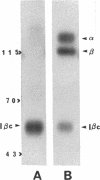
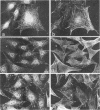
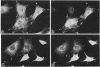
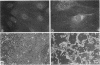
Abstract
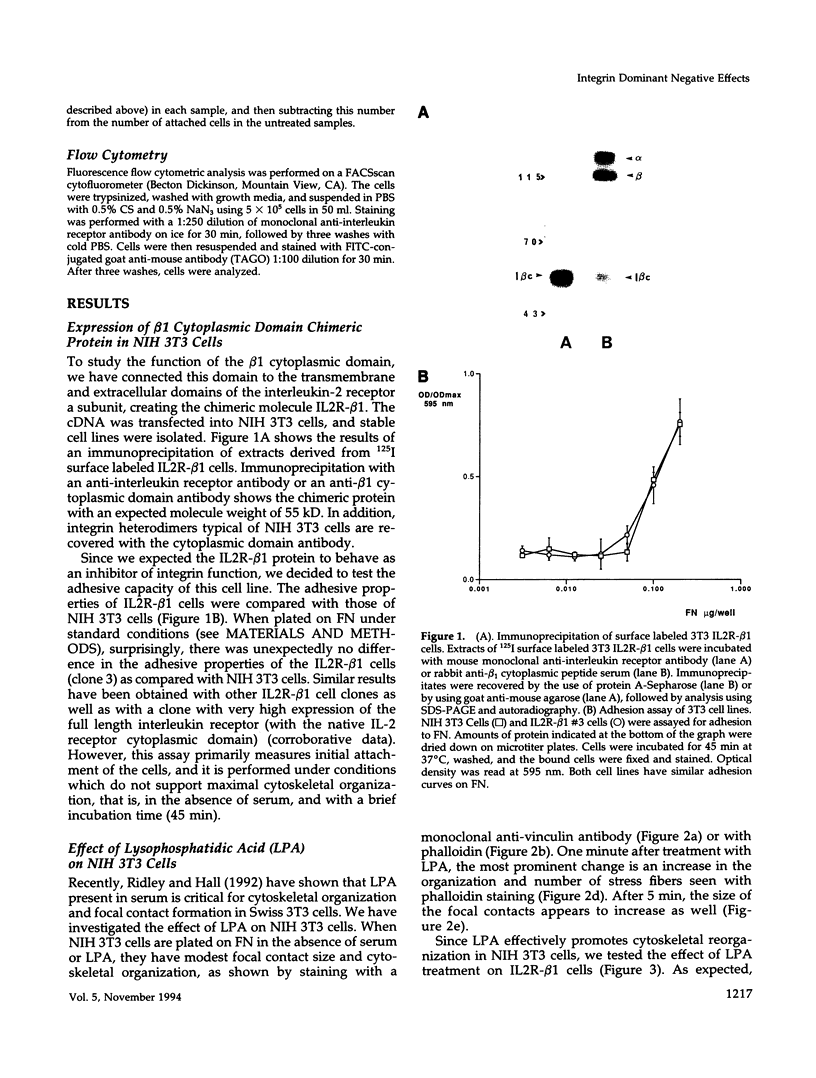
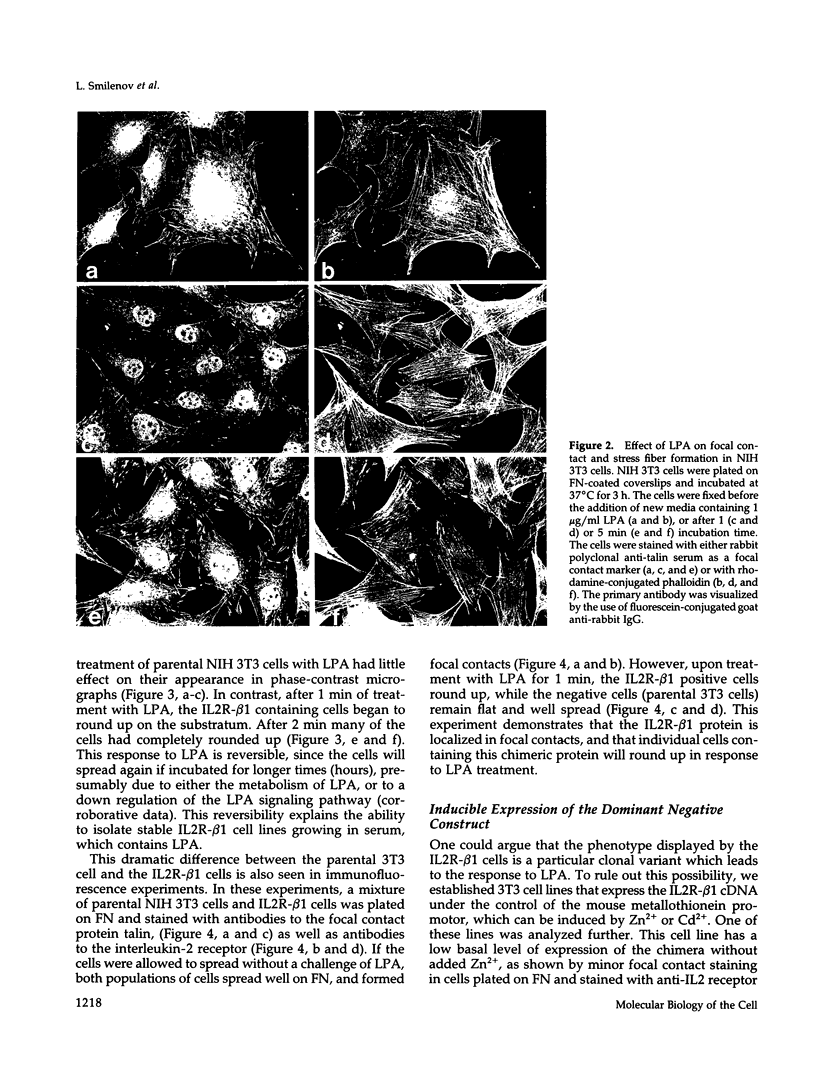
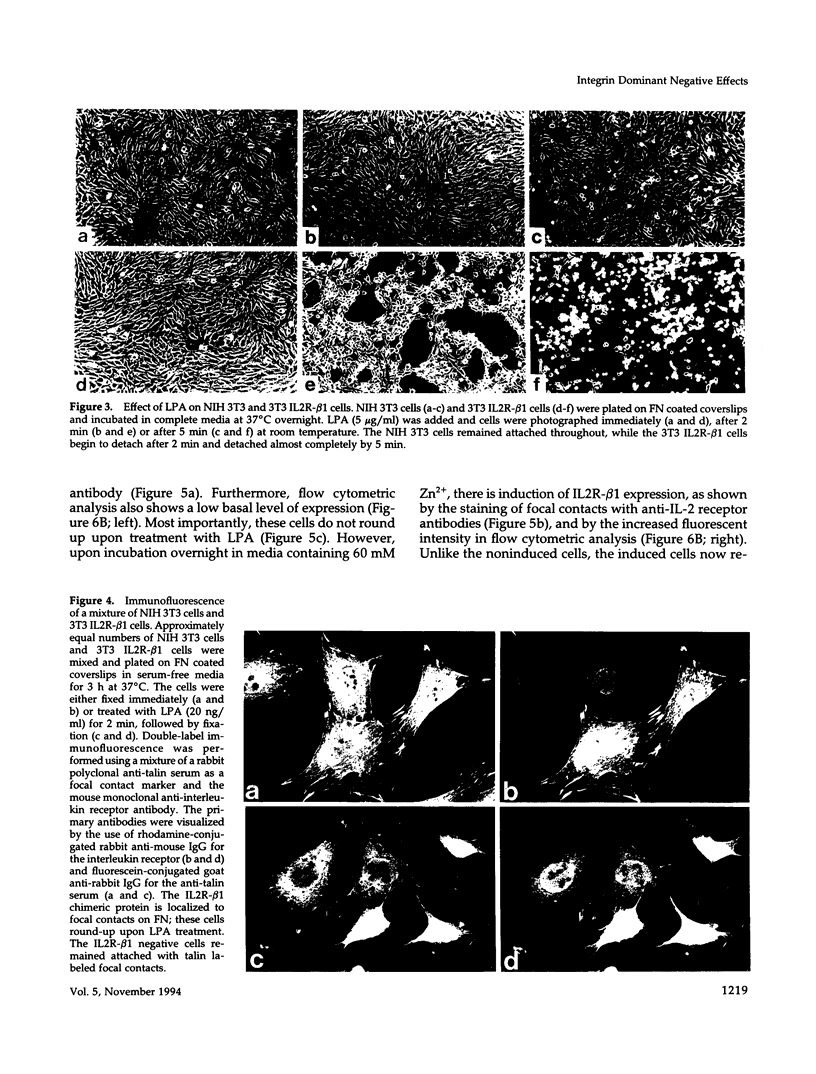
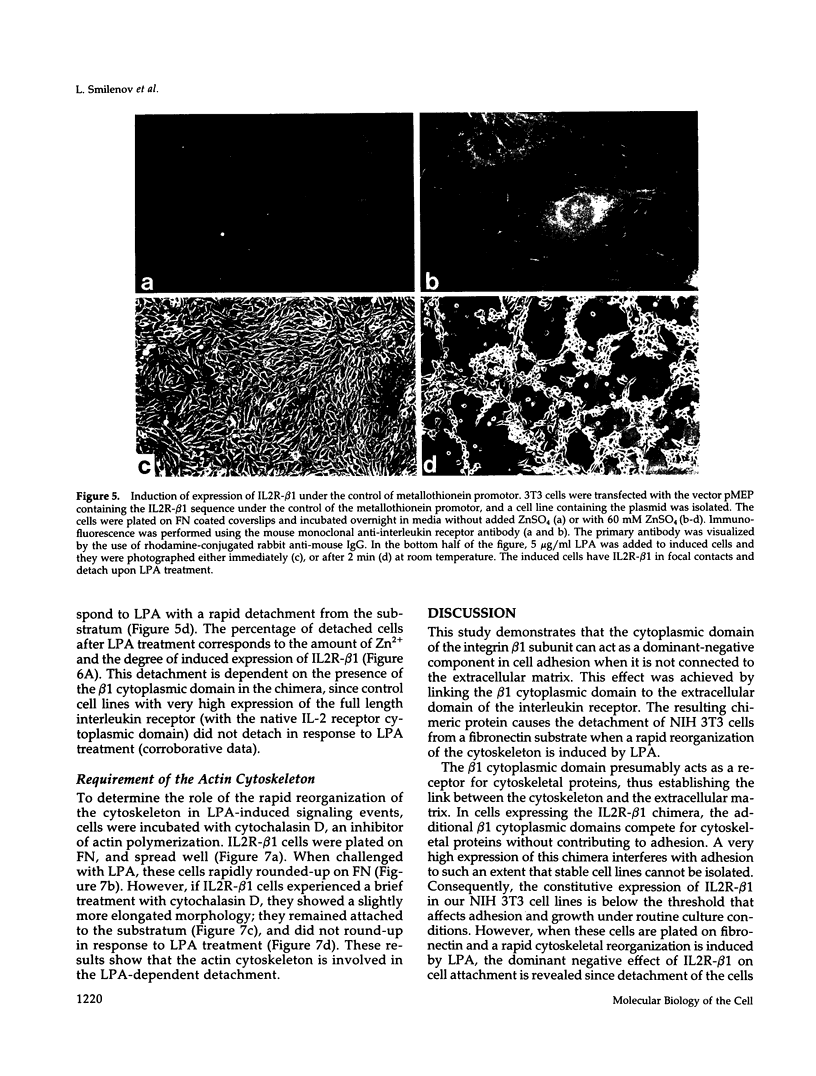
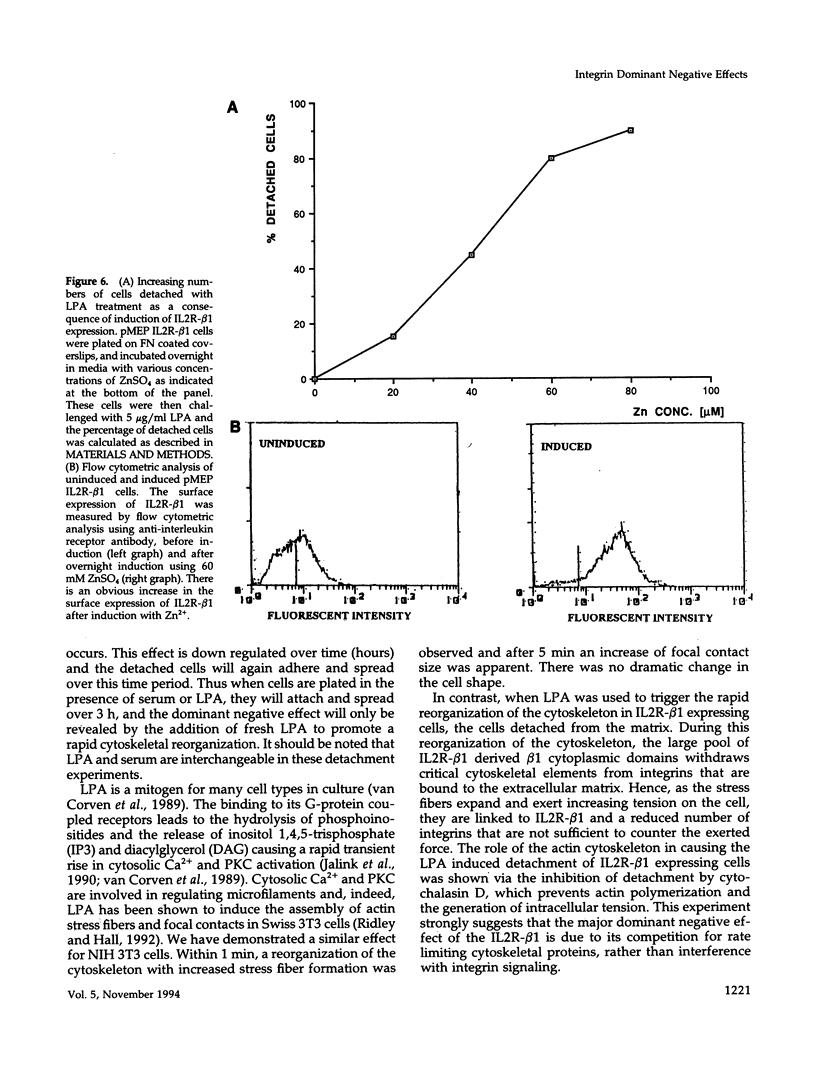
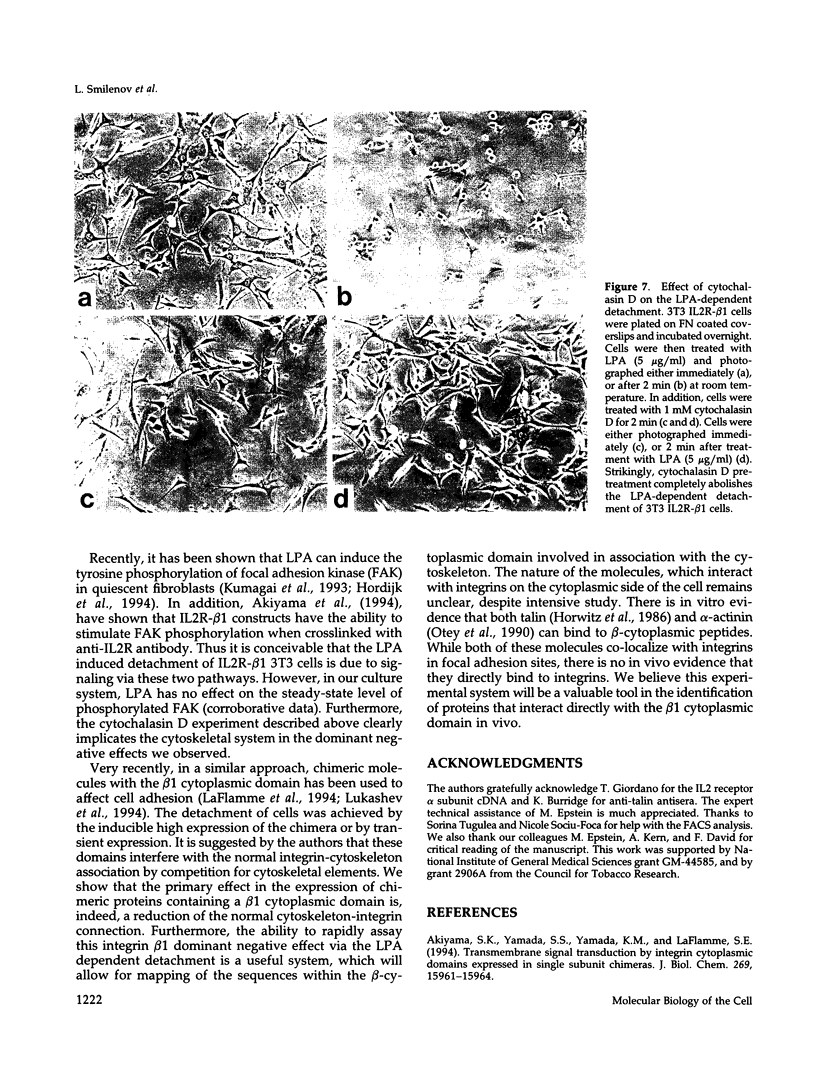
Integrin receptors localize to focal contact sites and interact with the cytoskeleton via the beta 1 cytoplasmic domain. To study the role of this domain in adhesion, we have expressed in NIH 3T3 cells a cDNA consisting of the interleukin 2 receptor alpha subunit extracellular and transmembrane domains, connected to the integrin beta 1 cytoplasmic domain (IL2R-beta 1). Since the extracellular domain of the chimeric protein has no role in adhesion, this protein could uncouple adhesion from intracellular events. As expected, in a cell line expressing IL2R-beta 1, this chimera was directed to focal contact sites. Unexpectedly, the cells exhibited normal adhesion to fibronectin (FN). However, when a rapid reorganization of the cytoskeleton was induced using lysophosphatidic acid (LPA), IL2R-beta 1 cells detached from FN in contrast to wild-type cells. The detachment in response to LPA could be prevented with cytochalasin D, an inhibitor of actin polymerization. These results imply that a beta 1 cytoplasmic domain, which is uncoupled from adhesion, can compete with the cytoplasmic domain of native integrin beta 1 for cytoskeletal proteins. As a consequence, the IL2R-beta 1 protein acts as a dominant negative effector of adhesion by disrupting the integrin-cytoskeleton connection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M., LaFlamme S. E. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1994 Jun 10;269(23):15961–15964. [PubMed] [Google Scholar]
- Aumailley M., Mann K., von der Mark H., Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989 Apr;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- Briesewitz R., Kern A., Marcantonio E. E. Ligand-dependent and -independent integrin focal contact localization: the role of the alpha chain cytoplasmic domain. Mol Biol Cell. 1993 Jun;4(6):593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Hasegawa E., Hasegawa T., Weinstock C., Yamada K. M. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985 Apr;100(4):1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Salomon D., Takeichi M., Hynes R. O. A chimeric N-cadherin/beta 1-integrin receptor which localizes to both cell-cell and cell-matrix adhesions. J Cell Sci. 1992 Dec;103(Pt 4):943–951. doi: 10.1242/jcs.103.4.943. [DOI] [PubMed] [Google Scholar]
- Giordano T., Howard T. H., Coleman J., Sakamoto K., Howard B. H. Isolation of a population of transiently transfected quiescent and senescent cells by magnetic affinity cell sorting. Exp Cell Res. 1991 Jan;192(1):193–197. doi: 10.1016/0014-4827(91)90175-t. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Haimovich B., Reszka A., Boettiger D., Horwitz A. Expression and function of chicken integrin beta 1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990 Jan;110(1):175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P. L., Verlaan I., van Corven E. J., Moolenaar W. H. Protein tyrosine phosphorylation induced by lysophosphatidic acid in Rat-1 fibroblasts. Evidence that phosphorylation of map kinase is mediated by the Gi-p21ras pathway. J Biol Chem. 1994 Jan 7;269(1):645–651. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Yoshimasa T., Nakao K., Narumiya S. Lysophosphatidic acid induces tyrosine phosphorylation and activation of MAP-kinase and focal adhesion kinase in cultured Swiss 3T3 cells. FEBS Lett. 1993 Aug 30;329(3):273–276. doi: 10.1016/0014-5793(93)80236-n. [DOI] [PubMed] [Google Scholar]
- LaFlamme S. E., Akiyama S. K., Yamada K. M. Regulation of fibronectin receptor distribution. J Cell Biol. 1992 Apr;117(2):437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme S. E., Thomas L. A., Yamada S. S., Yamada K. M. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994 Sep;126(5):1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukashev M. E., Sheppard D., Pytela R. Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed beta 1 integrin cytoplasmic domain. J Biol Chem. 1994 Jul 15;269(28):18311–18314. [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S., Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem. 1994 Jan 7;269(1):704–710. [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M., Gailit J., Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988 Jun;106(6):2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J., Zachary I., Valverde A. M., Rozengurt E. Bombesin stimulation of p125 focal adhesion kinase tyrosine phosphorylation. Role of protein kinase C, Ca2+ mobilization, and the actin cytoskeleton. J Biol Chem. 1993 Jul 5;268(19):14261–14268. [PubMed] [Google Scholar]
- Solowska J., Guan J. L., Marcantonio E. E., Trevithick J. E., Buck C. A., Hynes R. O. Expression of normal and mutant avian integrin subunits in rodent cells. J Cell Biol. 1989 Aug;109(2):853–861. doi: 10.1083/jcb.109.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Ylänne J., Chen Y., O'Toole T. E., Loftus J. C., Takada Y., Ginsberg M. H. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993 Jul;122(1):223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]