Abstract
In the title compound, [Mn(C13H14N3O3)2(H2O)2], the MnII ion is coordinated by four N atoms from two (±)-2-(5-isopropyl-5-methyl-4-oxo-4,5-dihydro-1H-imidazol-2-yl)nicotinate ligands and two water molecules in a distorted octahedral environment. Intermolecular O—H⋯O hydrogen bonds lead to a chain along [010]. Intramolecular N—H⋯O and O—H⋯O hydrogen bonds are observed.
Related literature
For coordination compounds with pyridinecarboxylic acids, see: Chatterjee et al. (1998 ▶); Nathan & Mai (2000 ▶); Park et al. (2007 ▶); Yang et al. (2002 ▶). For the synthesis of compounds containing imidazolidinone derivatives, see: Erre et al. (1998 ▶).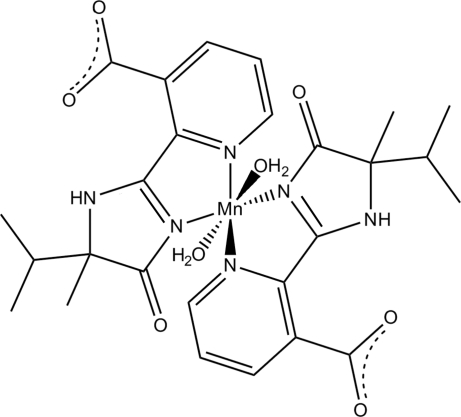
Experimental
Crystal data
[Mn(C13H14N3O3)2(H2O)2]
M r = 611.52
Orthorhombic,
a = 12.620 (3) Å
b = 19.753 (4) Å
c = 23.017 (5) Å
V = 5738 (2) Å3
Z = 8
Mo Kα radiation
μ = 0.52 mm−1
T = 298 K
0.50 × 0.48 × 0.35 mm
Data collection
Bruker SMART 1000 diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.782, T max = 0.839
25491 measured reflections
5057 independent reflections
3208 reflections with I > 2σ(I)
R int = 0.055
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.179
S = 1.06
5057 reflections
376 parameters
5 restraints
H-atom parameters constrained
Δρmax = 1.08 e Å−3
Δρmin = −0.47 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 1999 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810048506/hy2382sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810048506/hy2382Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O2 | 0.86 | 1.74 | 2.524 (5) | 151 |
| N5—H5⋯O5 | 0.86 | 1.76 | 2.535 (6) | 149 |
| O7—H7A⋯O3 | 0.85 | 2.09 | 2.838 (5) | 147 |
| O7—H7B⋯O1i | 0.85 | 1.80 | 2.638 (5) | 170 |
| O8—H8A⋯O6 | 0.85 | 2.06 | 2.791 (5) | 143 |
| O8—H8B⋯O4ii | 0.85 | 1.77 | 2.609 (5) | 171 |
Symmetry codes: (i) 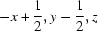 ; (ii)
; (ii) 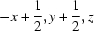 .
.
Acknowledgments
This work was supported by the Innovation Project of Guangxi University for Nationalities (gxun-chx2009080).
supplementary crystallographic information
Comment
(±)-2-(4-Isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl) nicotinic acid (imina) is a novel pyridylimidazolidinone ligand, which provides with efficient metal-chelating ability. The pyridine carboxylic acids have been extensively used in the design of coordination compounds, due to a variety of bonding modes and ability to form strong hydrogen bonds (Chatterjee et al., 1998; Nathan & Mai, 2000; Park et al., 2007; Yang et al., 2002). Imidazole group, which is one of the polydentate amine ligands, generally coordinates to metal ions using N atoms as donors. The synthesis of imina and its manganese(II) complex has been reported (Erre et al., 1998). Here we present the structure of a manganese(II) complex with 2-(5-isopropyl-5-methyl-4-oxo-4,5-dihydro-1H- imidazol-2-yl)nicotinate (L) ligand.
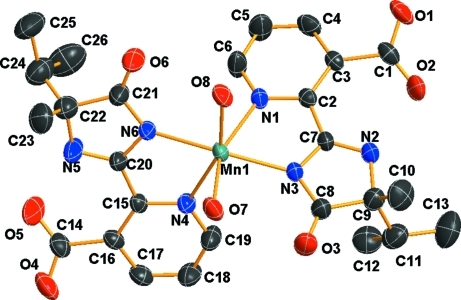
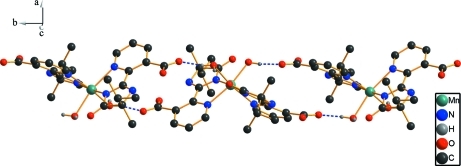
The molecular structure of the title complex is shown in Fig. 1. The asymmetric unit contains one MnII atom, two L ligands and two coordinated water molecules. The MnII atom exhibits a distorted octahedral geometry, defined by four N atoms from two L ligands and two O atoms from two water molecules. The dihedral angle between the two L planes in the complex is 61.58 (9)°. Intramolecular N—H···O and O—H···O hydrogen bonds are observed (Table 1). The complex molecules are connected via intermolecular O—H···O hydrogen bonds, forming a one-dimensional chain (Fig. 2).
Experimental
A mixture of Mn(CH3CO2)2.4H2O (0.122 g, 0.5 mmol), imina (0.392 g, 0.5 mmol), DMF (5 ml) and H2O (15 ml) was heated in a Teflon-lined steel bomb at 423 K for 3 d. Yellow crystals were obtained by slow evaporation of the solution at room temperature (yield: 78% ). Analysis, calculated for C26H32MnN6O8: C 51.07, H 5.27, N 13.74%; found: C 51.02, H 5.23, N 13.70%.
Refinement
H atoms on C and N atoms were positioned geometrically and refined using a riding model, with C—H = 0.93–0.98 Å and N—H = 0.86 Å and with Uiso(H) = 1.2(1.5 for methyl)Ueq(C,N). The water H atoms were located in a difference Fourier map and refined as riding atoms, with O—H = 0.85 Å and Uiso(H) = 1.2Ueq(O). The highest residual electron density was found 1.08 Å from C22 and the deepest hole 0.33 Å from N5.
Figures
Fig. 1.
Molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity.
Fig. 2.
Part of the chain structure in the title compound. Dashed lines indicate hydrongen bonds.
Crystal data
| [Mn(C13H14N3O3)2(H2O)2] | F(000) = 2552 |
| Mr = 611.52 | Dx = 1.416 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 5733 reflections |
| a = 12.620 (3) Å | θ = 2.4–27.9° |
| b = 19.753 (4) Å | µ = 0.52 mm−1 |
| c = 23.017 (5) Å | T = 298 K |
| V = 5738 (2) Å3 | Block, yellow |
| Z = 8 | 0.50 × 0.48 × 0.35 mm |
Data collection
| Bruker SMART 1000 diffractometer | 5057 independent reflections |
| Radiation source: fine-focus sealed tube | 3208 reflections with I > 2σ(I) |
| graphite | Rint = 0.055 |
| φ and ω scans | θmax = 25.0°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −14→15 |
| Tmin = 0.782, Tmax = 0.839 | k = −23→23 |
| 25491 measured reflections | l = −14→27 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.179 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0592P)2 + 17.1897P] where P = (Fo2 + 2Fc2)/3 |
| 5057 reflections | (Δ/σ)max < 0.001 |
| 376 parameters | Δρmax = 1.08 e Å−3 |
| 5 restraints | Δρmin = −0.47 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mn1 | 0.28498 (5) | 0.10909 (3) | 0.37476 (3) | 0.0311 (2) | |
| N1 | 0.1631 (3) | 0.19583 (18) | 0.39468 (16) | 0.0367 (9) | |
| N2 | 0.2232 (4) | 0.2701 (2) | 0.25716 (17) | 0.0490 (11) | |
| H2 | 0.1965 | 0.3100 | 0.2544 | 0.059* | |
| N3 | 0.2672 (3) | 0.17162 (18) | 0.29797 (15) | 0.0355 (9) | |
| N4 | 0.1688 (3) | 0.02058 (18) | 0.35275 (16) | 0.0365 (9) | |
| N5 | 0.2283 (4) | −0.0537 (2) | 0.49061 (18) | 0.0664 (15) | |
| H5 | 0.2023 | −0.0939 | 0.4929 | 0.080* | |
| N6 | 0.2631 (3) | 0.04717 (18) | 0.45175 (15) | 0.0350 (9) | |
| O1 | 0.0913 (3) | 0.42772 (17) | 0.36537 (17) | 0.0651 (11) | |
| O2 | 0.1213 (4) | 0.37586 (18) | 0.28288 (17) | 0.0650 (12) | |
| O3 | 0.3547 (3) | 0.12123 (19) | 0.22133 (16) | 0.0646 (11) | |
| O4 | 0.0995 (3) | −0.21079 (17) | 0.38402 (18) | 0.0673 (12) | |
| O5 | 0.1178 (5) | −0.1567 (2) | 0.46503 (19) | 0.110 (2) | |
| O6 | 0.3470 (3) | 0.09912 (18) | 0.52887 (15) | 0.0637 (11) | |
| O7 | 0.4045 (3) | 0.05333 (16) | 0.32650 (15) | 0.0475 (9) | |
| H7A | 0.3963 | 0.0573 | 0.2900 | 0.057* | |
| H7B | 0.4022 | 0.0115 | 0.3351 | 0.057* | |
| O8 | 0.3971 (2) | 0.16651 (15) | 0.42592 (14) | 0.0440 (8) | |
| H8A | 0.3819 | 0.1647 | 0.4619 | 0.053* | |
| H8B | 0.3977 | 0.2080 | 0.4162 | 0.053* | |
| C1 | 0.1092 (3) | 0.3765 (2) | 0.3365 (2) | 0.0391 (11) | |
| C2 | 0.1627 (3) | 0.2491 (2) | 0.35795 (19) | 0.0308 (10) | |
| C3 | 0.1138 (3) | 0.3108 (2) | 0.37203 (19) | 0.0301 (10) | |
| C4 | 0.0613 (4) | 0.3130 (2) | 0.4252 (2) | 0.0468 (13) | |
| H4 | 0.0279 | 0.3528 | 0.4366 | 0.056* | |
| C5 | 0.0578 (5) | 0.2576 (3) | 0.4611 (2) | 0.0643 (17) | |
| H5A | 0.0199 | 0.2587 | 0.4958 | 0.077* | |
| C6 | 0.1118 (5) | 0.2006 (3) | 0.4444 (2) | 0.0565 (15) | |
| H6 | 0.1123 | 0.1635 | 0.4693 | 0.068* | |
| C7 | 0.2186 (4) | 0.2319 (2) | 0.30315 (18) | 0.0341 (10) | |
| C8 | 0.3063 (4) | 0.1685 (2) | 0.2425 (2) | 0.0461 (12) | |
| C9 | 0.2812 (4) | 0.2346 (3) | 0.2106 (2) | 0.0473 (12) | |
| C10 | 0.3824 (5) | 0.2715 (3) | 0.1936 (3) | 0.0673 (17) | |
| H10A | 0.3653 | 0.3162 | 0.1803 | 0.101* | |
| H10B | 0.4173 | 0.2470 | 0.1631 | 0.101* | |
| H10C | 0.4284 | 0.2744 | 0.2267 | 0.101* | |
| C11 | 0.2073 (5) | 0.2211 (3) | 0.1594 (2) | 0.0609 (15) | |
| H11 | 0.2455 | 0.1906 | 0.1331 | 0.073* | |
| C12 | 0.1066 (5) | 0.1839 (3) | 0.1777 (3) | 0.0666 (17) | |
| H12A | 0.1252 | 0.1447 | 0.2002 | 0.100* | |
| H12B | 0.0682 | 0.1700 | 0.1437 | 0.100* | |
| H12C | 0.0632 | 0.2135 | 0.2006 | 0.100* | |
| C13 | 0.1825 (7) | 0.2845 (4) | 0.1247 (3) | 0.095 (2) | |
| H13A | 0.1352 | 0.2734 | 0.0935 | 0.142* | |
| H13B | 0.2469 | 0.3029 | 0.1091 | 0.142* | |
| H13C | 0.1497 | 0.3174 | 0.1497 | 0.142* | |
| C14 | 0.1122 (4) | −0.1588 (2) | 0.4119 (2) | 0.0452 (12) | |
| C15 | 0.1655 (3) | −0.0322 (2) | 0.39010 (18) | 0.0309 (10) | |
| C16 | 0.1191 (3) | −0.0941 (2) | 0.3754 (2) | 0.0339 (10) | |
| C17 | 0.0734 (4) | −0.0981 (2) | 0.3206 (2) | 0.0443 (12) | |
| H17 | 0.0420 | −0.1385 | 0.3088 | 0.053* | |
| C18 | 0.0735 (5) | −0.0442 (3) | 0.2836 (2) | 0.0543 (14) | |
| H18 | 0.0406 | −0.0469 | 0.2475 | 0.065* | |
| C19 | 0.1235 (4) | 0.0142 (3) | 0.3011 (2) | 0.0518 (14) | |
| H19 | 0.1256 | 0.0508 | 0.2756 | 0.062* | |
| C20 | 0.2189 (4) | −0.0141 (2) | 0.44567 (19) | 0.0373 (11) | |
| C21 | 0.3027 (5) | 0.0501 (3) | 0.5075 (2) | 0.0505 (13) | |
| C22 | 0.2893 (5) | −0.0204 (3) | 0.5368 (2) | 0.0533 (12) | |
| C23 | 0.3975 (5) | −0.0538 (3) | 0.5459 (3) | 0.0712 (17) | |
| H23A | 0.3879 | −0.0982 | 0.5620 | 0.107* | |
| H23B | 0.4390 | −0.0269 | 0.5721 | 0.107* | |
| H23C | 0.4336 | −0.0573 | 0.5093 | 0.107* | |
| C24 | 0.2253 (5) | −0.0186 (4) | 0.5912 (3) | 0.0735 (16) | |
| H24 | 0.2100 | −0.0655 | 0.6021 | 0.088* | |
| C25 | 0.2858 (7) | 0.0140 (4) | 0.6421 (3) | 0.101 (3) | |
| H25A | 0.3512 | −0.0097 | 0.6482 | 0.152* | |
| H25B | 0.2434 | 0.0115 | 0.6767 | 0.152* | |
| H25C | 0.3004 | 0.0606 | 0.6332 | 0.152* | |
| C26 | 0.1188 (6) | 0.0170 (4) | 0.5812 (3) | 0.100 (2) | |
| H26A | 0.1310 | 0.0639 | 0.5726 | 0.150* | |
| H26B | 0.0760 | 0.0132 | 0.6155 | 0.150* | |
| H26C | 0.0828 | −0.0039 | 0.5491 | 0.150* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn1 | 0.0387 (4) | 0.0209 (3) | 0.0337 (4) | −0.0009 (3) | −0.0012 (3) | 0.0038 (3) |
| N1 | 0.047 (2) | 0.029 (2) | 0.034 (2) | 0.0061 (17) | 0.0065 (18) | 0.0057 (17) |
| N2 | 0.078 (3) | 0.031 (2) | 0.037 (2) | 0.015 (2) | 0.004 (2) | 0.0046 (19) |
| N3 | 0.049 (2) | 0.0263 (19) | 0.031 (2) | 0.0067 (17) | 0.0038 (17) | 0.0039 (17) |
| N4 | 0.044 (2) | 0.032 (2) | 0.033 (2) | −0.0081 (17) | −0.0043 (18) | 0.0033 (17) |
| N5 | 0.115 (4) | 0.046 (3) | 0.039 (2) | −0.034 (3) | −0.013 (3) | 0.012 (2) |
| N6 | 0.047 (2) | 0.0271 (19) | 0.0307 (19) | −0.0084 (17) | −0.0055 (17) | 0.0025 (17) |
| O1 | 0.097 (3) | 0.0228 (18) | 0.076 (3) | 0.0018 (19) | 0.020 (2) | −0.0028 (18) |
| O2 | 0.105 (3) | 0.038 (2) | 0.052 (2) | 0.029 (2) | −0.003 (2) | 0.0107 (18) |
| O3 | 0.097 (3) | 0.051 (2) | 0.046 (2) | 0.028 (2) | 0.019 (2) | 0.0007 (18) |
| O4 | 0.090 (3) | 0.0272 (19) | 0.085 (3) | −0.0018 (19) | −0.016 (2) | 0.003 (2) |
| O5 | 0.220 (6) | 0.056 (3) | 0.052 (3) | −0.075 (3) | −0.003 (3) | 0.016 (2) |
| O6 | 0.099 (3) | 0.050 (2) | 0.042 (2) | −0.032 (2) | −0.020 (2) | 0.0020 (18) |
| O7 | 0.056 (2) | 0.0297 (17) | 0.056 (2) | 0.0084 (16) | 0.0059 (18) | 0.0029 (16) |
| O8 | 0.052 (2) | 0.0286 (17) | 0.052 (2) | −0.0086 (15) | −0.0028 (17) | 0.0019 (16) |
| C1 | 0.031 (2) | 0.027 (3) | 0.059 (3) | 0.0029 (19) | −0.002 (2) | −0.001 (2) |
| C2 | 0.030 (2) | 0.028 (2) | 0.034 (2) | 0.0033 (18) | −0.0049 (19) | 0.0007 (19) |
| C3 | 0.025 (2) | 0.026 (2) | 0.039 (2) | 0.0009 (17) | −0.005 (2) | −0.001 (2) |
| C4 | 0.048 (3) | 0.036 (3) | 0.056 (3) | 0.013 (2) | 0.006 (3) | −0.006 (3) |
| C5 | 0.081 (4) | 0.060 (4) | 0.052 (3) | 0.025 (3) | 0.031 (3) | 0.009 (3) |
| C6 | 0.075 (4) | 0.045 (3) | 0.049 (3) | 0.018 (3) | 0.024 (3) | 0.016 (3) |
| C7 | 0.045 (3) | 0.025 (2) | 0.032 (2) | 0.003 (2) | −0.003 (2) | 0.004 (2) |
| C8 | 0.064 (3) | 0.038 (3) | 0.037 (3) | 0.011 (3) | 0.003 (2) | 0.002 (2) |
| C9 | 0.056 (3) | 0.046 (3) | 0.040 (3) | 0.006 (3) | 0.004 (2) | 0.003 (2) |
| C10 | 0.060 (4) | 0.073 (4) | 0.069 (4) | −0.018 (3) | 0.014 (3) | 0.013 (3) |
| C11 | 0.068 (4) | 0.069 (4) | 0.046 (3) | 0.010 (3) | −0.005 (3) | −0.001 (3) |
| C12 | 0.060 (4) | 0.072 (4) | 0.068 (4) | −0.012 (3) | −0.016 (3) | −0.005 (3) |
| C13 | 0.109 (6) | 0.103 (6) | 0.073 (5) | 0.009 (5) | −0.016 (4) | 0.032 (4) |
| C14 | 0.047 (3) | 0.033 (3) | 0.056 (3) | −0.012 (2) | −0.005 (3) | 0.005 (3) |
| C15 | 0.032 (2) | 0.027 (2) | 0.033 (2) | −0.0045 (18) | 0.0043 (19) | 0.0029 (19) |
| C16 | 0.032 (2) | 0.028 (2) | 0.042 (3) | −0.0045 (18) | 0.004 (2) | −0.003 (2) |
| C17 | 0.047 (3) | 0.033 (3) | 0.053 (3) | −0.009 (2) | 0.000 (2) | −0.007 (2) |
| C18 | 0.068 (4) | 0.055 (3) | 0.040 (3) | −0.021 (3) | −0.015 (3) | 0.001 (3) |
| C19 | 0.067 (4) | 0.050 (3) | 0.039 (3) | −0.021 (3) | −0.012 (3) | 0.010 (2) |
| C20 | 0.051 (3) | 0.030 (2) | 0.031 (2) | −0.009 (2) | 0.000 (2) | 0.003 (2) |
| C21 | 0.076 (4) | 0.042 (3) | 0.034 (3) | −0.013 (3) | −0.008 (3) | 0.004 (2) |
| C22 | 0.064 (3) | 0.051 (3) | 0.045 (3) | −0.006 (2) | −0.005 (2) | −0.001 (3) |
| C23 | 0.081 (3) | 0.066 (4) | 0.066 (4) | 0.016 (3) | −0.012 (3) | 0.015 (3) |
| C24 | 0.085 (4) | 0.084 (5) | 0.052 (3) | −0.014 (3) | 0.006 (3) | 0.001 (3) |
| C25 | 0.164 (8) | 0.097 (6) | 0.043 (3) | −0.030 (6) | −0.006 (4) | −0.004 (4) |
| C26 | 0.077 (4) | 0.148 (8) | 0.075 (5) | 0.003 (4) | 0.018 (3) | −0.010 (5) |
Geometric parameters (Å, °)
| Mn1—O8 | 2.163 (3) | C8—C9 | 1.533 (7) |
| Mn1—N3 | 2.168 (4) | C9—C10 | 1.520 (7) |
| Mn1—N6 | 2.171 (4) | C9—C11 | 1.525 (7) |
| Mn1—O7 | 2.173 (3) | C10—H10A | 0.9600 |
| Mn1—N4 | 2.337 (4) | C10—H10B | 0.9600 |
| Mn1—N1 | 2.348 (4) | C10—H10C | 0.9600 |
| N1—C6 | 1.318 (6) | C11—C13 | 1.519 (8) |
| N1—C2 | 1.350 (5) | C11—C12 | 1.527 (8) |
| N2—C7 | 1.301 (5) | C11—H11 | 0.9800 |
| N2—C9 | 1.475 (6) | C12—H12A | 0.9600 |
| N2—H2 | 0.8600 | C12—H12B | 0.9600 |
| N3—C7 | 1.345 (5) | C12—H12C | 0.9600 |
| N3—C8 | 1.369 (6) | C13—H13A | 0.9600 |
| N4—C19 | 1.325 (6) | C13—H13B | 0.9600 |
| N4—C15 | 1.351 (5) | C13—H13C | 0.9600 |
| N5—C20 | 1.303 (6) | C14—C16 | 1.531 (6) |
| N5—C22 | 1.468 (7) | C15—C16 | 1.398 (6) |
| N5—H5 | 0.8600 | C15—C20 | 1.489 (6) |
| N6—C20 | 1.340 (5) | C16—C17 | 1.390 (7) |
| N6—C21 | 1.377 (6) | C17—C18 | 1.363 (7) |
| O1—C1 | 1.231 (6) | C17—H17 | 0.9300 |
| O2—C1 | 1.243 (6) | C18—C19 | 1.376 (7) |
| O3—C8 | 1.218 (6) | C18—H18 | 0.9300 |
| O4—C14 | 1.222 (6) | C19—H19 | 0.9300 |
| O5—C14 | 1.225 (6) | C21—C22 | 1.556 (7) |
| O6—C21 | 1.222 (6) | C22—C24 | 1.492 (8) |
| O7—H7A | 0.8499 | C22—C23 | 1.531 (8) |
| O7—H7B | 0.8500 | C23—H23A | 0.9600 |
| O8—H8A | 0.8500 | C23—H23B | 0.9600 |
| O8—H8B | 0.8500 | C23—H23C | 0.9600 |
| C1—C3 | 1.536 (6) | C24—C26 | 1.534 (10) |
| C2—C3 | 1.403 (6) | C24—C25 | 1.539 (9) |
| C2—C7 | 1.485 (6) | C24—H24 | 0.9800 |
| C3—C4 | 1.393 (7) | C25—H25A | 0.9600 |
| C4—C5 | 1.372 (7) | C25—H25B | 0.9600 |
| C4—H4 | 0.9300 | C25—H25C | 0.9600 |
| C5—C6 | 1.372 (7) | C26—H26A | 0.9600 |
| C5—H5A | 0.9300 | C26—H26B | 0.9600 |
| C6—H6 | 0.9300 | C26—H26C | 0.9600 |
| O8—Mn1—N3 | 102.29 (13) | H10B—C10—H10C | 109.5 |
| O8—Mn1—N6 | 86.22 (13) | C13—C11—C9 | 112.7 (5) |
| N3—Mn1—N6 | 166.75 (15) | C13—C11—C12 | 111.7 (5) |
| O8—Mn1—O7 | 95.15 (13) | C9—C11—C12 | 112.4 (5) |
| N3—Mn1—O7 | 86.80 (13) | C13—C11—H11 | 106.5 |
| N6—Mn1—O7 | 102.69 (14) | C9—C11—H11 | 106.5 |
| O8—Mn1—N4 | 157.06 (13) | C12—C11—H11 | 106.5 |
| N3—Mn1—N4 | 100.64 (14) | C11—C12—H12A | 109.5 |
| N6—Mn1—N4 | 71.08 (13) | C11—C12—H12B | 109.5 |
| O7—Mn1—N4 | 86.87 (13) | H12A—C12—H12B | 109.5 |
| O8—Mn1—N1 | 86.55 (13) | C11—C12—H12C | 109.5 |
| N3—Mn1—N1 | 71.07 (13) | H12A—C12—H12C | 109.5 |
| N6—Mn1—N1 | 99.70 (14) | H12B—C12—H12C | 109.5 |
| O7—Mn1—N1 | 157.60 (13) | C11—C13—H13A | 109.5 |
| N4—Mn1—N1 | 100.21 (14) | C11—C13—H13B | 109.5 |
| C6—N1—C2 | 119.1 (4) | H13A—C13—H13B | 109.5 |
| C6—N1—Mn1 | 122.9 (3) | C11—C13—H13C | 109.5 |
| C2—N1—Mn1 | 116.7 (3) | H13A—C13—H13C | 109.5 |
| C7—N2—C9 | 109.8 (4) | H13B—C13—H13C | 109.5 |
| C7—N2—H2 | 125.1 | O4—C14—O5 | 124.1 (5) |
| C9—N2—H2 | 125.1 | O4—C14—C16 | 114.9 (5) |
| C7—N3—C8 | 106.7 (4) | O5—C14—C16 | 121.1 (5) |
| C7—N3—Mn1 | 118.7 (3) | N4—C15—C16 | 122.3 (4) |
| C8—N3—Mn1 | 134.2 (3) | N4—C15—C20 | 110.3 (4) |
| C19—N4—C15 | 118.9 (4) | C16—C15—C20 | 127.4 (4) |
| C19—N4—Mn1 | 122.4 (3) | C17—C16—C15 | 116.3 (4) |
| C15—N4—Mn1 | 117.3 (3) | C17—C16—C14 | 115.3 (4) |
| C20—N5—C22 | 110.7 (4) | C15—C16—C14 | 128.4 (4) |
| C20—N5—H5 | 124.7 | C18—C17—C16 | 121.5 (4) |
| C22—N5—H5 | 124.7 | C18—C17—H17 | 119.2 |
| C20—N6—C21 | 106.6 (4) | C16—C17—H17 | 119.2 |
| C20—N6—Mn1 | 118.5 (3) | C17—C18—C19 | 118.2 (5) |
| C21—N6—Mn1 | 133.7 (3) | C17—C18—H18 | 120.9 |
| Mn1—O7—H7A | 111.9 | C19—C18—H18 | 120.9 |
| Mn1—O7—H7B | 110.5 | N4—C19—C18 | 122.7 (5) |
| H7A—O7—H7B | 108.4 | N4—C19—H19 | 118.6 |
| Mn1—O8—H8A | 111.1 | C18—C19—H19 | 118.6 |
| Mn1—O8—H8B | 111.5 | N5—C20—N6 | 114.9 (4) |
| H8A—O8—H8B | 107.4 | N5—C20—C15 | 125.4 (4) |
| O1—C1—O2 | 124.6 (5) | N6—C20—C15 | 119.6 (4) |
| O1—C1—C3 | 114.4 (4) | O6—C21—N6 | 125.0 (5) |
| O2—C1—C3 | 121.0 (4) | O6—C21—C22 | 125.7 (4) |
| N1—C2—C3 | 122.3 (4) | N6—C21—C22 | 109.1 (4) |
| N1—C2—C7 | 110.6 (4) | N5—C22—C24 | 109.6 (5) |
| C3—C2—C7 | 127.2 (4) | N5—C22—C23 | 112.0 (5) |
| C4—C3—C2 | 116.1 (4) | C24—C22—C23 | 112.2 (5) |
| C4—C3—C1 | 115.1 (4) | N5—C22—C21 | 98.3 (4) |
| C2—C3—C1 | 128.9 (4) | C24—C22—C21 | 113.7 (5) |
| C5—C4—C3 | 121.3 (4) | C23—C22—C21 | 110.4 (5) |
| C5—C4—H4 | 119.3 | C22—C23—H23A | 109.5 |
| C3—C4—H4 | 119.3 | C22—C23—H23B | 109.5 |
| C4—C5—C6 | 118.0 (5) | H23A—C23—H23B | 109.5 |
| C4—C5—H5A | 121.0 | C22—C23—H23C | 109.5 |
| C6—C5—H5A | 121.0 | H23A—C23—H23C | 109.5 |
| N1—C6—C5 | 123.1 (5) | H23B—C23—H23C | 109.5 |
| N1—C6—H6 | 118.5 | C22—C24—C26 | 111.0 (5) |
| C5—C6—H6 | 118.5 | C22—C24—C25 | 112.4 (6) |
| N2—C7—N3 | 114.9 (4) | C26—C24—C25 | 111.0 (6) |
| N2—C7—C2 | 125.4 (4) | C22—C24—H24 | 107.4 |
| N3—C7—C2 | 119.7 (4) | C26—C24—H24 | 107.4 |
| O3—C8—N3 | 126.1 (4) | C25—C24—H24 | 107.4 |
| O3—C8—C9 | 124.4 (4) | C24—C25—H25A | 109.5 |
| N3—C8—C9 | 109.6 (4) | C24—C25—H25B | 109.5 |
| N2—C9—C10 | 112.1 (4) | H25A—C25—H25B | 109.5 |
| N2—C9—C11 | 110.0 (4) | C24—C25—H25C | 109.5 |
| C10—C9—C11 | 113.6 (5) | H25A—C25—H25C | 109.5 |
| N2—C9—C8 | 99.1 (4) | H25B—C25—H25C | 109.5 |
| C10—C9—C8 | 110.9 (5) | C24—C26—H26A | 109.5 |
| C11—C9—C8 | 110.3 (4) | C24—C26—H26B | 109.5 |
| C9—C10—H10A | 109.5 | H26A—C26—H26B | 109.5 |
| C9—C10—H10B | 109.5 | C24—C26—H26C | 109.5 |
| H10A—C10—H10B | 109.5 | H26A—C26—H26C | 109.5 |
| C9—C10—H10C | 109.5 | H26B—C26—H26C | 109.5 |
| H10A—C10—H10C | 109.5 | ||
| O8—Mn1—N1—C6 | −79.3 (4) | C3—C2—C7—N3 | 175.8 (4) |
| N3—Mn1—N1—C6 | 176.5 (5) | C7—N3—C8—O3 | −179.3 (5) |
| N6—Mn1—N1—C6 | 6.3 (4) | Mn1—N3—C8—O3 | 9.4 (9) |
| O7—Mn1—N1—C6 | −174.4 (4) | C7—N3—C8—C9 | 1.4 (6) |
| N4—Mn1—N1—C6 | 78.7 (4) | Mn1—N3—C8—C9 | −169.9 (3) |
| O8—Mn1—N1—C2 | 87.5 (3) | C7—N2—C9—C10 | −117.0 (5) |
| N3—Mn1—N1—C2 | −16.8 (3) | C7—N2—C9—C11 | 115.7 (5) |
| N6—Mn1—N1—C2 | 173.1 (3) | C7—N2—C9—C8 | 0.1 (5) |
| O7—Mn1—N1—C2 | −7.7 (6) | O3—C8—C9—N2 | 179.8 (5) |
| N4—Mn1—N1—C2 | −114.6 (3) | N3—C8—C9—N2 | −0.9 (5) |
| O8—Mn1—N3—C7 | −68.3 (3) | O3—C8—C9—C10 | −62.2 (7) |
| N6—Mn1—N3—C7 | 60.8 (8) | N3—C8—C9—C10 | 117.0 (5) |
| O7—Mn1—N3—C7 | −162.9 (3) | O3—C8—C9—C11 | 64.5 (7) |
| N4—Mn1—N3—C7 | 110.9 (3) | N3—C8—C9—C11 | −116.2 (5) |
| N1—Mn1—N3—C7 | 13.6 (3) | N2—C9—C11—C13 | 73.7 (6) |
| O8—Mn1—N3—C8 | 102.1 (5) | C10—C9—C11—C13 | −52.8 (7) |
| N6—Mn1—N3—C8 | −128.7 (6) | C8—C9—C11—C13 | −178.1 (5) |
| O7—Mn1—N3—C8 | 7.5 (5) | N2—C9—C11—C12 | −53.7 (6) |
| N4—Mn1—N3—C8 | −78.7 (5) | C10—C9—C11—C12 | 179.8 (5) |
| N1—Mn1—N3—C8 | −175.9 (5) | C8—C9—C11—C12 | 54.6 (6) |
| O8—Mn1—N4—C19 | −173.7 (4) | C19—N4—C15—C16 | 2.4 (7) |
| N3—Mn1—N4—C19 | 8.3 (4) | Mn1—N4—C15—C16 | −164.7 (3) |
| N6—Mn1—N4—C19 | 177.6 (4) | C19—N4—C15—C20 | −178.9 (4) |
| O7—Mn1—N4—C19 | −77.8 (4) | Mn1—N4—C15—C20 | 14.0 (5) |
| N1—Mn1—N4—C19 | 80.7 (4) | N4—C15—C16—C17 | −2.0 (7) |
| O8—Mn1—N4—C15 | −7.0 (6) | C20—C15—C16—C17 | 179.6 (4) |
| N3—Mn1—N4—C15 | 174.9 (3) | N4—C15—C16—C14 | 177.9 (4) |
| N6—Mn1—N4—C15 | −15.8 (3) | C20—C15—C16—C14 | −0.6 (8) |
| O7—Mn1—N4—C15 | 88.8 (3) | O4—C14—C16—C17 | 22.3 (6) |
| N1—Mn1—N4—C15 | −112.6 (3) | O5—C14—C16—C17 | −156.7 (6) |
| O8—Mn1—N6—C20 | −162.1 (4) | O4—C14—C16—C15 | −157.6 (5) |
| N3—Mn1—N6—C20 | 67.3 (7) | O5—C14—C16—C15 | 23.5 (8) |
| O7—Mn1—N6—C20 | −67.6 (4) | C15—C16—C17—C18 | −0.4 (7) |
| N4—Mn1—N6—C20 | 14.5 (3) | C14—C16—C17—C18 | 179.7 (5) |
| N1—Mn1—N6—C20 | 112.1 (4) | C16—C17—C18—C19 | 2.2 (8) |
| O8—Mn1—N6—C21 | 3.2 (5) | C15—N4—C19—C18 | −0.5 (8) |
| N3—Mn1—N6—C21 | −127.4 (6) | Mn1—N4—C19—C18 | 166.0 (4) |
| O7—Mn1—N6—C21 | 97.6 (5) | C17—C18—C19—N4 | −1.8 (9) |
| N4—Mn1—N6—C21 | 179.8 (5) | C22—N5—C20—N6 | −1.6 (7) |
| N1—Mn1—N6—C21 | −82.7 (5) | C22—N5—C20—C15 | 177.6 (5) |
| C6—N1—C2—C3 | 3.1 (7) | C21—N6—C20—N5 | −2.7 (6) |
| Mn1—N1—C2—C3 | −164.1 (3) | Mn1—N6—C20—N5 | 166.2 (4) |
| C6—N1—C2—C7 | −176.3 (4) | C21—N6—C20—C15 | 178.1 (4) |
| Mn1—N1—C2—C7 | 16.5 (5) | Mn1—N6—C20—C15 | −13.0 (6) |
| N1—C2—C3—C4 | −2.8 (6) | N4—C15—C20—N5 | 179.5 (5) |
| C7—C2—C3—C4 | 176.5 (4) | C16—C15—C20—N5 | −1.9 (8) |
| N1—C2—C3—C1 | 177.9 (4) | N4—C15—C20—N6 | −1.4 (6) |
| C7—C2—C3—C1 | −2.8 (7) | C16—C15—C20—N6 | 177.2 (4) |
| O1—C1—C3—C4 | 22.3 (6) | C20—N6—C21—O6 | −178.5 (6) |
| O2—C1—C3—C4 | −156.6 (5) | Mn1—N6—C21—O6 | 15.0 (9) |
| O1—C1—C3—C2 | −158.4 (5) | C20—N6—C21—C22 | 5.6 (6) |
| O2—C1—C3—C2 | 22.7 (7) | Mn1—N6—C21—C22 | −160.9 (4) |
| C2—C3—C4—C5 | −0.3 (7) | C20—N5—C22—C24 | 123.4 (6) |
| C1—C3—C4—C5 | 179.1 (5) | C20—N5—C22—C23 | −111.5 (6) |
| C3—C4—C5—C6 | 2.9 (9) | C20—N5—C22—C21 | 4.5 (6) |
| C2—N1—C6—C5 | −0.2 (9) | O6—C21—C22—N5 | 178.0 (6) |
| Mn1—N1—C6—C5 | 166.2 (5) | N6—C21—C22—N5 | −6.1 (6) |
| C4—C5—C6—N1 | −2.7 (10) | O6—C21—C22—C24 | 62.3 (8) |
| C9—N2—C7—N3 | 0.8 (6) | N6—C21—C22—C24 | −121.8 (5) |
| C9—N2—C7—C2 | −177.3 (4) | O6—C21—C22—C23 | −64.8 (8) |
| C8—N3—C7—N2 | −1.4 (6) | N6—C21—C22—C23 | 111.1 (5) |
| Mn1—N3—C7—N2 | 171.4 (3) | N5—C22—C24—C26 | −56.6 (7) |
| C8—N3—C7—C2 | 176.8 (4) | C23—C22—C24—C26 | 178.3 (6) |
| Mn1—N3—C7—C2 | −10.3 (5) | C21—C22—C24—C26 | 52.2 (7) |
| N1—C2—C7—N2 | 173.2 (5) | N5—C22—C24—C25 | 178.4 (5) |
| C3—C2—C7—N2 | −6.2 (8) | C23—C22—C24—C25 | 53.4 (8) |
| N1—C2—C7—N3 | −4.9 (6) | C21—C22—C24—C25 | −72.8 (7) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O2 | 0.86 | 1.74 | 2.524 (5) | 151 |
| N5—H5···O5 | 0.86 | 1.76 | 2.535 (6) | 149 |
| O7—H7A···O3 | 0.85 | 2.09 | 2.838 (5) | 147 |
| O7—H7B···O1i | 0.85 | 1.80 | 2.638 (5) | 170 |
| O8—H8A···O6 | 0.85 | 2.06 | 2.791 (5) | 143 |
| O8—H8B···O4ii | 0.85 | 1.77 | 2.609 (5) | 171 |
Symmetry codes: (i) −x+1/2, y−1/2, z; (ii) −x+1/2, y+1/2, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2382).
References
- Brandenburg, K. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2007). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chatterjee, M., Maji, M., Ghosh, S. & Mak, T. C. W. (1998). J. Chem. Soc. Dalton Trans. pp. 3641–3646.
- Erre, L. S., Garribba, E., Micera, G. & Sardone, N. (1998). Inorg. Chim. Acta, 272, 68–73.
- Nathan, L. C. & Mai, T. D. (2000). J. Chem. Crystallogr.30, 509–518.
- Park, H., Lough, A. J., Kim, J. C., Jeong, M. H. & Kang, Y. S. (2007). Inorg. Chim. Acta, 360, 2819–2823.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Yang, L., Crans, D. C., Miller, S. M., la Cour, A., Anderson, O. P., Kaszynski, P. M., Godzala, M. E. III, Austin, L. D. & Willsky, G. R. (2002). Inorg. Chem.41, 4859–4871. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810048506/hy2382sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810048506/hy2382Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report