C6 | 0.0340 (15) | 0.0374 (15) | 0.0542 (18) | 0.0006 (12) | 0.0034 (13) | 0.0025 (13) |
| C1 | 0.0362 (16) | 0.0384 (16) | 0.0526 (18) | 0.0021 (12) | 0.0010 (13) | 0.0010 (13) |
| C8 | 0.0357 (15) | 0.0362 (15) | 0.0507 (17) | 0.0023 (11) | −0.0009 (13) | 0.0001 (13) |
| C5 | 0.0374 (17) | 0.0458 (18) | 0.073 (2) | −0.0046 (13) | 0.0014 (15) | −0.0021 (15) |
| N1 | 0.0332 (13) | 0.0357 (13) | 0.0680 (17) | −0.0008 (10) | −0.0049 (11) | 0.0009 (11) |
| O3 | 0.0340 (12) | 0.0536 (14) | 0.127 (2) | −0.0050 (10) | −0.0155 (12) | −0.0091 (14) |
| C7 | 0.0396 (16) | 0.0347 (15) | 0.0564 (19) | −0.0002 (12) | 0.0015 (14) | −0.0025 (13) |
| C2 | 0.0428 (18) | 0.0371 (16) | 0.071 (2) | 0.0018 (12) | 0.0008 (15) | 0.0003 (14) |
| C9 | 0.0339 (16) | 0.0423 (16) | 0.065 (2) | 0.0019 (12) | −0.0004 (14) | −0.0012 (14) |
| C10 | 0.0336 (16) | 0.0424 (17) | 0.069 (2) | 0.0006 (12) | −0.0035 (14) | 0.0018 (15) |
| O1 | 0.0428 (12) | 0.0353 (11) | 0.0905 (17) | 0.0076 (9) | −0.0075 (11) | −0.0010 (10) |
| C11 | 0.0356 (16) | 0.052 (2) | 0.074 (2) | 0.0011 (14) | −0.0057 (15) | −0.0044 (16) |
| O2 | 0.0467 (15) | 0.0687 (17) | 0.143 (3) | 0.0075 (12) | −0.0295 (15) | 0.0194 (16) |
| C3 | 0.0420 (18) | 0.0458 (18) | 0.083 (2) | 0.0102 (14) | −0.0017 (16) | 0.0066 (16) |
| C12 | 0.038 (2) | 0.081 (3) | 0.138 (4) | −0.0081 (18) | −0.020 (2) | −0.014 (3) |
| C4 | 0.0324 (16) | 0.055 (2) | 0.081 (2) | 0.0031 (13) | 0.0010 (15) | 0.0037 (17) |
| C13 | 0.078 (3) | 0.088 (4) | 0.207 (6) | −0.031 (3) | −0.015 (3) | −0.020 (4) |
Geometric parameters (Å, °)
| N2—C8 | 1.287 (3) | C9—C10 | 1.511 (4) |
| N2—C1 | 1.402 (3) | C9—H9A | 0.9700 |
| C6—N1 | 1.382 (3) | C9—H9B | 0.9700 |
| C6—C5 | 1.389 (4) | C10—C11 | 1.494 (4) |
| C6—C1 | 1.390 (4) | C10—H10A | 0.9700 |
| C1—C2 | 1.394 (4) | C10—H10B | 0.9700 |
| C8—C7 | 1.478 (4) | C11—O2 | 1.193 (4) |
| C8—C9 | 1.495 (4) | C3—C4 | 1.387 (4) |
| C5—C4 | 1.373 (4) | C3—H3 | 0.9300 |
| C5—H5 | 0.9300 | C12—C13 | 1.375 (6) |
| N1—C7 | 1.349 (3) | C12—H12A | 0.9700 |
| N1—H1N | 0.96 (6) | C12—H12B | 0.9700 |
| O3—C11 | 1.316 (4) | C4—H4 | 0.9300 |
| O3—C12 | 1.453 (4) | C13—H13C | 0.9600 |
| C7—O1 | 1.242 (3) | C13—H13A | 0.9600 |
| C2—C3 | 1.375 (4) | C13—H13B | 0.9600 |
| C2—H2 | 0.9300 | ||
| C8—N2—C1 | 118.5 (2) | H9A—C9—H9B | 107.6 |
| N1—C6—C5 | 121.0 (2) | C11—C10—C9 | 111.3 (2) |
| N1—C6—C1 | 118.5 (2) | C11—C10—H10A | 109.4 |
| C5—C6—C1 | 120.5 (3) | C9—C10—H10A | 109.4 |
| C6—C1—C2 | 119.3 (3) | C11—C10—H10B | 109.4 |
| C6—C1—N2 | 121.2 (2) | C9—C10—H10B | 109.4 |
| C2—C1—N2 | 119.5 (2) | H10A—C10—H10B | 108.0 |
| N2—C8—C7 | 123.6 (3) | O2—C11—O3 | 122.4 (3) |
| N2—C8—C9 | 121.0 (2) | O2—C11—C10 | 125.8 (3) |
| C7—C8—C9 | 115.4 (2) | O3—C11—C10 | 111.8 (3) |
| C4—C5—C6 | 119.7 (3) | C2—C3—C4 | 120.6 (3) |
| C4—C5—H5 | 120.2 | C2—C3—H3 | 119.7 |
| C6—C5—H5 | 120.2 | C4—C3—H3 | 119.7 |
| C7—N1—C6 | 122.6 (2) | C13—C12—O3 | 110.1 (4) |
| C7—N1—H1N | 118 (3) | C13—C12—H12A | 109.6 |
| C6—N1—H1N | 119 (3) | O3—C12—H12A | 109.6 |
| C11—O3—C12 | 115.2 (3) | C13—C12—H12B | 109.6 |
| O1—C7—N1 | 121.8 (2) | O3—C12—H12B | 109.6 |
| O1—C7—C8 | 122.7 (3) | H12A—C12—H12B | 108.2 |
| N1—C7—C8 | 115.5 (2) | C5—C4—C3 | 120.1 (3) |
| C3—C2—C1 | 119.8 (3) | C5—C4—H4 | 119.9 |
| C3—C2—H2 | 120.1 | C3—C4—H4 | 119.9 |
| C1—C2—H2 | 120.1 | C12—C13—H13C | 109.5 |
| C8—C9—C10 | 114.4 (2) | C12—C13—H13A | 109.5 |
| C8—C9—H9A | 108.7 | H13C—C13—H13A | 109.5 |
| C10—C9—H9A | 108.7 | C12—C13—H13B | 109.5 |
| C8—C9—H9B | 108.7 | H13C—C13—H13B | 109.5 |
| C10—C9—H9B | 108.7 | H13A—C13—H13B | 109.5 |
| N1—C6—C1—C2 | 178.5 (3) | N2—C8—C7—N1 | −0.2 (4) |
| C5—C6—C1—C2 | −0.4 (5) | C9—C8—C7—N1 | 179.5 (3) |
| N1—C6—C1—N2 | −0.8 (4) | C6—C1—C2—C3 | 0.4 (5) |
| C5—C6—C1—N2 | −179.7 (3) | N2—C1—C2—C3 | 179.6 (3) |
| C8—N2—C1—C6 | 1.3 (4) | N2—C8—C9—C10 | 0.1 (4) |
| C8—N2—C1—C2 | −177.9 (3) | C7—C8—C9—C10 | −179.6 (3) |
| C1—N2—C8—C7 | −0.8 (4) | C8—C9—C10—C11 | 177.0 (3) |
| C1—N2—C8—C9 | 179.5 (3) | C12—O3—C11—O2 | 1.7 (5) |
| N1—C6—C5—C4 | −178.4 (3) | C12—O3—C11—C10 | −179.6 (3) |
| C1—C6—C5—C4 | 0.5 (5) | C9—C10—C11—O2 | −8.1 (5) |
| C5—C6—N1—C7 | 178.6 (3) | C9—C10—C11—O3 | 173.2 (3) |
| C1—C6—N1—C7 | −0.3 (4) | C1—C2—C3—C4 | −0.4 (5) |
| C6—N1—C7—O1 | −177.9 (3) | C11—O3—C12—C13 | −172.8 (4) |
| C6—N1—C7—C8 | 0.8 (4) | C6—C5—C4—C3 | −0.5 (5) |
| N2—C8—C7—O1 | 178.5 (3) | C2—C3—C4—C5 | 0.5 (5) |
| C9—C8—C7—O1 | −1.8 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O1i | 0.96 (6) | 1.87 (6) | 2.827 (3) | 179 (5) |
| C3—H3···O3ii | 0.93 | 2.51 | 3.426 (4) | 170 |
Symmetry codes: (i) −x+1, −y+1, −z; (ii) −x+1, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5740).
References
- Benbow, J. W., Chu-Moyer, Y. M. & Kung, D. W. (2007). Patent No. US 7 202 245 B2,
- Bruker (2007). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cai, S. X., Huang, J. C., Espitia, S. A., Tran, M., Ilyin, V. I., Hawkinson, J. E., Woodward, R. M., Weber, E. & Keana, F. M. (1997). J Med. Chem.40, 3679–3686. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Sarges, R., Howard, H. R., Browne, R. G., Lebel, L. A., Seymour, P. A. & Koe, B. K. (1990). J. Med. Chem.33, 2240–2254. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Smits, R. A., Lim, H. D., Hanzer, A., Zuiderveld, O. P., Guaita, E., Adami, M., Coruzzi, G., Leurs, R. & de Esch, T. J. P. (2008). J. Med. Chem.51, 2457–2467. [DOI] [PubMed]
- Sona, P., Carta, A. Loriga, M., Zanetti, S., & Sechi, L. (1998). Farmaco, 53, 455–461. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tandon, V. K., Yadav, D. B., Maurya, H. K., Chaturvedi, A. K. & Shukla, P. K. (2006). Bioorg. Med. Chem. Lett.14, 6120–6126. [DOI] [PubMed]
- Taylor, E. C., McKillop, A. & Ross, R. E. (1965). J. Am. Chem. Soc.87, 1990–1995.
- Toshima, K., Kimura, T., Takano, O. T., Shima, Y., Umerzawa, K. & Matsumura, S. (2003). Tetrahedron, 59, 7057–7067.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810047094/hb5740sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810047094/hb5740Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




