Abstract
The asymmetric unit of the title compound, C26H36N2O8, comprises two independent molecules. In each molecule, the two pyrrole rings are linked by a –CH2CH2– bridge, with dihedral angles between the two pyrrole rings of 14.5 (3) and 16.4 (3)° in the two molecules. Each pyrrole ring carries 2- and 5-methyl substituents and ethoxycarbonyl groups at the 3- and 5-positions.
Related literature
For background to the biological applications of bispyrrole and its derivatives, see: Dairi et al. (2006 ▶); Bordner & Rapoport (1965 ▶); Rapoport & Castagnoli (1962 ▶). For the synthesis and biological properties of pyrrole derivatives containing N-substituent groups, see: Banik et al. (2004 ▶); Sagyam et al. (2007 ▶). For details of the the Paal–Knorr condensation reaction, see Amarnath et al. (1991 ▶). For representative bond-length data, see: Allen et al. (1987 ▶).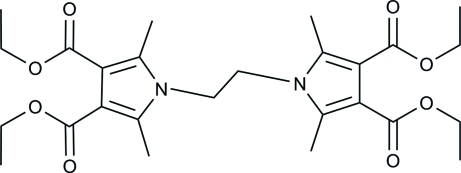
Experimental
Crystal data
C26H36N2O8
M r = 504.57
Monoclinic,
a = 12.891 (3) Å
b = 13.743 (3) Å
c = 16.717 (3) Å
β = 113.350 (14)°
V = 2719.0 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 298 K
0.20 × 0.18 × 0.17 mm
Data collection
Bruker SMART 1000 CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2001 ▶) T min = 0.982, T max = 0.985
5726 measured reflections
5726 independent reflections
3020 reflections with I > 2σ(I)
R int = 0.000
Refinement
R[F 2 > 2σ(F 2)] = 0.062
wR(F 2) = 0.194
S = 0.99
5726 reflections
663 parameters
2 restraints
H-atom parameters constrained
Δρmax = 0.29 e Å−3
Δρmin = −0.18 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810044119/sj5043sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810044119/sj5043Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This research was supported financially by the National Key Technology R&D Program of China (grant No. 2007BAI27B05).
supplementary crystallographic information
Comment
Bis-pyrrole and its derivatives play important roles in some bioactive pyrrole natural products, (Dairi et al., 2006; Bordner & Rapoport, 1965; Rapoport & Castagnoli, 1962). Recently, the synthesis of pyrrole derivatives with N-substituent groups aroused great interest because of their significant biological activity (Banik et al., 2004; Sagyam et al., 2007). As an intermediate for further synthesis of pyrrole derivatives containing N-substituent groups, we have prepared the title compound by the Paal-Knorr condensation reaction (Amarnath et al., 1991) and obtained its strcuture is reported here.
In the asymmetric unit of the title compound, Fig. 1, there are two independent molecules, A and B. The dihedral angle between the two pyrrole rings in one molecule is 14.5 (3)°, and that in the other molecule is 16.4 (3)°. All the bond lengths are within normal ranges (Allen et al., 1987).
Experimental
12.8 ml of ethyl acetoacetate (0.10 mol) and 2.28 g (0.10 mol) of sodium metal were added into 300 ml dry ether under stirring at room temperature. Then, the mixed solution was refluxed for 24 h till the Na was depleted. 100 ml dry ether solution contained 12.5 g (0.050 mol) I2 was added dropwise. After all of the iodine solution had been added, the reaction mixture was refluxed for 12 h and then cooled to room temperature. The undissolved solid was filtered and the ether solution was evaporated to yield diacetyl butanedioic acid diethyl ester as a gray solid (8.64 g, 67%). 5.20 g (20 mmol) of diacetyl butanedioic acid diethyl ester and 0.60 g (10 mmol) of ethylenediamine were dissolved into the mixed solution of ethanol and acetic acid (v/v, 5:1). The mixture was then refluxed for 6 h and evaporated to remove the ethanol. The residue was poured into water to give the title compound as a white solid (4.91 g, 97%). A little of the solid was dissolved in mixed solvent of acetone-water (v/v, 20:1). After standing in air over a period of about five days, the acetone is evaporated, colourless crystals suitable for X-ray diffraction analysis were formed at the bottom of the vessel.
Refinement
H atoms were positioned geometrically and refined using the riding-model approximation, with C–H = 0.93–0.97 Å, and Uiso(H) = 1.2Ueq(C) or Uiso(H) = 1.5Ueq(methyl C). In the absence of significant anomalous dispersion effects, Friedel pairs were averaged. Displacement parameters on some of the atoms particularly of the ethyl groups of the ethylcarboxylate substituents were unusually large. However, a suitable disorder model could not be found for them.
Figures
Fig. 1.
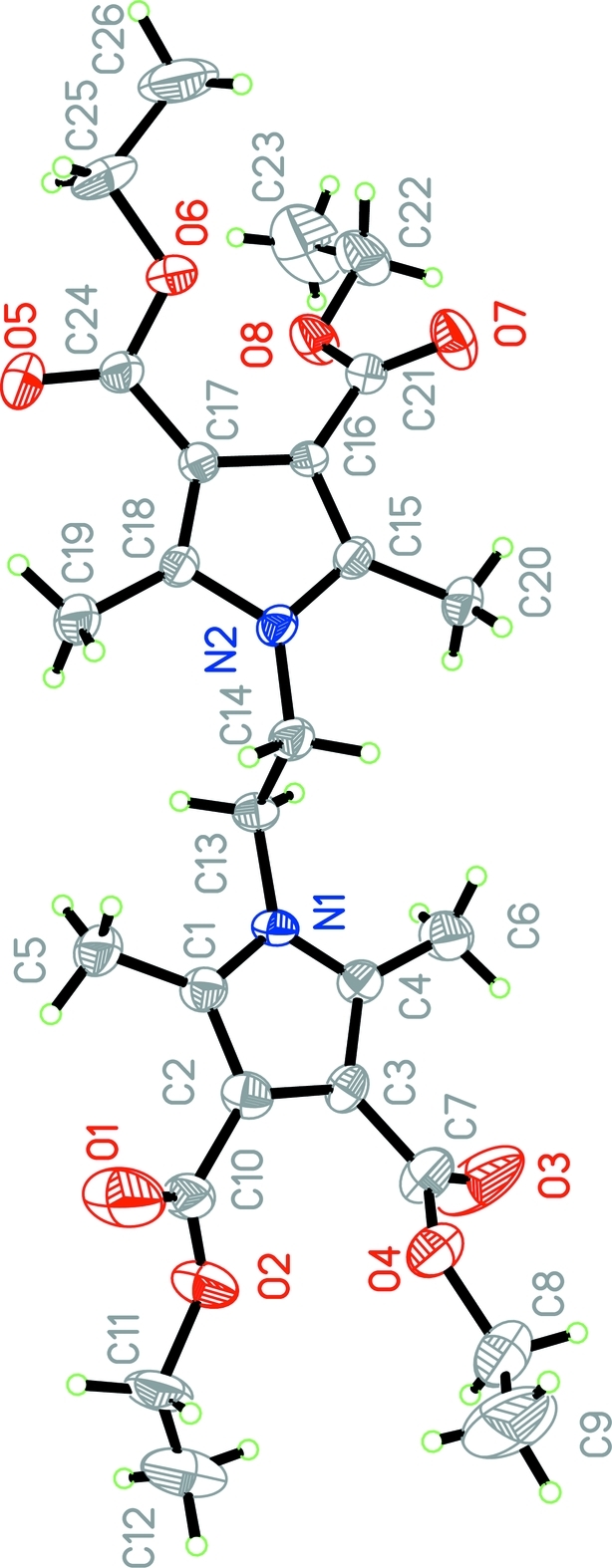
The structure of molecule A of the title compound with atom labels and 30% probability displacement ellipsoids for non-H atoms.
Fig. 2.
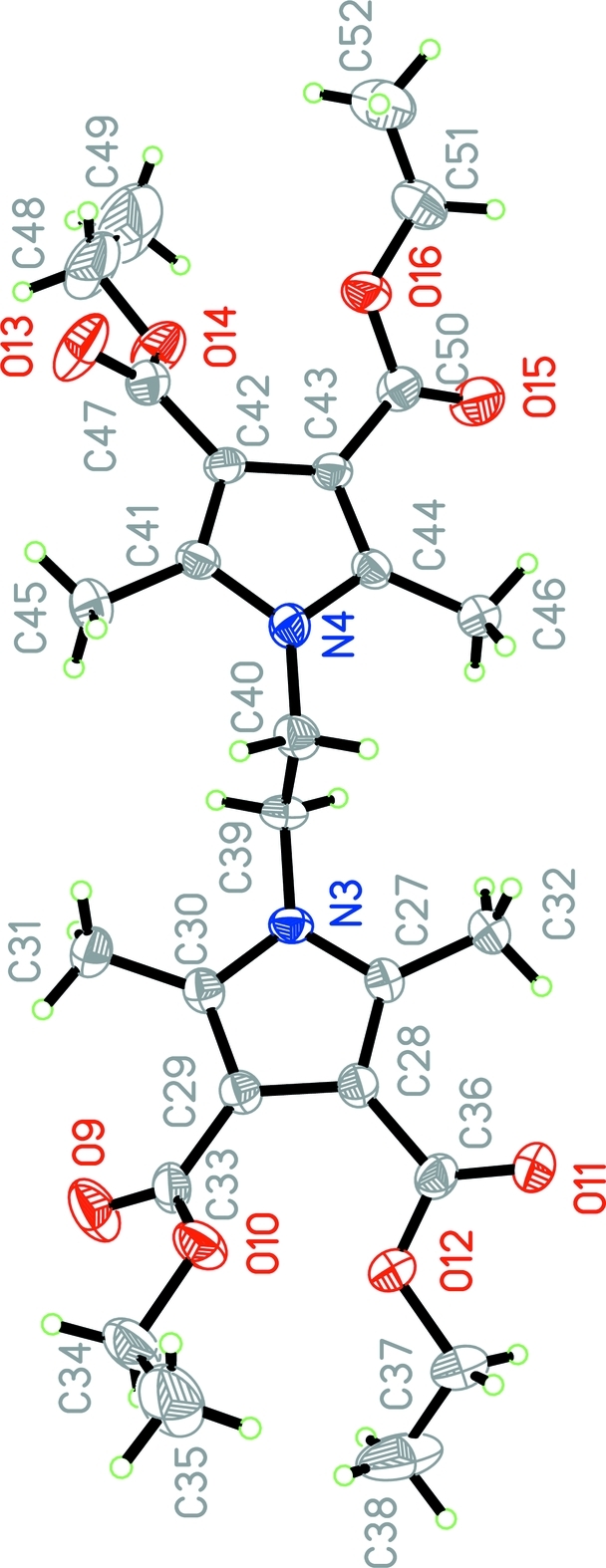
The structure of molecule B of the title compound with atom labels and 30% probability displacement ellipsoids for non-H atoms.
Crystal data
| C26H36N2O8 | F(000) = 1080 |
| Mr = 504.57 | Dx = 1.233 Mg m−3 |
| Monoclinic, Pc | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P -2yc | Cell parameters from 2429 reflections |
| a = 12.891 (3) Å | θ = 2.5–24.5° |
| b = 13.743 (3) Å | µ = 0.09 mm−1 |
| c = 16.717 (3) Å | T = 298 K |
| β = 113.350 (14)° | Block, colourless |
| V = 2719.0 (10) Å3 | 0.20 × 0.18 × 0.17 mm |
| Z = 4 |
Data collection
| Bruker SMART 1000 CCD diffractometer | 5726 independent reflections |
| Radiation source: fine-focus sealed tube | 3020 reflections with I > 2σ(I) |
| graphite | Rint = 0.0000 |
| ω scans | θmax = 27.0°, θmin = 1.5° |
| Absorption correction: multi-scan (SADABS; Bruker, 2001) | h = −16→15 |
| Tmin = 0.982, Tmax = 0.985 | k = 0→17 |
| 5726 measured reflections | l = 0→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.062 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.194 | H-atom parameters constrained |
| S = 0.99 | w = 1/[σ2(Fo2) + (0.1027P)2] where P = (Fo2 + 2Fc2)/3 |
| 5726 reflections | (Δ/σ)max = 0.001 |
| 663 parameters | Δρmax = 0.29 e Å−3 |
| 2 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.6385 (7) | 0.3819 (5) | 0.1367 (4) | 0.133 (3) | |
| O2 | 0.6668 (5) | 0.4974 (4) | 0.2316 (3) | 0.0819 (15) | |
| O3 | 0.7288 (7) | 0.4428 (7) | 0.4836 (4) | 0.173 (5) | |
| O4 | 0.8112 (5) | 0.4230 (4) | 0.3945 (3) | 0.0933 (16) | |
| O5 | 0.0805 (4) | −0.1379 (4) | 0.0950 (3) | 0.0823 (15) | |
| O6 | 0.1269 (4) | −0.2452 (3) | 0.2036 (3) | 0.0707 (13) | |
| O7 | 0.3049 (4) | −0.2263 (4) | 0.4369 (3) | 0.0765 (14) | |
| O8 | 0.1351 (3) | −0.1624 (3) | 0.3687 (3) | 0.0678 (12) | |
| N1 | 0.4959 (5) | 0.2503 (4) | 0.3016 (3) | 0.0456 (13) | |
| N2 | 0.3699 (5) | 0.0020 (4) | 0.2865 (3) | 0.0449 (13) | |
| C1 | 0.5129 (6) | 0.2823 (5) | 0.2310 (4) | 0.0522 (17) | |
| C2 | 0.5991 (6) | 0.3500 (5) | 0.2616 (4) | 0.0565 (19) | |
| C3 | 0.6301 (6) | 0.3564 (5) | 0.3544 (4) | 0.0519 (17) | |
| C4 | 0.5637 (5) | 0.2912 (5) | 0.3764 (4) | 0.0505 (17) | |
| C5 | 0.4492 (7) | 0.2512 (6) | 0.1411 (4) | 0.075 (2) | |
| H5A | 0.4740 | 0.1876 | 0.1326 | 0.112* | |
| H5B | 0.4618 | 0.2964 | 0.1020 | 0.112* | |
| H5C | 0.3701 | 0.2493 | 0.1295 | 0.112* | |
| C6 | 0.5673 (7) | 0.2669 (6) | 0.4622 (5) | 0.078 (2) | |
| H6A | 0.6291 | 0.3005 | 0.5060 | 0.117* | |
| H6B | 0.5774 | 0.1980 | 0.4714 | 0.117* | |
| H6C | 0.4977 | 0.2861 | 0.4658 | 0.117* | |
| C7 | 0.7231 (7) | 0.4142 (7) | 0.4190 (6) | 0.077 (3) | |
| C8 | 0.9070 (9) | 0.4896 (9) | 0.4463 (6) | 0.130 (4) | |
| H8A | 0.8798 | 0.5560 | 0.4420 | 0.156* | |
| H8B | 0.9374 | 0.4707 | 0.5072 | 0.156* | |
| C9 | 0.9903 (9) | 0.4839 (11) | 0.4143 (11) | 0.196 (7) | |
| H9A | 1.0497 | 0.5290 | 0.4451 | 0.294* | |
| H9B | 0.9590 | 0.4998 | 0.3534 | 0.294* | |
| H9C | 1.0203 | 0.4191 | 0.4221 | 0.294* | |
| C10 | 0.6377 (7) | 0.4101 (6) | 0.2051 (5) | 0.065 (2) | |
| C11 | 0.7157 (8) | 0.5562 (7) | 0.1838 (5) | 0.103 (3) | |
| H11A | 0.7847 | 0.5262 | 0.1856 | 0.124* | |
| H11B | 0.6633 | 0.5614 | 0.1234 | 0.124* | |
| C12 | 0.7393 (9) | 0.6497 (7) | 0.2222 (8) | 0.126 (4) | |
| H12A | 0.6773 | 0.6926 | 0.1919 | 0.189* | |
| H12B | 0.8068 | 0.6747 | 0.2185 | 0.189* | |
| H12C | 0.7499 | 0.6454 | 0.2823 | 0.189* | |
| C13 | 0.4084 (5) | 0.1770 (4) | 0.2939 (4) | 0.0460 (16) | |
| H13A | 0.3417 | 0.1892 | 0.2414 | 0.055* | |
| H13B | 0.3874 | 0.1826 | 0.3434 | 0.055* | |
| C14 | 0.4509 (5) | 0.0752 (5) | 0.2907 (4) | 0.0518 (18) | |
| H14A | 0.4695 | 0.0694 | 0.2400 | 0.062* | |
| H14B | 0.5196 | 0.0644 | 0.3420 | 0.062* | |
| C15 | 0.3637 (5) | −0.0487 (5) | 0.3554 (4) | 0.0467 (16) | |
| C16 | 0.2756 (5) | −0.1119 (4) | 0.3244 (3) | 0.0388 (14) | |
| C17 | 0.2231 (5) | −0.0999 (5) | 0.2330 (4) | 0.0431 (16) | |
| C18 | 0.2818 (5) | −0.0299 (5) | 0.2104 (4) | 0.0443 (16) | |
| C19 | 0.2710 (7) | 0.0107 (6) | 0.1232 (4) | 0.071 (2) | |
| H19A | 0.2518 | 0.0785 | 0.1201 | 0.106* | |
| H19B | 0.2127 | −0.0238 | 0.0772 | 0.106* | |
| H19C | 0.3414 | 0.0031 | 0.1170 | 0.106* | |
| C20 | 0.4437 (6) | −0.0241 (6) | 0.4472 (4) | 0.063 (2) | |
| H20A | 0.4261 | −0.0633 | 0.4877 | 0.094* | |
| H20B | 0.4360 | 0.0434 | 0.4585 | 0.094* | |
| H20C | 0.5200 | −0.0368 | 0.4539 | 0.094* | |
| C21 | 0.2419 (6) | −0.1726 (5) | 0.3819 (4) | 0.0485 (16) | |
| C22 | 0.0935 (7) | −0.2215 (6) | 0.4220 (6) | 0.089 (2) | |
| H22A | 0.0929 | −0.2895 | 0.4063 | 0.107* | |
| H22B | 0.1432 | −0.2144 | 0.4830 | 0.107* | |
| C23 | −0.0193 (8) | −0.1911 (9) | 0.4089 (8) | 0.152 (5) | |
| H23A | −0.0162 | −0.1285 | 0.4352 | 0.228* | |
| H23B | −0.0517 | −0.2376 | 0.4351 | 0.228* | |
| H23C | −0.0649 | −0.1870 | 0.3476 | 0.228* | |
| C24 | 0.1352 (6) | −0.1593 (5) | 0.1703 (4) | 0.0509 (18) | |
| C25 | 0.0423 (7) | −0.3109 (6) | 0.1489 (6) | 0.098 (3) | |
| H25A | 0.0602 | −0.3318 | 0.1005 | 0.117* | |
| H25B | −0.0310 | −0.2794 | 0.1259 | 0.117* | |
| C26 | 0.0408 (9) | −0.3958 (7) | 0.2035 (7) | 0.127 (4) | |
| H26A | 0.1167 | −0.4177 | 0.2358 | 0.191* | |
| H26B | −0.0026 | −0.4474 | 0.1668 | 0.191* | |
| H26C | 0.0072 | −0.3770 | 0.2432 | 0.191* | |
| O9 | 0.5561 (4) | 0.9699 (4) | 0.1424 (3) | 0.0949 (18) | |
| O10 | 0.7295 (4) | 0.9161 (3) | 0.2169 (3) | 0.0663 (12) | |
| O11 | 0.7871 (4) | 0.8930 (4) | 0.4889 (3) | 0.0920 (17) | |
| O12 | 0.7420 (4) | 0.9967 (3) | 0.3800 (3) | 0.0659 (12) | |
| O13 | 0.1588 (5) | 0.2854 (4) | 0.1302 (3) | 0.0999 (18) | |
| O14 | 0.0572 (4) | 0.3395 (4) | 0.2025 (3) | 0.0784 (14) | |
| O15 | 0.2457 (5) | 0.3679 (4) | 0.4697 (3) | 0.0921 (17) | |
| O16 | 0.2027 (4) | 0.2573 (3) | 0.3677 (3) | 0.0643 (12) | |
| N3 | 0.4994 (4) | 0.7494 (4) | 0.3027 (3) | 0.0445 (13) | |
| N4 | 0.3782 (5) | 0.5013 (4) | 0.2989 (3) | 0.0490 (14) | |
| C27 | 0.5838 (5) | 0.7816 (5) | 0.3766 (4) | 0.0465 (17) | |
| C28 | 0.6412 (5) | 0.8515 (5) | 0.3522 (4) | 0.0469 (17) | |
| C29 | 0.5865 (5) | 0.8617 (5) | 0.2585 (4) | 0.0467 (15) | |
| C30 | 0.4989 (5) | 0.7976 (5) | 0.2309 (4) | 0.0466 (15) | |
| C31 | 0.4202 (6) | 0.7749 (5) | 0.1407 (4) | 0.0597 (19) | |
| H31A | 0.4222 | 0.7064 | 0.1303 | 0.090* | |
| H31B | 0.4425 | 0.8102 | 0.1005 | 0.090* | |
| H31C | 0.3450 | 0.7935 | 0.1326 | 0.090* | |
| C32 | 0.6010 (6) | 0.7457 (5) | 0.4635 (4) | 0.063 (2) | |
| H32A | 0.6363 | 0.6829 | 0.4725 | 0.095* | |
| H32B | 0.5294 | 0.7406 | 0.4683 | 0.095* | |
| H32C | 0.6487 | 0.7902 | 0.5068 | 0.095* | |
| C33 | 0.6200 (6) | 0.9234 (6) | 0.2012 (4) | 0.0560 (18) | |
| C34 | 0.7673 (6) | 0.9755 (6) | 0.1622 (5) | 0.086 (2) | |
| H34A | 0.7564 | 1.0438 | 0.1710 | 0.103* | |
| H34B | 0.7245 | 0.9599 | 0.1013 | 0.103* | |
| C35 | 0.8860 (7) | 0.9555 (8) | 0.1858 (6) | 0.117 (3) | |
| H35A | 0.9284 | 0.9768 | 0.2445 | 0.176* | |
| H35B | 0.9116 | 0.9895 | 0.1469 | 0.176* | |
| H35C | 0.8967 | 0.8868 | 0.1817 | 0.176* | |
| C36 | 0.7301 (5) | 0.9140 (6) | 0.4137 (4) | 0.0539 (19) | |
| C37 | 0.8286 (7) | 1.0645 (6) | 0.4332 (5) | 0.097 (3) | |
| H37A | 0.8177 | 1.0789 | 0.4861 | 0.116* | |
| H37B | 0.9025 | 1.0349 | 0.4495 | 0.116* | |
| C38 | 0.8238 (8) | 1.1506 (6) | 0.3875 (8) | 0.129 (4) | |
| H38A | 0.8778 | 1.1961 | 0.4248 | 0.193* | |
| H38B | 0.7493 | 1.1778 | 0.3685 | 0.193* | |
| H38C | 0.8410 | 1.1370 | 0.3377 | 0.193* | |
| C39 | 0.4157 (5) | 0.6754 (4) | 0.3000 (4) | 0.0459 (16) | |
| H39A | 0.3479 | 0.6841 | 0.2474 | 0.055* | |
| H39B | 0.3955 | 0.6843 | 0.3495 | 0.055* | |
| C40 | 0.4601 (6) | 0.5742 (5) | 0.3016 (4) | 0.0532 (19) | |
| H40A | 0.4803 | 0.5655 | 0.2520 | 0.064* | |
| H40B | 0.5278 | 0.5656 | 0.3541 | 0.064* | |
| C41 | 0.3053 (6) | 0.4518 (5) | 0.2249 (4) | 0.0477 (16) | |
| C42 | 0.2423 (5) | 0.3905 (5) | 0.2500 (4) | 0.0458 (16) | |
| C43 | 0.2723 (5) | 0.4021 (4) | 0.3397 (4) | 0.0422 (15) | |
| C44 | 0.3576 (5) | 0.4694 (4) | 0.3699 (3) | 0.0446 (16) | |
| C45 | 0.3057 (7) | 0.4778 (6) | 0.1375 (4) | 0.068 (2) | |
| H45A | 0.2878 | 0.5455 | 0.1258 | 0.103* | |
| H45B | 0.2504 | 0.4392 | 0.0930 | 0.103* | |
| H45C | 0.3791 | 0.4652 | 0.1378 | 0.103* | |
| C46 | 0.4253 (6) | 0.5073 (5) | 0.4589 (4) | 0.0613 (19) | |
| H46A | 0.4093 | 0.4697 | 0.5010 | 0.092* | |
| H46B | 0.4060 | 0.5742 | 0.4624 | 0.092* | |
| H46C | 0.5043 | 0.5026 | 0.4705 | 0.092* | |
| C47 | 0.1503 (7) | 0.3337 (6) | 0.1879 (4) | 0.062 (2) | |
| C48 | −0.0347 (8) | 0.2802 (9) | 0.1476 (6) | 0.123 (4) | |
| H48A | −0.0690 | 0.3093 | 0.0902 | 0.148* | |
| H48B | −0.0069 | 0.2162 | 0.1415 | 0.148* | |
| C49 | −0.1135 (10) | 0.2716 (11) | 0.1827 (9) | 0.194 (7) | |
| H49A | −0.0957 | 0.3158 | 0.2308 | 0.291* | |
| H49B | −0.1135 | 0.2062 | 0.2028 | 0.291* | |
| H49C | −0.1869 | 0.2869 | 0.1392 | 0.291* | |
| C50 | 0.2382 (6) | 0.3448 (5) | 0.3992 (4) | 0.0548 (18) | |
| C51 | 0.1578 (7) | 0.1949 (6) | 0.4156 (5) | 0.089 (2) | |
| H51A | 0.2116 | 0.1905 | 0.4757 | 0.106* | |
| H51B | 0.0886 | 0.2231 | 0.4154 | 0.106* | |
| C52 | 0.1351 (8) | 0.1011 (6) | 0.3792 (6) | 0.099 (3) | |
| H52A | 0.0690 | 0.1030 | 0.3257 | 0.148* | |
| H52B | 0.1224 | 0.0575 | 0.4192 | 0.148* | |
| H52C | 0.1984 | 0.0787 | 0.3677 | 0.148* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.213 (8) | 0.129 (5) | 0.093 (4) | −0.075 (5) | 0.100 (5) | −0.036 (4) |
| O2 | 0.117 (4) | 0.063 (3) | 0.089 (3) | −0.018 (3) | 0.067 (3) | −0.002 (3) |
| O3 | 0.164 (7) | 0.287 (11) | 0.093 (4) | −0.142 (7) | 0.079 (5) | −0.110 (6) |
| O4 | 0.076 (3) | 0.111 (4) | 0.080 (3) | −0.031 (3) | 0.016 (3) | −0.005 (3) |
| O5 | 0.075 (3) | 0.097 (4) | 0.052 (3) | −0.018 (3) | 0.001 (2) | 0.001 (2) |
| O6 | 0.069 (3) | 0.050 (3) | 0.075 (3) | −0.019 (2) | 0.009 (2) | 0.002 (2) |
| O7 | 0.071 (3) | 0.097 (4) | 0.066 (3) | 0.014 (3) | 0.031 (2) | 0.035 (3) |
| O8 | 0.050 (3) | 0.079 (3) | 0.082 (3) | 0.005 (2) | 0.034 (2) | 0.031 (2) |
| N1 | 0.055 (3) | 0.034 (3) | 0.052 (3) | −0.007 (3) | 0.026 (3) | −0.003 (2) |
| N2 | 0.048 (3) | 0.049 (3) | 0.036 (3) | −0.010 (3) | 0.015 (2) | −0.006 (2) |
| C1 | 0.058 (4) | 0.052 (4) | 0.050 (4) | −0.006 (4) | 0.025 (3) | −0.006 (3) |
| C2 | 0.063 (4) | 0.061 (5) | 0.058 (4) | −0.002 (4) | 0.037 (3) | −0.001 (3) |
| C3 | 0.051 (4) | 0.061 (4) | 0.042 (3) | −0.002 (4) | 0.017 (3) | −0.014 (3) |
| C4 | 0.048 (4) | 0.061 (4) | 0.049 (4) | −0.006 (3) | 0.026 (3) | −0.003 (3) |
| C5 | 0.086 (5) | 0.086 (5) | 0.046 (4) | −0.027 (4) | 0.020 (3) | −0.009 (4) |
| C6 | 0.101 (6) | 0.083 (5) | 0.058 (4) | −0.022 (5) | 0.039 (4) | −0.006 (4) |
| C7 | 0.059 (5) | 0.106 (8) | 0.066 (5) | −0.016 (5) | 0.024 (4) | −0.019 (5) |
| C8 | 0.089 (7) | 0.171 (11) | 0.097 (6) | −0.036 (7) | 0.004 (5) | 0.006 (7) |
| C9 | 0.076 (7) | 0.241 (17) | 0.268 (18) | −0.045 (8) | 0.064 (10) | 0.011 (13) |
| C10 | 0.076 (5) | 0.072 (6) | 0.054 (4) | −0.020 (4) | 0.034 (4) | −0.003 (4) |
| C11 | 0.143 (7) | 0.101 (7) | 0.085 (5) | −0.062 (6) | 0.065 (5) | 0.003 (5) |
| C12 | 0.132 (8) | 0.086 (7) | 0.193 (11) | −0.014 (6) | 0.099 (8) | 0.028 (7) |
| C13 | 0.051 (4) | 0.034 (4) | 0.055 (4) | −0.001 (3) | 0.024 (3) | −0.007 (3) |
| C14 | 0.048 (4) | 0.061 (5) | 0.048 (4) | −0.012 (4) | 0.021 (3) | −0.002 (3) |
| C15 | 0.041 (3) | 0.048 (4) | 0.046 (3) | −0.002 (3) | 0.012 (3) | −0.004 (3) |
| C16 | 0.045 (3) | 0.030 (3) | 0.038 (3) | −0.003 (3) | 0.012 (3) | −0.003 (3) |
| C17 | 0.041 (3) | 0.040 (4) | 0.045 (3) | 0.000 (3) | 0.014 (3) | 0.001 (3) |
| C18 | 0.041 (3) | 0.049 (4) | 0.038 (3) | −0.004 (3) | 0.011 (3) | −0.003 (3) |
| C19 | 0.084 (5) | 0.083 (5) | 0.041 (3) | −0.024 (4) | 0.021 (3) | −0.004 (3) |
| C20 | 0.064 (4) | 0.074 (5) | 0.040 (3) | −0.008 (4) | 0.009 (3) | −0.003 (3) |
| C21 | 0.050 (4) | 0.044 (4) | 0.047 (3) | −0.003 (3) | 0.014 (3) | 0.002 (3) |
| C22 | 0.094 (6) | 0.097 (6) | 0.094 (5) | −0.003 (5) | 0.057 (5) | 0.030 (4) |
| C23 | 0.097 (7) | 0.218 (13) | 0.183 (10) | 0.016 (7) | 0.100 (7) | 0.072 (9) |
| C24 | 0.050 (4) | 0.053 (5) | 0.049 (4) | −0.012 (3) | 0.018 (3) | −0.001 (3) |
| C25 | 0.084 (6) | 0.083 (6) | 0.109 (6) | −0.050 (5) | 0.020 (5) | −0.027 (5) |
| C26 | 0.138 (9) | 0.084 (7) | 0.156 (9) | −0.064 (6) | 0.052 (7) | −0.029 (6) |
| O9 | 0.062 (3) | 0.142 (5) | 0.073 (3) | 0.011 (3) | 0.019 (3) | 0.056 (3) |
| O10 | 0.057 (3) | 0.079 (3) | 0.070 (3) | −0.001 (2) | 0.034 (2) | 0.023 (2) |
| O11 | 0.085 (4) | 0.114 (4) | 0.051 (3) | −0.044 (3) | −0.001 (3) | 0.011 (3) |
| O12 | 0.061 (3) | 0.070 (3) | 0.058 (2) | −0.023 (2) | 0.014 (2) | −0.003 (2) |
| O13 | 0.095 (4) | 0.127 (4) | 0.073 (3) | −0.027 (3) | 0.029 (3) | −0.055 (3) |
| O14 | 0.049 (3) | 0.102 (4) | 0.083 (3) | −0.020 (2) | 0.025 (2) | −0.025 (3) |
| O15 | 0.137 (5) | 0.094 (4) | 0.065 (3) | −0.050 (3) | 0.062 (3) | −0.025 (3) |
| O16 | 0.081 (3) | 0.057 (3) | 0.063 (3) | −0.019 (2) | 0.037 (2) | 0.000 (2) |
| N3 | 0.039 (3) | 0.042 (3) | 0.053 (3) | −0.005 (2) | 0.019 (2) | 0.004 (2) |
| N4 | 0.053 (3) | 0.057 (4) | 0.041 (3) | −0.006 (3) | 0.023 (2) | −0.003 (2) |
| C27 | 0.049 (4) | 0.051 (4) | 0.040 (3) | 0.002 (3) | 0.018 (3) | 0.003 (3) |
| C28 | 0.037 (3) | 0.061 (4) | 0.042 (3) | −0.002 (3) | 0.015 (3) | 0.004 (3) |
| C29 | 0.038 (3) | 0.057 (4) | 0.047 (3) | 0.001 (3) | 0.020 (3) | 0.006 (3) |
| C30 | 0.041 (3) | 0.058 (4) | 0.045 (3) | 0.003 (3) | 0.021 (3) | 0.004 (3) |
| C31 | 0.054 (4) | 0.069 (5) | 0.047 (3) | −0.002 (3) | 0.010 (3) | −0.004 (3) |
| C32 | 0.070 (4) | 0.068 (5) | 0.047 (3) | −0.012 (4) | 0.018 (3) | 0.011 (3) |
| C33 | 0.049 (4) | 0.078 (5) | 0.037 (3) | −0.002 (4) | 0.013 (3) | 0.006 (3) |
| C34 | 0.078 (5) | 0.111 (7) | 0.079 (5) | −0.011 (4) | 0.044 (4) | 0.028 (4) |
| C35 | 0.079 (6) | 0.188 (10) | 0.098 (6) | −0.007 (6) | 0.049 (5) | 0.032 (6) |
| C36 | 0.037 (4) | 0.070 (6) | 0.050 (4) | −0.007 (3) | 0.012 (3) | −0.001 (3) |
| C37 | 0.106 (6) | 0.081 (6) | 0.088 (5) | −0.046 (5) | 0.023 (5) | −0.008 (4) |
| C38 | 0.086 (6) | 0.058 (5) | 0.216 (11) | −0.019 (5) | 0.031 (7) | 0.005 (6) |
| C39 | 0.045 (4) | 0.032 (4) | 0.064 (4) | −0.002 (3) | 0.025 (3) | 0.003 (3) |
| C40 | 0.046 (4) | 0.065 (5) | 0.054 (4) | −0.004 (4) | 0.026 (3) | 0.006 (3) |
| C41 | 0.060 (4) | 0.040 (4) | 0.049 (3) | −0.006 (3) | 0.029 (3) | 0.001 (3) |
| C42 | 0.056 (4) | 0.038 (3) | 0.048 (3) | −0.011 (3) | 0.025 (3) | −0.005 (3) |
| C43 | 0.046 (4) | 0.040 (4) | 0.044 (3) | −0.007 (3) | 0.021 (3) | −0.002 (3) |
| C44 | 0.053 (4) | 0.043 (4) | 0.042 (3) | −0.001 (3) | 0.023 (3) | 0.004 (3) |
| C45 | 0.083 (5) | 0.081 (5) | 0.046 (4) | −0.004 (4) | 0.032 (3) | 0.003 (3) |
| C46 | 0.069 (4) | 0.060 (4) | 0.049 (3) | −0.015 (3) | 0.017 (3) | 0.002 (3) |
| C47 | 0.076 (5) | 0.063 (5) | 0.050 (4) | −0.015 (4) | 0.028 (4) | −0.005 (3) |
| C48 | 0.081 (6) | 0.192 (11) | 0.090 (6) | −0.079 (7) | 0.025 (5) | −0.036 (6) |
| C49 | 0.118 (10) | 0.270 (18) | 0.150 (11) | −0.095 (11) | 0.007 (9) | −0.029 (11) |
| C50 | 0.059 (4) | 0.062 (5) | 0.052 (4) | −0.010 (3) | 0.031 (3) | −0.010 (3) |
| C51 | 0.107 (6) | 0.087 (6) | 0.082 (5) | −0.023 (5) | 0.047 (4) | 0.018 (5) |
| C52 | 0.123 (7) | 0.070 (6) | 0.116 (7) | −0.006 (5) | 0.061 (6) | 0.010 (5) |
Geometric parameters (Å, °)
| O1—C10 | 1.210 (9) | O9—C33 | 1.187 (8) |
| O2—C10 | 1.282 (9) | O10—C33 | 1.333 (8) |
| O2—C11 | 1.444 (8) | O10—C34 | 1.446 (7) |
| O3—C7 | 1.123 (9) | O11—C36 | 1.212 (8) |
| O4—C7 | 1.356 (10) | O12—C36 | 1.305 (8) |
| O4—C8 | 1.505 (11) | O12—C37 | 1.454 (8) |
| O5—C24 | 1.210 (7) | O13—C47 | 1.211 (9) |
| O6—C24 | 1.327 (8) | O14—C47 | 1.318 (9) |
| O6—C25 | 1.432 (7) | O14—C48 | 1.431 (8) |
| O7—C21 | 1.208 (7) | O15—C50 | 1.186 (8) |
| O8—C21 | 1.314 (8) | O16—C50 | 1.321 (8) |
| O8—C22 | 1.456 (7) | O16—C51 | 1.443 (8) |
| N1—C4 | 1.332 (8) | N3—C27 | 1.356 (7) |
| N1—C1 | 1.357 (9) | N3—C30 | 1.368 (8) |
| N1—C13 | 1.480 (8) | N3—C39 | 1.470 (8) |
| N2—C15 | 1.375 (8) | N4—C44 | 1.386 (8) |
| N2—C18 | 1.399 (7) | N4—C41 | 1.397 (8) |
| N2—C14 | 1.432 (8) | N4—C40 | 1.443 (9) |
| C1—C2 | 1.384 (10) | C27—C28 | 1.369 (8) |
| C1—C5 | 1.462 (9) | C27—C32 | 1.466 (9) |
| C2—C3 | 1.443 (9) | C28—C29 | 1.448 (8) |
| C2—C10 | 1.482 (10) | C28—C36 | 1.475 (9) |
| C3—C4 | 1.386 (9) | C29—C30 | 1.361 (9) |
| C3—C7 | 1.486 (10) | C29—C33 | 1.467 (10) |
| C4—C6 | 1.456 (9) | C30—C31 | 1.479 (8) |
| C5—H5A | 0.9600 | C31—H31A | 0.9600 |
| C5—H5B | 0.9600 | C31—H31B | 0.9600 |
| C5—H5C | 0.9600 | C31—H31C | 0.9600 |
| C6—H6A | 0.9600 | C32—H32A | 0.9600 |
| C6—H6B | 0.9600 | C32—H32B | 0.9600 |
| C6—H6C | 0.9600 | C32—H32C | 0.9600 |
| C8—C9 | 1.378 (15) | C34—C35 | 1.447 (11) |
| C8—H8A | 0.9700 | C34—H34A | 0.9700 |
| C8—H8B | 0.9700 | C34—H34B | 0.9700 |
| C9—H9A | 0.9600 | C35—H35A | 0.9600 |
| C9—H9B | 0.9600 | C35—H35B | 0.9600 |
| C9—H9C | 0.9600 | C35—H35C | 0.9600 |
| C11—C12 | 1.416 (12) | C37—C38 | 1.397 (11) |
| C11—H11A | 0.9700 | C37—H37A | 0.9700 |
| C11—H11B | 0.9700 | C37—H37B | 0.9700 |
| C12—H12A | 0.9600 | C38—H38A | 0.9600 |
| C12—H12B | 0.9600 | C38—H38B | 0.9600 |
| C12—H12C | 0.9600 | C38—H38C | 0.9600 |
| C13—C14 | 1.510 (7) | C39—C40 | 1.500 (7) |
| C13—H13A | 0.9700 | C39—H39A | 0.9700 |
| C13—H13B | 0.9700 | C39—H39B | 0.9700 |
| C14—H14A | 0.9700 | C40—H40A | 0.9700 |
| C14—H14B | 0.9700 | C40—H40B | 0.9700 |
| C15—C16 | 1.359 (8) | C41—C42 | 1.347 (8) |
| C15—C20 | 1.510 (8) | C41—C45 | 1.507 (9) |
| C16—C17 | 1.415 (8) | C42—C43 | 1.401 (8) |
| C16—C21 | 1.461 (9) | C42—C47 | 1.455 (9) |
| C17—C18 | 1.366 (8) | C43—C44 | 1.371 (8) |
| C17—C24 | 1.453 (8) | C43—C50 | 1.466 (9) |
| C18—C19 | 1.515 (9) | C44—C46 | 1.489 (8) |
| C19—H19A | 0.9600 | C45—H45A | 0.9600 |
| C19—H19B | 0.9600 | C45—H45B | 0.9600 |
| C19—H19C | 0.9600 | C45—H45C | 0.9600 |
| C20—H20A | 0.9600 | C46—H46A | 0.9600 |
| C20—H20B | 0.9600 | C46—H46B | 0.9600 |
| C20—H20C | 0.9600 | C46—H46C | 0.9600 |
| C22—C23 | 1.443 (12) | C48—C49 | 1.365 (15) |
| C22—H22A | 0.9700 | C48—H48A | 0.9700 |
| C22—H22B | 0.9700 | C48—H48B | 0.9700 |
| C23—H23A | 0.9600 | C49—H49A | 0.9600 |
| C23—H23B | 0.9600 | C49—H49B | 0.9600 |
| C23—H23C | 0.9600 | C49—H49C | 0.9600 |
| C25—C26 | 1.485 (12) | C51—C52 | 1.407 (11) |
| C25—H25A | 0.9700 | C51—H51A | 0.9700 |
| C25—H25B | 0.9700 | C51—H51B | 0.9700 |
| C26—H26A | 0.9600 | C52—H52A | 0.9600 |
| C26—H26B | 0.9600 | C52—H52B | 0.9600 |
| C26—H26C | 0.9600 | C52—H52C | 0.9600 |
| C10—O2—C11 | 117.6 (6) | C33—O10—C34 | 115.4 (5) |
| C7—O4—C8 | 118.4 (7) | C36—O12—C37 | 119.2 (5) |
| C24—O6—C25 | 117.4 (5) | C47—O14—C48 | 115.5 (6) |
| C21—O8—C22 | 117.0 (5) | C50—O16—C51 | 118.1 (6) |
| C4—N1—C1 | 114.1 (5) | C27—N3—C30 | 111.4 (5) |
| C4—N1—C13 | 124.2 (5) | C27—N3—C39 | 124.6 (5) |
| C1—N1—C13 | 121.7 (5) | C30—N3—C39 | 124.0 (5) |
| C15—N2—C18 | 107.9 (5) | C44—N4—C41 | 108.3 (5) |
| C15—N2—C14 | 126.8 (5) | C44—N4—C40 | 125.4 (5) |
| C18—N2—C14 | 125.3 (5) | C41—N4—C40 | 126.4 (5) |
| N1—C1—C2 | 106.1 (5) | N3—C27—C28 | 106.9 (5) |
| N1—C1—C5 | 125.6 (6) | N3—C27—C32 | 123.0 (6) |
| C2—C1—C5 | 128.2 (6) | C28—C27—C32 | 130.0 (6) |
| C1—C2—C3 | 106.2 (6) | C27—C28—C29 | 107.6 (6) |
| C1—C2—C10 | 124.2 (6) | C27—C28—C36 | 124.3 (6) |
| C3—C2—C10 | 129.2 (7) | C29—C28—C36 | 127.4 (7) |
| C4—C3—C2 | 108.0 (6) | C30—C29—C28 | 106.6 (6) |
| C4—C3—C7 | 124.0 (6) | C30—C29—C33 | 124.9 (6) |
| C2—C3—C7 | 127.8 (7) | C28—C29—C33 | 128.3 (6) |
| N1—C4—C3 | 105.6 (5) | C29—C30—N3 | 107.5 (5) |
| N1—C4—C6 | 125.5 (6) | C29—C30—C31 | 128.5 (6) |
| C3—C4—C6 | 128.9 (6) | N3—C30—C31 | 123.8 (6) |
| C1—C5—H5A | 109.5 | C30—C31—H31A | 109.5 |
| C1—C5—H5B | 109.5 | C30—C31—H31B | 109.5 |
| H5A—C5—H5B | 109.5 | H31A—C31—H31B | 109.5 |
| C1—C5—H5C | 109.5 | C30—C31—H31C | 109.5 |
| H5A—C5—H5C | 109.5 | H31A—C31—H31C | 109.5 |
| H5B—C5—H5C | 109.5 | H31B—C31—H31C | 109.5 |
| C4—C6—H6A | 109.5 | C27—C32—H32A | 109.5 |
| C4—C6—H6B | 109.5 | C27—C32—H32B | 109.5 |
| H6A—C6—H6B | 109.5 | H32A—C32—H32B | 109.5 |
| C4—C6—H6C | 109.5 | C27—C32—H32C | 109.5 |
| H6A—C6—H6C | 109.5 | H32A—C32—H32C | 109.5 |
| H6B—C6—H6C | 109.5 | H32B—C32—H32C | 109.5 |
| O3—C7—O4 | 121.0 (8) | O9—C33—O10 | 122.5 (7) |
| O3—C7—C3 | 127.7 (8) | O9—C33—C29 | 124.3 (7) |
| O4—C7—C3 | 111.1 (7) | O10—C33—C29 | 113.0 (6) |
| C9—C8—O4 | 109.4 (10) | O10—C34—C35 | 107.7 (7) |
| C9—C8—H8A | 109.8 | O10—C34—H34A | 110.2 |
| O4—C8—H8A | 109.8 | C35—C34—H34A | 110.2 |
| C9—C8—H8B | 109.8 | O10—C34—H34B | 110.2 |
| O4—C8—H8B | 109.8 | C35—C34—H34B | 110.2 |
| H8A—C8—H8B | 108.3 | H34A—C34—H34B | 108.5 |
| C8—C9—H9A | 109.5 | C34—C35—H35A | 109.5 |
| C8—C9—H9B | 109.5 | C34—C35—H35B | 109.5 |
| H9A—C9—H9B | 109.5 | H35A—C35—H35B | 109.5 |
| C8—C9—H9C | 109.5 | C34—C35—H35C | 109.5 |
| H9A—C9—H9C | 109.5 | H35A—C35—H35C | 109.5 |
| H9B—C9—H9C | 109.5 | H35B—C35—H35C | 109.5 |
| O1—C10—O2 | 121.0 (7) | O11—C36—O12 | 121.9 (6) |
| O1—C10—C2 | 123.7 (8) | O11—C36—C28 | 124.6 (7) |
| O2—C10—C2 | 115.2 (6) | O12—C36—C28 | 113.5 (6) |
| C12—C11—O2 | 108.7 (7) | C38—C37—O12 | 110.6 (7) |
| C12—C11—H11A | 110.0 | C38—C37—H37A | 109.5 |
| O2—C11—H11A | 110.0 | O12—C37—H37A | 109.5 |
| C12—C11—H11B | 110.0 | C38—C37—H37B | 109.5 |
| O2—C11—H11B | 110.0 | O12—C37—H37B | 109.5 |
| H11A—C11—H11B | 108.3 | H37A—C37—H37B | 108.1 |
| C11—C12—H12A | 109.5 | C37—C38—H38A | 109.5 |
| C11—C12—H12B | 109.5 | C37—C38—H38B | 109.5 |
| H12A—C12—H12B | 109.5 | H38A—C38—H38B | 109.5 |
| C11—C12—H12C | 109.5 | C37—C38—H38C | 109.5 |
| H12A—C12—H12C | 109.5 | H38A—C38—H38C | 109.5 |
| H12B—C12—H12C | 109.5 | H38B—C38—H38C | 109.5 |
| N1—C13—C14 | 111.0 (4) | N3—C39—C40 | 111.8 (4) |
| N1—C13—H13A | 109.4 | N3—C39—H39A | 109.3 |
| C14—C13—H13A | 109.4 | C40—C39—H39A | 109.3 |
| N1—C13—H13B | 109.4 | N3—C39—H39B | 109.3 |
| C14—C13—H13B | 109.4 | C40—C39—H39B | 109.3 |
| H13A—C13—H13B | 108.0 | H39A—C39—H39B | 107.9 |
| N2—C14—C13 | 112.6 (5) | N4—C40—C39 | 112.0 (5) |
| N2—C14—H14A | 109.1 | N4—C40—H40A | 109.2 |
| C13—C14—H14A | 109.1 | C39—C40—H40A | 109.2 |
| N2—C14—H14B | 109.1 | N4—C40—H40B | 109.2 |
| C13—C14—H14B | 109.1 | C39—C40—H40B | 109.2 |
| H14A—C14—H14B | 107.8 | H40A—C40—H40B | 107.9 |
| C16—C15—N2 | 108.8 (5) | C42—C41—N4 | 107.9 (5) |
| C16—C15—C20 | 131.4 (6) | C42—C41—C45 | 133.2 (6) |
| N2—C15—C20 | 119.6 (6) | N4—C41—C45 | 118.9 (6) |
| C15—C16—C17 | 107.8 (6) | C41—C42—C43 | 108.5 (5) |
| C15—C16—C21 | 122.4 (5) | C41—C42—C47 | 122.3 (6) |
| C17—C16—C21 | 129.6 (5) | C43—C42—C47 | 128.8 (6) |
| C18—C17—C16 | 107.6 (5) | C44—C43—C42 | 108.2 (5) |
| C18—C17—C24 | 123.7 (6) | C44—C43—C50 | 121.6 (6) |
| C16—C17—C24 | 128.0 (6) | C42—C43—C50 | 129.5 (6) |
| C17—C18—N2 | 107.9 (5) | C43—C44—N4 | 107.2 (5) |
| C17—C18—C19 | 132.5 (5) | C43—C44—C46 | 132.2 (5) |
| N2—C18—C19 | 119.5 (6) | N4—C44—C46 | 120.6 (5) |
| C18—C19—H19A | 109.5 | C41—C45—H45A | 109.5 |
| C18—C19—H19B | 109.5 | C41—C45—H45B | 109.5 |
| H19A—C19—H19B | 109.5 | H45A—C45—H45B | 109.5 |
| C18—C19—H19C | 109.5 | C41—C45—H45C | 109.5 |
| H19A—C19—H19C | 109.5 | H45A—C45—H45C | 109.5 |
| H19B—C19—H19C | 109.5 | H45B—C45—H45C | 109.5 |
| C15—C20—H20A | 109.5 | C44—C46—H46A | 109.5 |
| C15—C20—H20B | 109.5 | C44—C46—H46B | 109.5 |
| H20A—C20—H20B | 109.5 | H46A—C46—H46B | 109.5 |
| C15—C20—H20C | 109.5 | C44—C46—H46C | 109.5 |
| H20A—C20—H20C | 109.5 | H46A—C46—H46C | 109.5 |
| H20B—C20—H20C | 109.5 | H46B—C46—H46C | 109.5 |
| O7—C21—O8 | 122.4 (6) | O13—C47—O14 | 123.9 (7) |
| O7—C21—C16 | 124.4 (7) | O13—C47—C42 | 123.8 (8) |
| O8—C21—C16 | 113.2 (5) | O14—C47—C42 | 112.3 (6) |
| C23—C22—O8 | 109.8 (7) | C49—C48—O14 | 110.0 (8) |
| C23—C22—H22A | 109.7 | C49—C48—H48A | 109.7 |
| O8—C22—H22A | 109.7 | O14—C48—H48A | 109.7 |
| C23—C22—H22B | 109.7 | C49—C48—H48B | 109.7 |
| O8—C22—H22B | 109.7 | O14—C48—H48B | 109.7 |
| H22A—C22—H22B | 108.2 | H48A—C48—H48B | 108.2 |
| C22—C23—H23A | 109.5 | C48—C49—H49A | 109.5 |
| C22—C23—H23B | 109.5 | C48—C49—H49B | 109.5 |
| H23A—C23—H23B | 109.5 | H49A—C49—H49B | 109.5 |
| C22—C23—H23C | 109.5 | C48—C49—H49C | 109.5 |
| H23A—C23—H23C | 109.5 | H49A—C49—H49C | 109.5 |
| H23B—C23—H23C | 109.5 | H49B—C49—H49C | 109.5 |
| O5—C24—O6 | 122.9 (6) | O15—C50—O16 | 121.2 (6) |
| O5—C24—C17 | 125.5 (6) | O15—C50—C43 | 127.1 (6) |
| O6—C24—C17 | 111.5 (6) | O16—C50—C43 | 111.6 (5) |
| O6—C25—C26 | 107.2 (7) | C52—C51—O16 | 111.3 (7) |
| O6—C25—H25A | 110.3 | C52—C51—H51A | 109.4 |
| C26—C25—H25A | 110.3 | O16—C51—H51A | 109.4 |
| O6—C25—H25B | 110.3 | C52—C51—H51B | 109.4 |
| C26—C25—H25B | 110.3 | O16—C51—H51B | 109.4 |
| H25A—C25—H25B | 108.5 | H51A—C51—H51B | 108.0 |
| C25—C26—H26A | 109.5 | C51—C52—H52A | 109.5 |
| C25—C26—H26B | 109.5 | C51—C52—H52B | 109.5 |
| H26A—C26—H26B | 109.5 | H52A—C52—H52B | 109.5 |
| C25—C26—H26C | 109.5 | C51—C52—H52C | 109.5 |
| H26A—C26—H26C | 109.5 | H52A—C52—H52C | 109.5 |
| H26B—C26—H26C | 109.5 | H52B—C52—H52C | 109.5 |
| C4—N1—C1—C2 | 0.0 (8) | C30—N3—C27—C28 | −1.1 (7) |
| C13—N1—C1—C2 | 179.5 (6) | C39—N3—C27—C28 | −178.5 (6) |
| C4—N1—C1—C5 | −178.7 (7) | C30—N3—C27—C32 | 177.2 (7) |
| C13—N1—C1—C5 | 0.8 (11) | C39—N3—C27—C32 | −0.1 (10) |
| N1—C1—C2—C3 | −0.8 (8) | N3—C27—C28—C29 | 0.7 (7) |
| C5—C1—C2—C3 | 177.9 (8) | C32—C27—C28—C29 | −177.5 (7) |
| N1—C1—C2—C10 | −174.1 (7) | N3—C27—C28—C36 | 171.8 (6) |
| C5—C1—C2—C10 | 4.6 (13) | C32—C27—C28—C36 | −6.4 (11) |
| C1—C2—C3—C4 | 1.3 (8) | C27—C28—C29—C30 | 0.0 (7) |
| C10—C2—C3—C4 | 174.1 (8) | C36—C28—C29—C30 | −170.8 (7) |
| C1—C2—C3—C7 | 175.9 (8) | C27—C28—C29—C33 | −176.4 (7) |
| C10—C2—C3—C7 | −11.2 (13) | C36—C28—C29—C33 | 12.9 (12) |
| C1—N1—C4—C3 | 0.8 (8) | C28—C29—C30—N3 | −0.6 (7) |
| C13—N1—C4—C3 | −178.7 (6) | C33—C29—C30—N3 | 175.9 (7) |
| C1—N1—C4—C6 | −176.8 (7) | C28—C29—C30—C31 | −176.1 (7) |
| C13—N1—C4—C6 | 3.7 (11) | C33—C29—C30—C31 | 0.4 (11) |
| C2—C3—C4—N1 | −1.3 (7) | C27—N3—C30—C29 | 1.1 (7) |
| C7—C3—C4—N1 | −176.2 (7) | C39—N3—C30—C29 | 178.5 (6) |
| C2—C3—C4—C6 | 176.2 (8) | C27—N3—C30—C31 | 176.9 (6) |
| C7—C3—C4—C6 | 1.3 (12) | C39—N3—C30—C31 | −5.8 (10) |
| C8—O4—C7—O3 | −12.6 (15) | C34—O10—C33—O9 | 5.2 (11) |
| C8—O4—C7—C3 | 172.6 (8) | C34—O10—C33—C29 | −180.0 (6) |
| C4—C3—C7—O3 | −31.7 (16) | C30—C29—C33—O9 | 46.6 (11) |
| C2—C3—C7—O3 | 154.5 (11) | C28—C29—C33—O9 | −137.6 (8) |
| C4—C3—C7—O4 | 142.8 (7) | C30—C29—C33—O10 | −128.1 (7) |
| C2—C3—C7—O4 | −31.1 (12) | C28—C29—C33—O10 | 47.7 (10) |
| C7—O4—C8—C9 | 176.5 (10) | C33—O10—C34—C35 | −177.7 (7) |
| C11—O2—C10—O1 | −8.6 (13) | C37—O12—C36—O11 | 2.0 (11) |
| C11—O2—C10—C2 | 174.0 (7) | C37—O12—C36—C28 | −178.6 (6) |
| C1—C2—C10—O1 | −34.0 (13) | C27—C28—C36—O11 | 24.6 (11) |
| C3—C2—C10—O1 | 154.3 (9) | C29—C28—C36—O11 | −166.1 (7) |
| C1—C2—C10—O2 | 143.3 (8) | C27—C28—C36—O12 | −154.7 (6) |
| C3—C2—C10—O2 | −28.4 (12) | C29—C28—C36—O12 | 14.5 (10) |
| C10—O2—C11—C12 | 178.7 (8) | C36—O12—C37—C38 | −176.6 (8) |
| C4—N1—C13—C14 | −98.2 (6) | C27—N3—C39—C40 | −83.5 (7) |
| C1—N1—C13—C14 | 82.3 (7) | C30—N3—C39—C40 | 99.5 (6) |
| C15—N2—C14—C13 | −95.1 (7) | C44—N4—C40—C39 | −81.7 (7) |
| C18—N2—C14—C13 | 84.4 (7) | C41—N4—C40—C39 | 97.3 (7) |
| N1—C13—C14—N2 | 177.8 (6) | N3—C39—C40—N4 | −180.0 (6) |
| C18—N2—C15—C16 | 0.8 (7) | C44—N4—C41—C42 | −0.5 (7) |
| C14—N2—C15—C16 | −179.7 (6) | C40—N4—C41—C42 | −179.7 (6) |
| C18—N2—C15—C20 | −175.6 (6) | C44—N4—C41—C45 | 176.3 (6) |
| C14—N2—C15—C20 | 4.0 (10) | C40—N4—C41—C45 | −2.9 (10) |
| N2—C15—C16—C17 | −0.9 (7) | N4—C41—C42—C43 | 1.5 (7) |
| C20—C15—C16—C17 | 174.9 (7) | C45—C41—C42—C43 | −174.7 (7) |
| N2—C15—C16—C21 | −176.2 (6) | N4—C41—C42—C47 | 174.4 (6) |
| C20—C15—C16—C21 | −0.4 (11) | C45—C41—C42—C47 | −1.7 (12) |
| C15—C16—C17—C18 | 0.7 (7) | C41—C42—C43—C44 | −1.9 (7) |
| C21—C16—C17—C18 | 175.5 (7) | C47—C42—C43—C44 | −174.3 (7) |
| C15—C16—C17—C24 | 171.3 (7) | C41—C42—C43—C50 | −172.0 (7) |
| C21—C16—C17—C24 | −13.8 (12) | C47—C42—C43—C50 | 15.6 (12) |
| C16—C17—C18—N2 | −0.2 (7) | C42—C43—C44—N4 | 1.5 (7) |
| C24—C17—C18—N2 | −171.4 (6) | C50—C43—C44—N4 | 172.6 (6) |
| C16—C17—C18—C19 | 176.3 (7) | C42—C43—C44—C46 | −177.1 (7) |
| C24—C17—C18—C19 | 5.1 (11) | C50—C43—C44—C46 | −6.0 (11) |
| C15—N2—C18—C17 | −0.3 (7) | C41—N4—C44—C43 | −0.6 (7) |
| C14—N2—C18—C17 | −179.9 (6) | C40—N4—C44—C43 | 178.5 (6) |
| C15—N2—C18—C19 | −177.4 (6) | C41—N4—C44—C46 | 178.2 (6) |
| C14—N2—C18—C19 | 3.0 (9) | C40—N4—C44—C46 | −2.7 (10) |
| C22—O8—C21—O7 | −1.9 (10) | C48—O14—C47—O13 | 3.0 (12) |
| C22—O8—C21—C16 | 179.0 (6) | C48—O14—C47—C42 | −175.9 (7) |
| C15—C16—C21—O7 | −54.0 (10) | C41—C42—C47—O13 | 49.1 (11) |
| C17—C16—C21—O7 | 131.8 (8) | C43—C42—C47—O13 | −139.4 (8) |
| C15—C16—C21—O8 | 125.2 (7) | C41—C42—C47—O14 | −131.9 (7) |
| C17—C16—C21—O8 | −49.0 (9) | C43—C42—C47—O14 | 39.5 (10) |
| C21—O8—C22—C23 | 171.4 (8) | C47—O14—C48—C49 | 163.8 (11) |
| C25—O6—C24—O5 | −5.4 (11) | C51—O16—C50—O15 | 8.7 (11) |
| C25—O6—C24—C17 | 179.3 (6) | C51—O16—C50—C43 | −175.2 (6) |
| C18—C17—C24—O5 | −23.1 (11) | C44—C43—C50—O15 | 28.1 (12) |
| C16—C17—C24—O5 | 167.7 (7) | C42—C43—C50—O15 | −163.0 (8) |
| C18—C17—C24—O6 | 152.1 (6) | C44—C43—C50—O16 | −147.8 (6) |
| C16—C17—C24—O6 | −17.2 (10) | C42—C43—C50—O16 | 21.2 (10) |
| C24—O6—C25—C26 | −173.5 (7) | C50—O16—C51—C52 | −173.6 (7) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5043).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Amarnath, V., Anthony, D. C., Amarnath, K., Valentine, W. M., Lawrence, A., Wetterau, L. A. & Graham, D. G. (1991). J. Org. Chem.56, 6924–6931.
- Banik, B. K., Samajdar, S. & Banik, I. (2004). J. Org. Chem.69, 213–216. [DOI] [PubMed]
- Bordner, J. & Rapoport, H. (1965). J. Org. Chem.30, 3824–3828.
- Bruker (2001). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2007). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Dairi, K., Tripathy, S., Attardo, G. & Lavallée, J. F. (2006). Tetrahedron Lett.47, 2605–2606.
- Rapoport, H. & Castagnoli, N. (1962). J. Am. Chem. Soc.84, 2178–2181.
- Sagyam, R. R., Padi, P. R., Ghanta, M. R. & Vurimidi, H. (2007). J. Heterocycl. Chem.44, 923–926.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810044119/sj5043sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810044119/sj5043Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


