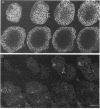
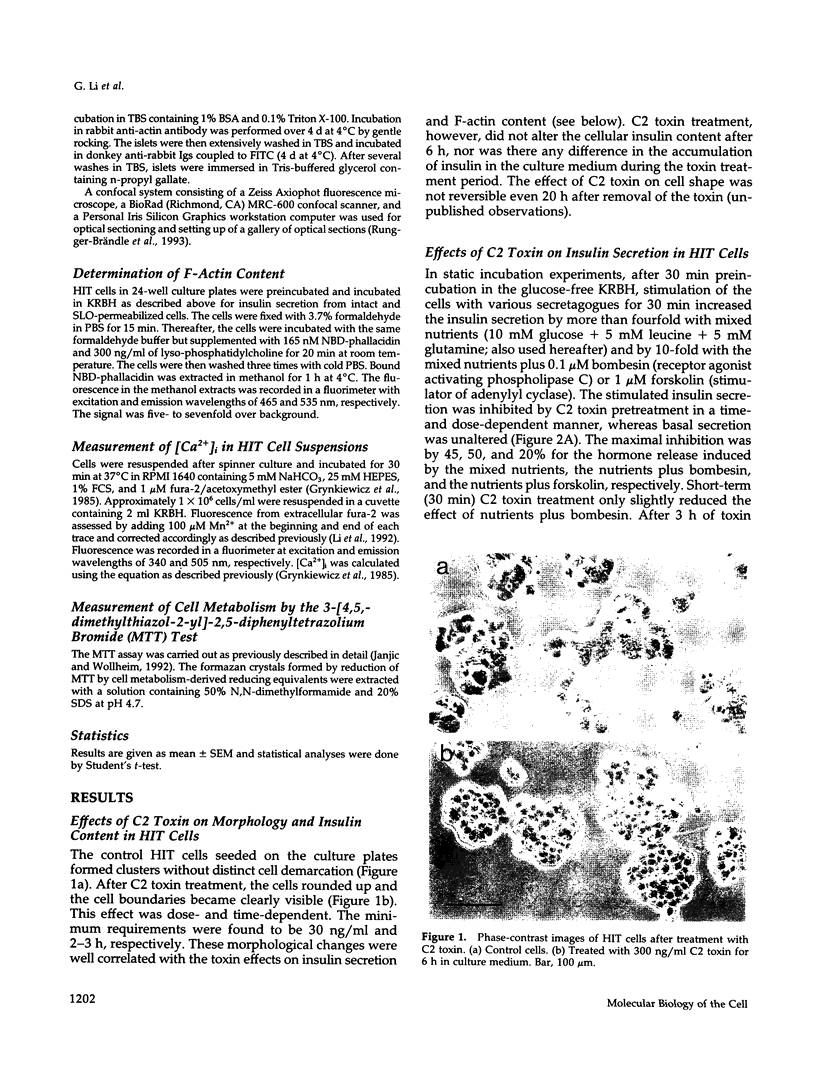
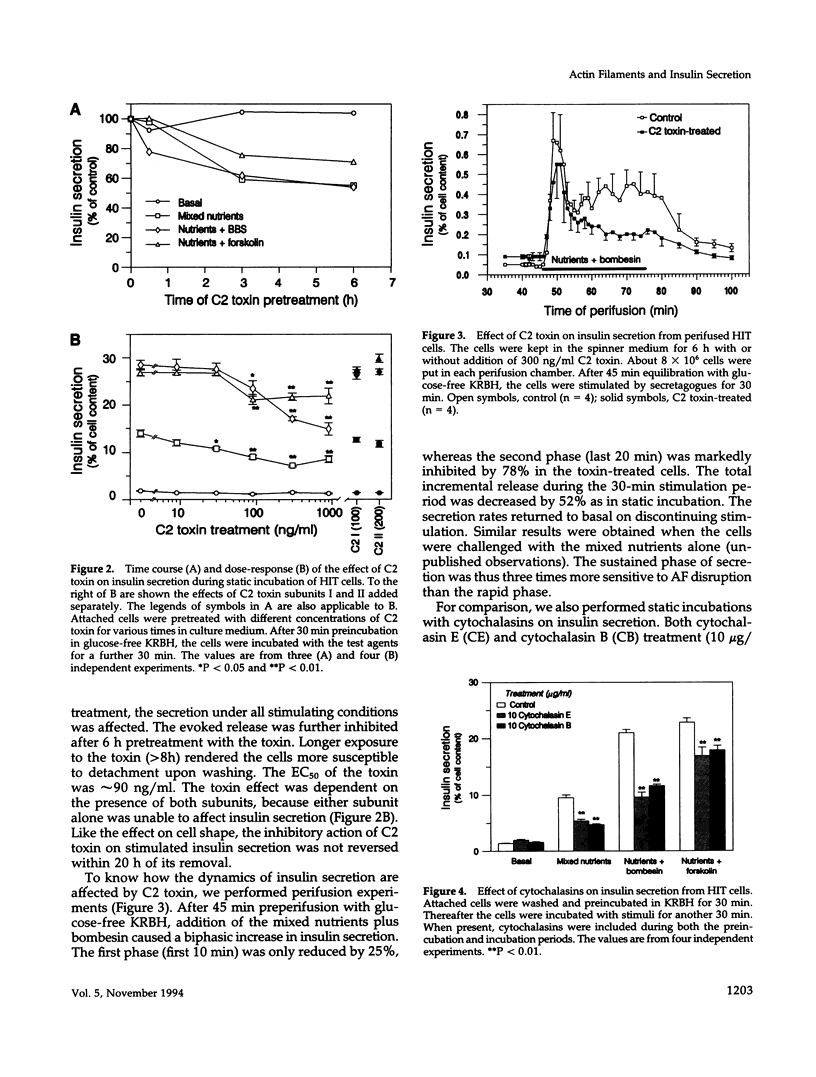
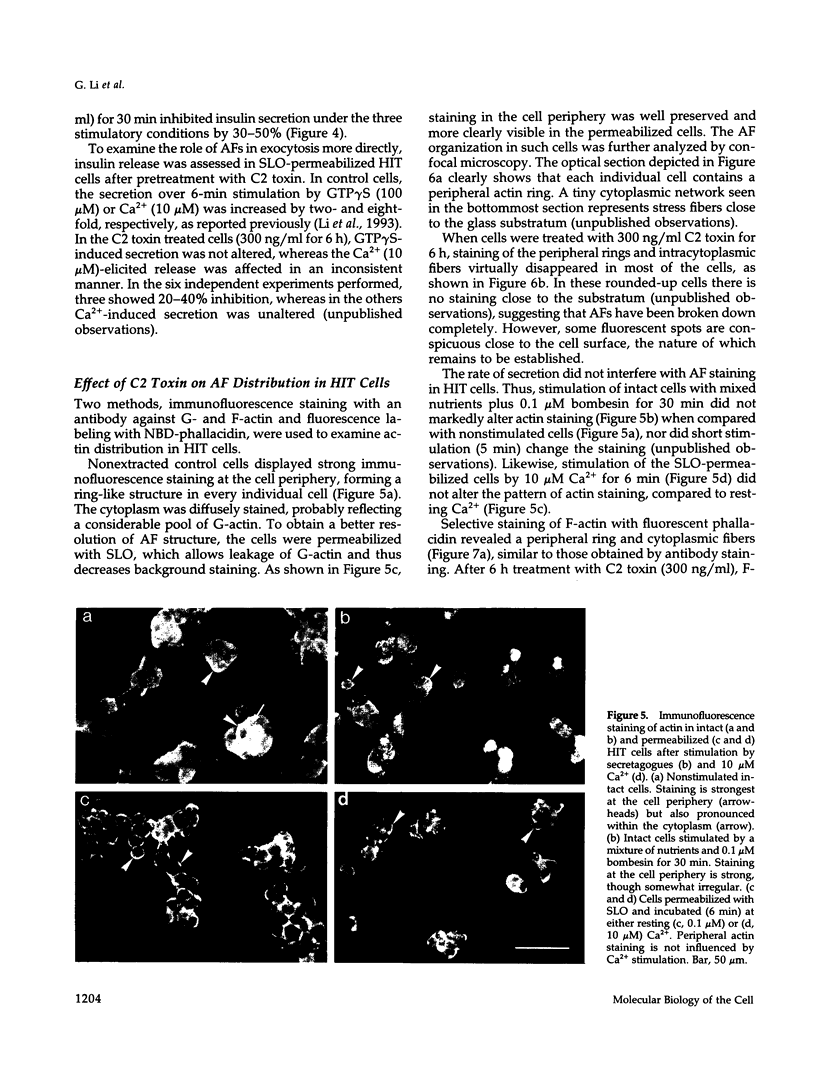
Abstract
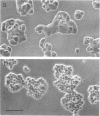
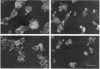
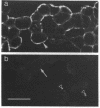
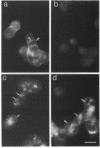
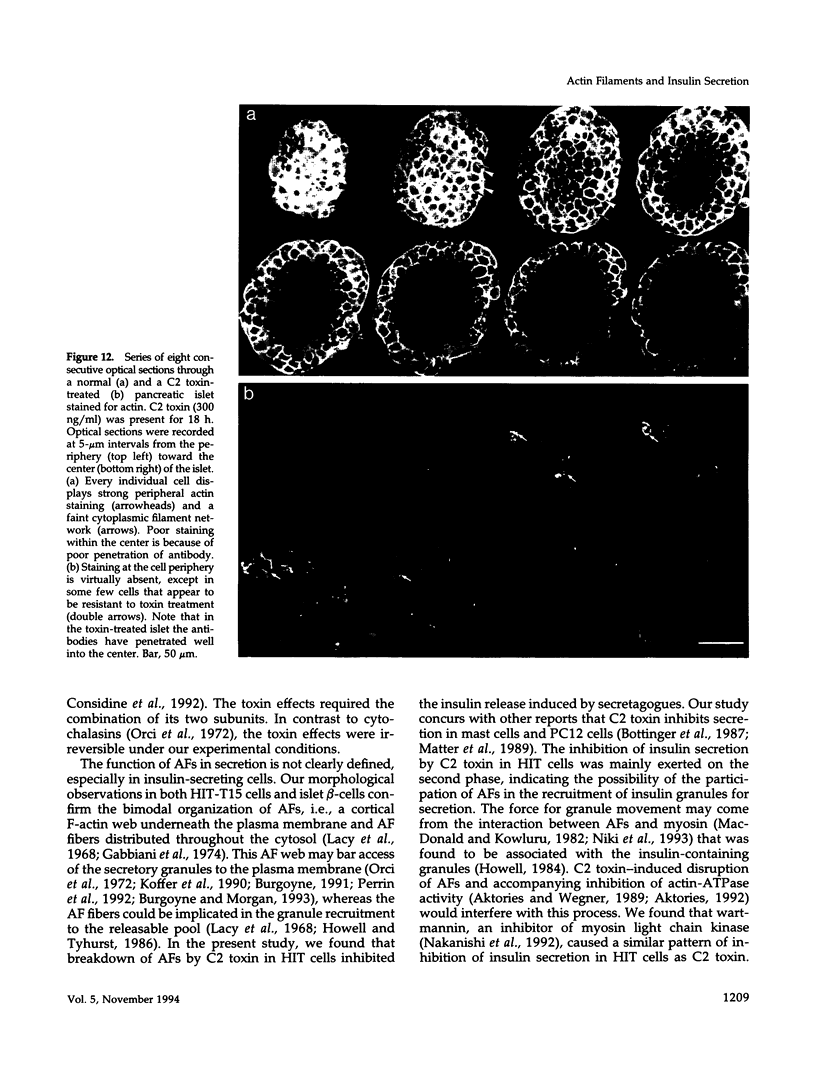
To examine their role in insulin secretion, actin filaments (AFs) were disrupted by Clostridium botulinum C2 toxin that ADP-ribosylates G-actin. Ribosylation also prevents polymerization of G-actin to F-actin and inhibits AF assembly by capping the fast-growing end of F-actin. Pretreatment of HIT-T15 cells with the toxin inhibited stimulated insulin secretion in a time- and dose-dependent manner. The toxin did not affect cellular insulin content or nonstimulated secretion. In static incubation, toxin treatment caused 45-50% inhibition of secretion induced by nutrients alone (10 mM glucose + 5 mM glutamine + 5 mM leucine) or combined with bombesin (phospholipase C-activator) and 20% reduction of that potentiated by forskolin (stimulator of adenylyl cyclase). In perifusion, the stimulated secretion during the first phase was marginally diminished, whereas the second phase was inhibited by approximately 80%. Pretreatment of HIT cells with wartmannin, a myosin light chain kinase inhibitor, caused a similar pattern of inhibition of the biphasic insulin release as C2 toxin. Nutrient metabolism and bombesin-evoked rise in cytosolic free Ca2+ were not affected by C2 toxin, indicating that nutrient recognition and the coupling between receptor activation and second messenger generation was not changed. In the toxin-treated cells, the AF web beneath the plasma membrane and the diffuse cytoplasmic F-actin fibers disappeared, as shown both by staining with an antibody against G- and F-actin and by staining F-actin with fluorescent phallacidin. C2 toxin dose-dependently reduced cellular F-actin content. Stimulation of insulin secretion was not associated with changes in F-actin content and organization. Treatment of cells with cytochalasin E and B, which shorten AFs, inhibited the stimulated insulin release by 30-50% although differing in their effects on F-actin content. In contrast to HIT-T15 cells, insulin secretion was potentiated in isolated rat islets after disruption of microfilaments with C2 toxin, most notably during the first phase. This effect was, however, diminished, and the second phase became slightly inhibited when the islets were degranulated. These results indicate an important role for AFs in insulin secretion. In the poorly granulated HIT-T15 cells actin-myosin interactions may participate in the recruitment of secretory granules to the releasable pool. In native islet beta-cells the predominant function of AFs appears to be the limitation of the access of granules to the plasma membrane.
Full text
PDF


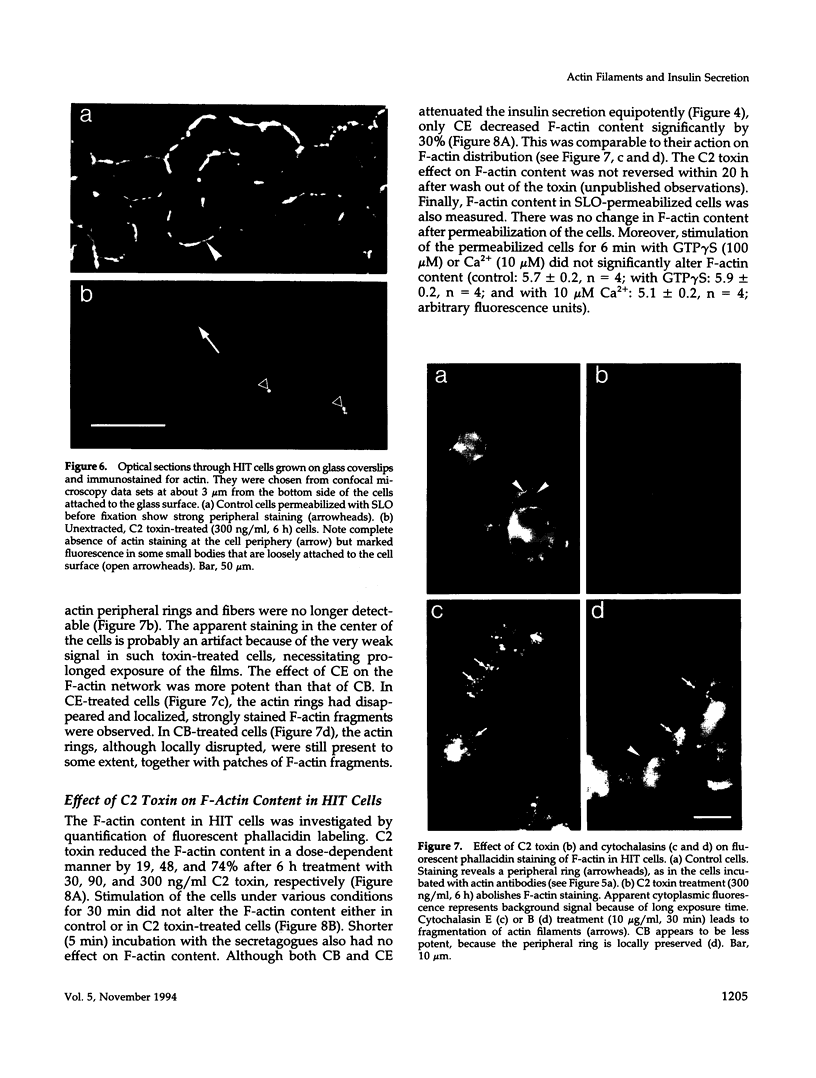
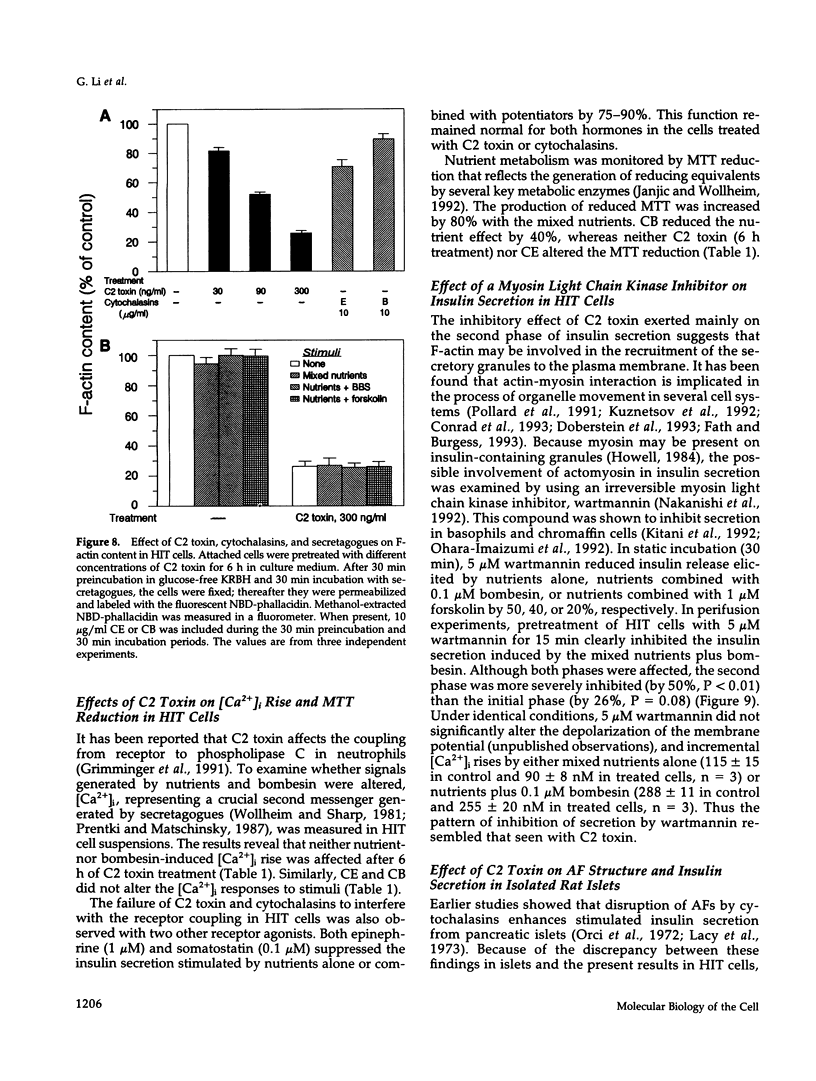
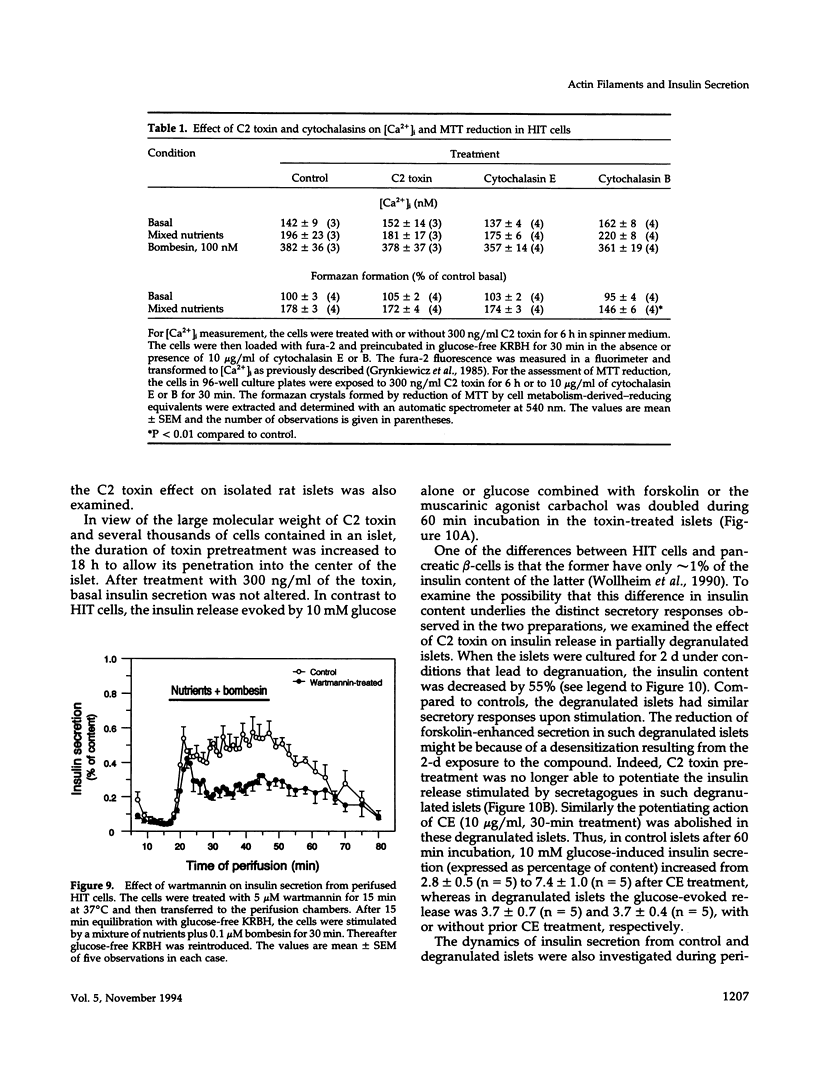
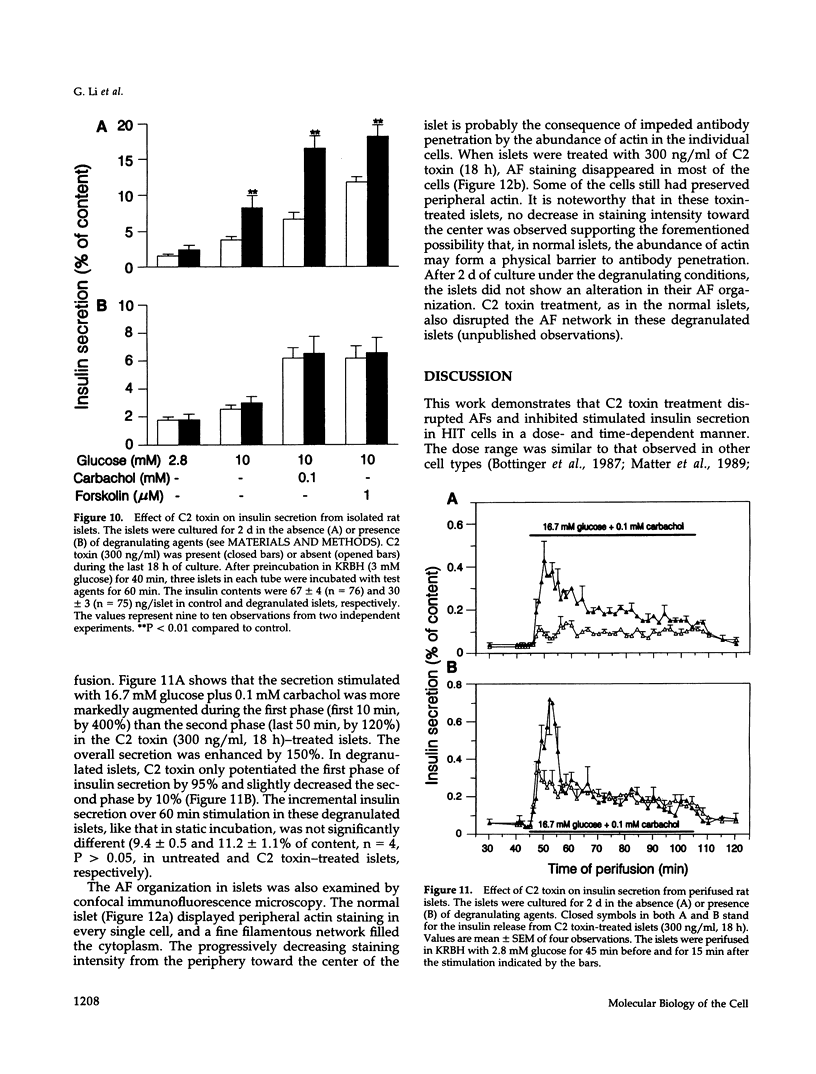




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Aktories K., Wegner A. ADP-ribosylation of actin by clostridial toxins. J Cell Biol. 1989 Oct;109(4 Pt 1):1385–1387. doi: 10.1083/jcb.109.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K., Wille M., Just I. Clostridial actin-ADP-ribosylating toxins. Curr Top Microbiol Immunol. 1992;175:97–113. doi: 10.1007/978-3-642-76966-5_5. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Stubbs M. The glucose sensor in HIT cells is the glucose transporter. FEBS Lett. 1987 Jul 27;219(2):311–315. doi: 10.1016/0014-5793(87)80242-9. [DOI] [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré M. H., Kreis T. E., Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J Cell Biol. 1987 Sep;105(3):1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttinger H., Reuner K. H., Aktories K. Inhibition of histamine release from rat mast cells by botulinum C2 toxin. Int Arch Allergy Appl Immunol. 1987;84(4):380–384. doi: 10.1159/000234453. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Gabbiani G. Gelsolin modulation in epithelial and stromal cells of mammary carcinoma. Am J Pathol. 1989 Mar;134(3):597–603. [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 1986 Oct 20;207(1):110–114. doi: 10.1016/0014-5793(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Conrad P. A., Giuliano K. A., Fisher G., Collins K., Matsudaira P. T., Taylor D. L. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993 Mar;120(6):1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine R. V., Simpson L. L., Sherwin J. R. Botulinum C2 toxin and steroid production in adrenal Y-1 cells: the role of microfilaments in the toxin-induced increase in steroid release. J Pharmacol Exp Ther. 1992 Feb;260(2):859–864. [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein S. K., Baines I. C., Wiegand G., Korn E. D., Pollard T. D. Inhibition of contractile vacuole function in vivo by antibodies against myosin-I. Nature. 1993 Oct 28;365(6449):841–843. doi: 10.1038/365841a0. [DOI] [PubMed] [Google Scholar]
- Fath K. R., Burgess D. R. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J Cell Biol. 1993 Jan;120(1):117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Gabbiani G., Malaisse-Lagae F., Blondel B., Orci L. Actin in pancreatic islet cells. Endocrinology. 1974 Dec;95(6):1630–1635. doi: 10.1210/endo-95-6-1630. [DOI] [PubMed] [Google Scholar]
- Goddette D. W., Frieden C. Actin polymerization. The mechanism of action of cytochalasin D. J Biol Chem. 1986 Dec 5;261(34):15974–15980. [PubMed] [Google Scholar]
- Grimminger F., Sibelius U., Aktories K., Just I., Seeger W. Suppression of cytoskeletal rearrangement in activated human neutrophils by botulinum C2 toxin. Impact on cellular signal transduction. J Biol Chem. 1991 Oct 15;266(29):19276–19282. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Howell S. L. The mechanism of insulin secretion. Diabetologia. 1984 May;26(5):319–327. doi: 10.1007/BF00266030. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Tyhurst M. Regulation of actin polymerizaton in rat islets of Langerhans. Biochem J. 1980 Oct 15;192(1):381–383. doi: 10.1042/bj1920381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Tyhurst M. The cytoskeleton and insulin secretion. Diabetes Metab Rev. 1986;2(1-2):107–123. doi: 10.1002/dmr.5610020107. [DOI] [PubMed] [Google Scholar]
- Janjic D., Wollheim C. B. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992 May;35(5):482–485. doi: 10.1007/BF02342448. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Rabinovitch A., Blackard W. G., Renold A. E. Perifusion of pancreas fragments. A system for the study of dynamic aspects of insulin secretion. Diabetes. 1974 Jun;23(6):550–559. doi: 10.2337/diab.23.6.550. [DOI] [PubMed] [Google Scholar]
- Kitani S., Teshima R., Morita Y., Ito K., Matsuda Y., Nonomura Y. Inhibition of IgE-mediated histamine release by myosin light chain kinase inhibitors. Biochem Biophys Res Commun. 1992 Feb 28;183(1):48–54. doi: 10.1016/0006-291x(92)91607-r. [DOI] [PubMed] [Google Scholar]
- Koffer A., Tatham P. E., Gomperts B. D. Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J Cell Biol. 1990 Sep;111(3):919–927. doi: 10.1083/jcb.111.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Klein N. J., Fink C. J. Effect of cytochalasin B on the biphasic release of insulin in perifused rat islets. Endocrinology. 1973 May;92(5):1458–1468. doi: 10.1210/endo-92-5-1458. [DOI] [PubMed] [Google Scholar]
- Li G. D., Regazzi R., Ullrich S., Pralong W. F., Wollheim C. B. Potentiation of stimulus-induced insulin secretion in protein kinase C-deficient RINm5F cells. Biochem J. 1990 Dec 15;272(3):637–645. doi: 10.1042/bj2720637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hidaka H., Wollheim C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol Pharmacol. 1992 Sep;42(3):489–488. [PubMed] [Google Scholar]
- Li G., Regazzi R., Balch W. E., Wollheim C. B. Stimulation of insulin release from permeabilized HIT-T15 cells by a synthetic peptide corresponding to the effector domain of the small GTP-binding protein rab3. FEBS Lett. 1993 Jul 26;327(2):145–149. doi: 10.1016/0014-5793(93)80159-r. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Kowluru A. Calcium-calmodulin-dependent myosin phosphorylation by pancreatic islets. Diabetes. 1982 Jun;31(6 Pt 1):566–570. doi: 10.2337/diab.31.6.566. [DOI] [PubMed] [Google Scholar]
- Matter K., Dreyer F., Aktories K. Actin involvement in exocytosis from PC12 cells: studies on the influence of botulinum C2 toxin on stimulated noradrenaline release. J Neurochem. 1989 Feb;52(2):370–376. doi: 10.1111/j.1471-4159.1989.tb09131.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Kakita S., Takahashi I., Kawahara K., Tsukuda E., Sano T., Yamada K., Yoshida M., Kase H., Matsuda Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992 Feb 5;267(4):2157–2163. [PubMed] [Google Scholar]
- Nelson T. Y., Boyd A. E., 3rd Gelsolin, a Ca2+-dependent actin-binding protein in a hamster insulin-secreting cell line. J Clin Invest. 1985 Mar;75(3):1015–1022. doi: 10.1172/JCI111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki I., Okazaki K., Saitoh M., Niki A., Niki H., Tamagawa T., Iguchi A., Hidaka H. Presence and possible involvement of Ca/calmodulin-dependent protein kinases in insulin release from the rat pancreatic beta cell. Biochem Biophys Res Commun. 1993 Feb 26;191(1):255–261. doi: 10.1006/bbrc.1993.1210. [DOI] [PubMed] [Google Scholar]
- Norgauer J., Kownatzki E., Seifert R., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and enhances O2- production and secretion but inhibits migration of activated human neutrophils. J Clin Invest. 1988 Oct;82(4):1376–1382. doi: 10.1172/JCI113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M., Sakurai T., Nakamura S., Nakanishi S., Matsuda Y., Muramatsu S., Nonomura Y., Kumakura K. Inhibition of Ca(2+)-dependent catecholamine release by myosin light chain kinase inhibitor, wortmannin, in adrenal chromaffin cells. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1016–1021. doi: 10.1016/0006-291x(92)91728-9. [DOI] [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Perrin D., Möller K., Hanke K., Söling H. D. cAMP and Ca(2+)-mediated secretion in parotid acinar cells is associated with reversible changes in the organization of the cytoskeleton. J Cell Biol. 1992 Jan;116(1):127–134. doi: 10.1083/jcb.116.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Kipnis D. M. Microtubule assembly and the intracellular transport of secretory granules in pancreatic islets. Science. 1976 Jan 9;191(4222):88–90. doi: 10.1126/science.1108194. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Doberstein S. K., Zot H. G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Reuner K. H., Presek P., Boschek C. B., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur J Cell Biol. 1987 Feb;43(1):134–140. [PubMed] [Google Scholar]
- Rungger-Brändle E., Messerli J. M., Niemeyer G., Eppenberger H. M. Confocal microscopy and computer-assisted image reconstruction of astrocytes in the mammalian retina. Eur J Neurosci. 1993 Aug 1;5(8):1093–1106. doi: 10.1111/j.1460-9568.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Schelling J. R., Hanson A. S., Marzec R., Linas S. L. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992 Dec;90(6):2472–2480. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. I. The binary toxin produced by Clostridium botulinum enters cells by receptor-mediated endocytosis to exert its pharmacologic effects. J Pharmacol Exp Ther. 1989 Dec;251(3):1223–1228. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd Increased filamentous actin in islets of Langerhans from fasted hamsters. Biochem Biophys Res Commun. 1982 Jan 15;104(1):207–211. doi: 10.1016/0006-291x(82)91960-x. [DOI] [PubMed] [Google Scholar]
- Sontag J. M., Aunis D., Bader M. F. Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur J Cell Biol. 1988 Jun;46(2):316–326. [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Stutchfield J., Howell S. L. The effect of phalloidin on insulin secretion from islets of Langerhans isolated from rat pancreas. FEBS Lett. 1984 Oct 1;175(2):393–396. doi: 10.1016/0014-5793(84)80775-9. [DOI] [PubMed] [Google Scholar]
- Sutton R., Peters M., McShane P., Gray D. W., Morris P. J. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986 Dec;42(6):689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Polley M., Seybold J., Schnittler H., Seeger W., Grimminger F., Aktories K. Adenosine diphosphate-ribosylation of G-actin by botulinum C2 toxin increases endothelial permeability in vitro. J Clin Invest. 1991 May;87(5):1575–1584. doi: 10.1172/JCI115171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanston-Flatt S. K., Carlsson L., Gylfe E. Actin filament formation in pancreatic beta-cells during glucose stimulation of insulin secretion. FEBS Lett. 1980 Aug 11;117(1):299–302. doi: 10.1016/0014-5793(80)80966-5. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Vandekerckhove J., Schering B., Bärmann M., Aktories K. Botulinum C2 toxin ADP-ribosylates cytoplasmic beta/gamma-actin in arginine 177. J Biol Chem. 1988 Jan 15;263(2):696–700. [PubMed] [Google Scholar]
- Vaziri C., Downes C. P. Association of a receptor and G-protein-regulated phospholipase C with the cytoskeleton. J Biol Chem. 1992 Nov 15;267(32):22973–22981. [PubMed] [Google Scholar]
- Vitale M. L., Rodríguez Del Castillo A., Tchakarov L., Trifaró J. M. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol. 1991 Jun;113(5):1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Easom R. A., Hughes J. H., McDaniel M. L. Evidence for a role of microfilaments in insulin release from purified beta-cells. Biochem Biophys Res Commun. 1990 Aug 31;171(1):424–430. doi: 10.1016/0006-291x(90)91410-t. [DOI] [PubMed] [Google Scholar]
- Weeds A., Maciver S. F-actin capping proteins. Curr Opin Cell Biol. 1993 Feb;5(1):63–69. doi: 10.1016/s0955-0674(05)80009-2. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Meda P., Halban P. A. Establishment and culture of insulin-secreting beta cell lines. Methods Enzymol. 1990;192:223–235. doi: 10.1016/0076-6879(90)92072-l. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]