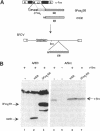
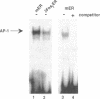
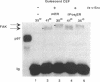
Abstract
v-Src activity results in both morphological transformation and reentry of quiescent chick embryo fibroblasts (CEF) into cell cycle. We have previously used temperature-sensitive v-Src mutants to show that enhanced activity of cellular AP-1 in the first few hours after activation of v-Src invariably precedes the biological consequences. Here we have investigated whether the early activation of AP-1 is essential for any or all of the v-Src responses by using a mutant c-Fos that comprises the leucine zipper and a disrupted basic region. Expression of the c-Fos mutant partially reduced cellular AP-1 activity in exponentially growing cells. However, in CEF that had been made quiescent by serum deprivation, v-Src-induced stimulation of AP-1 DNA binding activity was substantially reduced. In addition, quiescent CEF stably transfected with this mutant show an impaired mitogenic response to v-Src, indicating that the AP-1 stimulation is a necessary prerequisite for cell-cycle reentry. The ability of v-Src to morphologically transform quiescent CEF was not impaired by the inhibition of AP-1 stimulation, indicating that the mitogenic and morphological consequences of v-Src have distinguishable biochemical mediators. Focal adhesion kinase, a recently identified determinant of cell morphology, undergoes a gel mobility shift, characteristic of its hyperphosphorylated state, in response to v-Src activation in cells expressing the inhibitory AP-1 protein. This provides further evidence that the pathways that regulate morphological transformation are independent of AP-1.
Full text
PDF



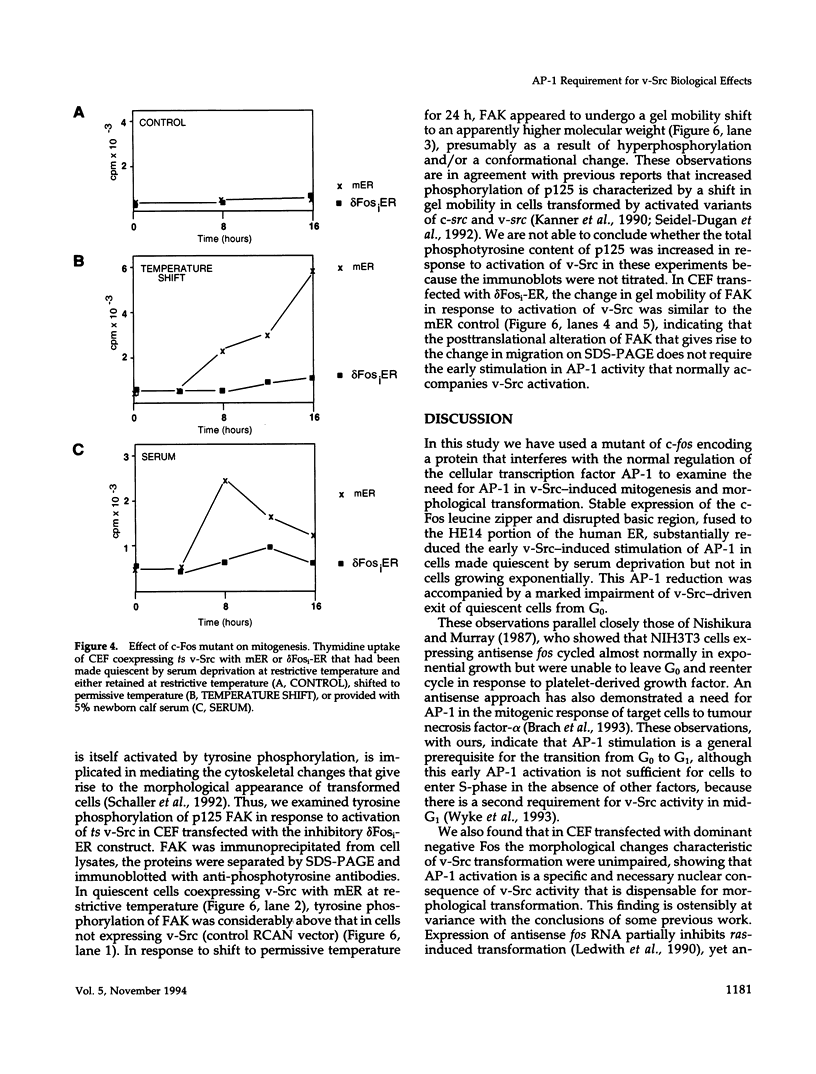


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. G., Wyke J. A., Macpherson I. A. Transformation by a temperature sensitive mutant of Rous sarcoma virus in the absence of serum. J Gen Virol. 1975 May;27(2):127–134. doi: 10.1099/0022-1317-27-2-127. [DOI] [PubMed] [Google Scholar]
- Beug H., Claviez M., Jockusch B. M., Graf T. Differential expression of Rous Sarcoma virus-specific transformation parameters in enucleated cells. Cell. 1978 Aug;14(4):843–856. doi: 10.1016/0092-8674(78)90340-9. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Characteristics of a conditional mutant of Rous sarcoma virus defective in ability to transform cells at high temperature. Virology. 1972 Feb;47(2):444–455. doi: 10.1016/0042-6822(72)90280-2. [DOI] [PubMed] [Google Scholar]
- Black E. J., Street A. J., Gillespie D. A. Protein phosphatase 2A reverses phosphorylation of c-Jun specified by the delta domain in vitro: correlation with oncogenic activation and deregulated transactivation activity of v-Jun. Oncogene. 1991 Nov;6(11):1949–1958. [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brach M. A., Gruss H. J., Sott C., Herrmann F. The mitogenic response to tumor necrosis factor alpha requires c-Jun/AP-1. Mol Cell Biol. 1993 Jul;13(7):4284–4290. doi: 10.1128/mcb.13.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Alani R., Preis L. H., Szabo E., Birrer M. J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993 Apr;8(4):877–886. [PubMed] [Google Scholar]
- Calothy G., Laugier D., Cross F. R., Jove R., Hanafusa T., Hanafusa H. The membrane-binding domain and myristylation of p60v-src are not essential for stimulation of cell proliferation. J Virol. 1987 May;61(5):1678–1681. doi: 10.1128/jvi.61.5.1678-1681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling A. D., Wyke J. A., Frame M. C. Mitogenesis of quiescent chick fibroblasts by v-Src: dependence on events at the membrane leading to early changes in AP-1. Oncogene. 1993 Jul;8(7):1875–1886. [PubMed] [Google Scholar]
- Crouch D. H., Lang C., Gillespie D. A. The leucine zipper domain of avian cMyc is required for transformation and autoregulation. Oncogene. 1990 May;5(5):683–689. [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Partial characterization of the mitogenic action of pp60v-src, the oncogenic protein product of the src gene of avian sarcoma virus. J Cell Physiol. 1984 Aug;120(2):135–145. doi: 10.1002/jcp.1041200205. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Frame M. C., Wilkie N. M., Darling A. J., Chudleigh A., Pintzas A., Lang J. C., Gillespie D. A. Regulation of AP-1/DNA complex formation in vitro. Oncogene. 1991 Feb;6(2):205–209. [PubMed] [Google Scholar]
- Fuerstenberg S., Beug H., Introna M., Khazaie K., Muñoz A., Ness S., Nordström K., Sap J., Stanley I., Zenke M. Ectopic expression of the erythrocyte band 3 anion exchange protein, using a new avian retrovirus vector. J Virol. 1990 Dec;64(12):5891–5902. doi: 10.1128/jvi.64.12.5891-5902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986 Sep;5(9):2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Ledwith B. J., Manam S., Kraynak A. R., Nichols W. W., Bradley M. O. Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1545–1555. doi: 10.1128/mcb.10.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Frost J., Deng T., Smeal T., al-Alawi N., Kikkawa U., Hunter T., Brenner D., Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992 Sep 4;70(5):777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- Lloyd A., Yancheva N., Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991 Aug 15;352(6336):635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Miao G. G., Curran T. Cell transformation by c-fos requires an extended period of expression and is independent of the cell cycle. Mol Cell Biol. 1994 Jun;14(6):4295–4310. doi: 10.1128/mcb.14.6.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H., Suzuki T., Yoshida T., Hashimoto Y., Curran T., Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991 Sep;6(9):1491–1497. [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Visvader J., Wamsley P., Verma I. M. Trans-dominant negative mutants of Fos and Jun. Proc Natl Acad Sci U S A. 1990 May;87(10):3806–3810. doi: 10.1073/pnas.87.10.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994 Mar;14(3):1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993 Aug;3(8):258–262. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Seidel-Dugan C., Meyer B. E., Thomas S. M., Brugge J. S. Effects of SH2 and SH3 deletions on the functional activities of wild-type and transforming variants of c-Src. Mol Cell Biol. 1992 Apr;12(4):1835–1845. doi: 10.1128/mcb.12.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T., Angel P., Meek J., Karin M. Different requirements for formation of Jun: Jun and Jun: Fos complexes. Genes Dev. 1989 Dec;3(12B):2091–2100. doi: 10.1101/gad.3.12b.2091. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A., Lang A., Wyke A. W. Mitogenesis induced by pp60v-src is not accompanied by increased expression of immediate early response genes. Oncogene. 1990 Feb;5(2):161–169. [PubMed] [Google Scholar]
- Wick M., Lucibello F. C., Müller R. Inhibition of Fos- and Ras-induced transformation by mutant Fos proteins with structural alterations in functionally different domains. Oncogene. 1992 May;7(5):859–867. [PubMed] [Google Scholar]
- Wyke A. W., Cushley W., Wyke J. A. Mitogenesis by v-Src: a need for active oncoprotein both in leaving G0 and in completing G1 phases of the cell cycle. Cell Growth Differ. 1993 Aug;4(8):671–678. [PubMed] [Google Scholar]
- Yoshida T., Shindo Y., Ohta K., Iba H. Identification of a small region of the v-fos gene product that is sufficient for transforming potential and growth-stimulating activity. Oncogene Res. 1989;5(2):79–89. [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]