Abstract
The structure of the title compound, (C12H24N)2[Sn2(C6H5)4(C2O4)3(H2O)2], consists of a bischelating oxalate ion, located on an inversion center, which is linked to two SnPh2 groups. The coordination sphere of the Sn(IV) ion is completed by a monochelating oxalate anion and a water molecule. The Sn(IV) atoms are thus seven-coordinated. The discrete binuclear units are further connected by hydrogen bonds, leading to a supramolecular crystal structure. The asymmetric unit contains one half dianion and one (Cy2NH2)+ cation.
Related literature
For background to organotin(IV) chemistry, see: Ballmann et al. (2009 ▶); Diallo et al. (2007 ▶); Diassé-Sarr et al. (1997 ▶); Ng et al. (1992 ▶); Singh et al. (2008 ▶); de Sousa et al. (2007 ▶); Wang et al. (2009 ▶); Xanthopoulou et al. (2007 ▶, 2008 ▶); Zia-ur-Rahman et al. (2007 ▶). For related Sn(IV) structures, see: Diop et al. (2002 ▶, 2003 ▶).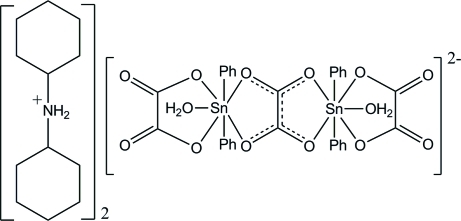
Experimental
Crystal data
(C12H24N)2[Sn2(C6H5)4(C2O4)3(H2O)2]
M r = 1210.52
Monoclinic,

a = 13.1725 (4) Å
b = 14.6121 (4) Å
c = 14.1139 (4) Å
β = 100.869 (2)°
V = 2667.88 (13) Å3
Z = 2
Mo Kα radiation
μ = 1.00 mm−1
T = 150 K
0.2 × 0.2 × 0.2 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1995 ▶) T min = 0.825, T max = 0.825
48411 measured reflections
6114 independent reflections
4069 reflections with I > 2σ(I)
R int = 0.126
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.082
S = 1.01
6114 reflections
341 parameters
2 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.54 e Å−3
Δρmin = −0.72 e Å−3
Data collection: COLLECT (Nonius, 2000 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶) and SCALEPACK; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810046738/bh2310sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810046738/bh2310Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O7—H7B⋯O4i | 0.90 (4) | 1.77 (4) | 2.663 (3) | 175 (4) |
| N—H1A⋯O3ii | 0.84 (4) | 2.12 (3) | 2.910 (4) | 155 (3) |
| N—H1A⋯O4ii | 0.84 (4) | 2.37 (4) | 2.986 (4) | 130 (3) |
| N—H1B⋯O6iii | 0.91 (4) | 2.08 (4) | 2.960 (4) | 164 (4) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
supplementary crystallographic information
Comment
In the dynamic of our research work on organotin(IV) chemistry (Diallo et al., 2007; Diassé-Sarr et al., 1997) because of several applications found (Xanthopoulou et al., 2007, 2008; Zia-ur-Rahman et al., 2007; Singh et al., 2008; Wang et al., 2009; Ballmann et al., 2009; de Sousa et al., 2007) and our interest in the coordinating behaviour of oxyanions in this family of compounds, we had yet reported the crystal structures of C2O4(SnPh3)2 (Diop et al., 2003) and SO4(SnPh3)2.H2O (Diop et al., 2002) and have initiated here the study of the interactions between (Cy2NH2)2C2O4.2H2O and C2O4(SnPh3)2 which has yielded the studied compound.
The asymmetric unit consists of one half of the molecule, located about an inversion centre at the mid-point of the C3—C3i bond (symmetry code i: -x, 1 - y, -z). In its units structure two SnPh2 residues are linked by a central bichelating oxalate ion [O5O6:O5O6] and every SnPh2 residue is then linked to another monochelating anion [O1, O2]. A water molecule completes the tin centre coordination to seven, which can be described as a distorted trans-C2SnO5 pentagonal bipyramidal geometry [C—Sn—C angle: 168.75 (13)°]. Within the bridging carboxylate all the C—O bonds are equal within experimental error, implying complete delocalization of double-bond character within this residue. The bond lengths C1—O1 and C2—O2, [1.273 (4) Å], and C2—O3, C1—O4 [1.225 (4) and 1.247 (4) Å] indicate respectively a single and double bond character; the bond length C1—O4 results from involvement of O4 in two distinct hydrogen bonds. Among the Sn—O bonds, Sn1—O6 is notably longer, O6 being the only oxygen of this kind involved in hydrogen bonding.
Every moiety is then connected to its neighbour by three types of hydrogen bonds: one O—H···O type involving an H atom of the water molecule and one O atom of monochelating oxalate [O7—H7B···O4], one N—H···O contact involving one O atom of the bichelating oxalate anion and the cation [N—H1B···O6] and a third bifurcated one also involving the cation [N—H1A···O3 and N—H1A···O4], giving a supramolecular crystal structure.
A similar structure, bearing butyl groups in place of phenyl, was previously reported (Ng et al., 1992).
Experimental
Crystals of the title compound were obtained by allowing (Cy2NH2)2C2O4.2H2O (90 mmol in 15 ml e thanol) to react with C2O4(SnPh3)2 (45 mmol in 15 ml e thanol). The mixture was stirred during several hours and slow solvent evaporation afforded crystals suitable for X-rays studies.
Refinement
All C–bonded H atoms were placed in idealized positions (C—H in the range 0.95 to 1.00 Å), while H atoms bonded to N and O atoms were considered as free atoms. Isotropic displacement parameters for H atoms were calculated from Ueq of their parent atoms.
Figures
Fig. 1.
A part of the crystal structure of the title compound, with the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level. Dashed bonds represent hydrogen bonds.
Crystal data
| (C12H24N)2[Sn2(C6H5)4(C2O4)3(H2O)2] | F(000) = 1244 |
| Mr = 1210.52 | Dx = 1.507 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 29450 reflections |
| a = 13.1725 (4) Å | θ = 2.9–27.5° |
| b = 14.6121 (4) Å | µ = 1.00 mm−1 |
| c = 14.1139 (4) Å | T = 150 K |
| β = 100.869 (2)° | Irregular, colourless |
| V = 2667.88 (13) Å3 | 0.2 × 0.2 × 0.2 mm |
| Z = 2 |
Data collection
| Nonius KappaCCD diffractometer | 6114 independent reflections |
| Radiation source: fine-focus sealed tube | 4069 reflections with I > 2σ(I) |
| graphite | Rint = 0.126 |
| 293 2.0 degree images with φ and ω scans | θmax = 27.5°, θmin = 4.1° |
| Absorption correction: multi-scan (SORTAV;Blessing, 1995) | h = −17→17 |
| Tmin = 0.825, Tmax = 0.825 | k = −18→18 |
| 48411 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.082 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0335P)2 + 0.6086P] where P = (Fo2 + 2Fc2)/3 |
| 6114 reflections | (Δ/σ)max = 0.001 |
| 341 parameters | Δρmax = 0.54 e Å−3 |
| 2 restraints | Δρmin = −0.72 e Å−3 |
| 0 constraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Sn | 0.208644 (18) | 0.515508 (15) | 0.133115 (17) | 0.02034 (8) | |
| O1 | 0.27245 (18) | 0.37817 (14) | 0.16466 (16) | 0.0235 (5) | |
| O2 | 0.35282 (17) | 0.53257 (14) | 0.24319 (16) | 0.0242 (5) | |
| O3 | 0.47106 (18) | 0.45574 (15) | 0.34724 (17) | 0.0285 (6) | |
| O4 | 0.38762 (18) | 0.29623 (15) | 0.26721 (17) | 0.0275 (6) | |
| O5 | 0.09563 (17) | 0.41884 (14) | 0.04184 (16) | 0.0230 (5) | |
| O6 | 0.06436 (17) | 0.60103 (14) | 0.04207 (15) | 0.0210 (5) | |
| O7 | 0.2413 (2) | 0.67274 (16) | 0.18266 (19) | 0.0292 (6) | |
| H7A | 0.292 (2) | 0.669 (3) | 0.233 (2) | 0.052 (14)* | |
| H7B | 0.197 (3) | 0.712 (3) | 0.202 (3) | 0.074 (16)* | |
| N | −0.0015 (2) | 0.2042 (2) | −0.0277 (2) | 0.0214 (6) | |
| H1A | −0.019 (3) | 0.170 (2) | −0.076 (3) | 0.027 (10)* | |
| H1B | −0.027 (3) | 0.261 (3) | −0.044 (3) | 0.038 (11)* | |
| C1 | 0.3500 (3) | 0.3702 (2) | 0.2332 (2) | 0.0216 (8) | |
| C2 | 0.3979 (3) | 0.4602 (2) | 0.2795 (2) | 0.0211 (7) | |
| C3 | 0.0088 (3) | 0.4480 (2) | −0.0001 (2) | 0.0196 (7) | |
| C4 | 0.1110 (3) | 0.5052 (2) | 0.2372 (2) | 0.0225 (7) | |
| C5 | 0.0459 (3) | 0.5767 (2) | 0.2523 (3) | 0.0277 (8) | |
| H5 | 0.0467 | 0.6321 | 0.2172 | 0.033* | |
| C6 | −0.0202 (3) | 0.5686 (3) | 0.3177 (3) | 0.0331 (9) | |
| H6 | −0.0632 | 0.6185 | 0.3277 | 0.040* | |
| C7 | −0.0237 (3) | 0.4886 (3) | 0.3681 (3) | 0.0356 (9) | |
| H7 | −0.0690 | 0.4828 | 0.4127 | 0.043* | |
| C8 | 0.0399 (3) | 0.4162 (3) | 0.3531 (3) | 0.0359 (9) | |
| H8 | 0.0380 | 0.3607 | 0.3880 | 0.043* | |
| C9 | 0.1059 (3) | 0.4239 (2) | 0.2882 (3) | 0.0295 (8) | |
| H9 | 0.1482 | 0.3735 | 0.2781 | 0.035* | |
| C10 | 0.2855 (3) | 0.5464 (2) | 0.0178 (3) | 0.0276 (8) | |
| C11 | 0.3816 (3) | 0.5881 (3) | 0.0352 (3) | 0.0453 (11) | |
| H11 | 0.4136 | 0.6027 | 0.0995 | 0.054* | |
| C12 | 0.4320 (4) | 0.6091 (4) | −0.0408 (4) | 0.0664 (15) | |
| H12 | 0.4979 | 0.6378 | −0.0276 | 0.080* | |
| C13 | 0.3872 (4) | 0.5888 (3) | −0.1337 (3) | 0.0533 (13) | |
| H13 | 0.4220 | 0.6032 | −0.1850 | 0.064* | |
| C14 | 0.2922 (4) | 0.5477 (3) | −0.1528 (3) | 0.0485 (11) | |
| H14 | 0.2609 | 0.5336 | −0.2174 | 0.058* | |
| C15 | 0.2416 (3) | 0.5266 (3) | −0.0778 (3) | 0.0377 (9) | |
| H15 | 0.1756 | 0.4981 | −0.0919 | 0.045* | |
| C16 | 0.1143 (2) | 0.2108 (2) | −0.0052 (2) | 0.0218 (7) | |
| H16 | 0.1344 | 0.2502 | 0.0533 | 0.026* | |
| C17 | 0.1521 (3) | 0.2565 (2) | −0.0890 (2) | 0.0256 (8) | |
| H17A | 0.1338 | 0.2183 | −0.1476 | 0.031* | |
| H17B | 0.1180 | 0.3168 | −0.1024 | 0.031* | |
| C18 | 0.2684 (3) | 0.2693 (2) | −0.0643 (3) | 0.0308 (9) | |
| H18A | 0.2863 | 0.3099 | −0.0075 | 0.037* | |
| H18B | 0.2927 | 0.2986 | −0.1192 | 0.037* | |
| C19 | 0.3215 (3) | 0.1774 (3) | −0.0425 (3) | 0.0355 (9) | |
| H19A | 0.3969 | 0.1869 | −0.0231 | 0.043* | |
| H19B | 0.3092 | 0.1392 | −0.1015 | 0.043* | |
| C20 | 0.2811 (3) | 0.1276 (3) | 0.0380 (3) | 0.0327 (9) | |
| H20A | 0.3134 | 0.0663 | 0.0471 | 0.039* | |
| H20B | 0.3018 | 0.1621 | 0.0990 | 0.039* | |
| C21 | 0.1647 (3) | 0.1171 (2) | 0.0165 (3) | 0.0270 (8) | |
| H21A | 0.1413 | 0.0894 | 0.0727 | 0.032* | |
| H21B | 0.1439 | 0.0761 | −0.0396 | 0.032* | |
| C22 | −0.0561 (3) | 0.1646 (2) | 0.0476 (2) | 0.0234 (8) | |
| H22 | −0.0323 | 0.1001 | 0.0613 | 0.028* | |
| C23 | −0.1710 (3) | 0.1642 (3) | 0.0064 (3) | 0.0332 (9) | |
| H23A | −0.1847 | 0.1254 | −0.0521 | 0.040* | |
| H23B | −0.1942 | 0.2272 | −0.0123 | 0.040* | |
| C24 | −0.2322 (3) | 0.1273 (3) | 0.0812 (3) | 0.0368 (10) | |
| H24A | −0.3071 | 0.1303 | 0.0542 | 0.044* | |
| H24B | −0.2137 | 0.0623 | 0.0953 | 0.044* | |
| C25 | −0.2087 (3) | 0.1823 (3) | 0.1736 (3) | 0.0371 (9) | |
| H25A | −0.2462 | 0.1557 | 0.2216 | 0.045* | |
| H25B | −0.2330 | 0.2461 | 0.1607 | 0.045* | |
| C26 | −0.0935 (3) | 0.1823 (3) | 0.2141 (3) | 0.0402 (10) | |
| H26A | −0.0706 | 0.1190 | 0.2321 | 0.048* | |
| H26B | −0.0795 | 0.2203 | 0.2732 | 0.048* | |
| C27 | −0.0316 (3) | 0.2196 (3) | 0.1407 (2) | 0.0324 (9) | |
| H27A | −0.0494 | 0.2848 | 0.1271 | 0.039* | |
| H27B | 0.0433 | 0.2158 | 0.1678 | 0.039* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sn | 0.01965 (13) | 0.01890 (12) | 0.02124 (13) | 0.00066 (11) | 0.00070 (8) | 0.00046 (11) |
| O1 | 0.0233 (14) | 0.0203 (12) | 0.0240 (13) | 0.0026 (10) | −0.0033 (11) | −0.0020 (10) |
| O2 | 0.0238 (13) | 0.0161 (13) | 0.0292 (13) | 0.0016 (9) | −0.0043 (10) | 0.0021 (10) |
| O3 | 0.0284 (14) | 0.0251 (13) | 0.0266 (14) | −0.0012 (10) | −0.0089 (11) | 0.0018 (10) |
| O4 | 0.0286 (14) | 0.0193 (13) | 0.0314 (14) | 0.0034 (10) | −0.0025 (11) | 0.0037 (10) |
| O5 | 0.0225 (13) | 0.0177 (12) | 0.0262 (13) | 0.0014 (10) | −0.0020 (11) | −0.0027 (10) |
| O6 | 0.0217 (13) | 0.0192 (12) | 0.0201 (12) | 0.0006 (10) | −0.0009 (10) | −0.0002 (10) |
| O7 | 0.0260 (16) | 0.0237 (14) | 0.0353 (16) | 0.0013 (11) | −0.0007 (13) | −0.0018 (12) |
| N | 0.0246 (17) | 0.0186 (17) | 0.0208 (17) | −0.0001 (12) | 0.0042 (14) | −0.0004 (14) |
| C1 | 0.021 (2) | 0.0227 (19) | 0.0225 (19) | 0.0008 (14) | 0.0082 (16) | −0.0007 (15) |
| C2 | 0.0206 (19) | 0.0233 (19) | 0.0203 (18) | −0.0011 (14) | 0.0060 (15) | −0.0010 (14) |
| C3 | 0.023 (2) | 0.0193 (17) | 0.0166 (16) | 0.0008 (14) | 0.0031 (14) | −0.0021 (13) |
| C4 | 0.0218 (17) | 0.027 (2) | 0.0175 (17) | −0.0008 (14) | −0.0009 (13) | −0.0022 (14) |
| C5 | 0.024 (2) | 0.029 (2) | 0.028 (2) | 0.0001 (15) | −0.0010 (16) | 0.0000 (15) |
| C6 | 0.021 (2) | 0.045 (2) | 0.033 (2) | −0.0008 (17) | 0.0036 (17) | −0.0033 (18) |
| C7 | 0.029 (2) | 0.048 (2) | 0.032 (2) | −0.0127 (18) | 0.0105 (17) | −0.0046 (19) |
| C8 | 0.036 (2) | 0.038 (2) | 0.034 (2) | −0.0119 (18) | 0.0066 (19) | 0.0024 (18) |
| C9 | 0.031 (2) | 0.027 (2) | 0.031 (2) | −0.0032 (16) | 0.0058 (17) | −0.0017 (16) |
| C10 | 0.029 (2) | 0.0265 (19) | 0.029 (2) | 0.0055 (15) | 0.0077 (16) | 0.0054 (15) |
| C11 | 0.030 (2) | 0.070 (3) | 0.036 (2) | −0.007 (2) | 0.0053 (19) | 0.008 (2) |
| C12 | 0.033 (3) | 0.105 (4) | 0.065 (4) | −0.011 (3) | 0.017 (3) | 0.019 (3) |
| C13 | 0.050 (3) | 0.072 (3) | 0.045 (3) | 0.015 (2) | 0.028 (2) | 0.016 (2) |
| C14 | 0.069 (3) | 0.048 (3) | 0.031 (2) | 0.004 (2) | 0.015 (2) | 0.0013 (19) |
| C15 | 0.048 (3) | 0.035 (2) | 0.033 (2) | −0.0038 (18) | 0.0124 (19) | −0.0001 (18) |
| C16 | 0.0198 (19) | 0.0236 (18) | 0.0212 (18) | 0.0027 (14) | 0.0020 (15) | 0.0001 (14) |
| C17 | 0.029 (2) | 0.0263 (19) | 0.0211 (19) | 0.0022 (15) | 0.0042 (16) | 0.0009 (15) |
| C18 | 0.026 (2) | 0.040 (2) | 0.028 (2) | 0.0013 (16) | 0.0100 (17) | 0.0019 (17) |
| C19 | 0.024 (2) | 0.045 (2) | 0.036 (2) | 0.0081 (17) | 0.0031 (17) | −0.0076 (18) |
| C20 | 0.027 (2) | 0.035 (2) | 0.034 (2) | 0.0090 (16) | −0.0025 (17) | −0.0011 (17) |
| C21 | 0.028 (2) | 0.0224 (19) | 0.029 (2) | 0.0033 (15) | 0.0002 (16) | −0.0016 (15) |
| C22 | 0.027 (2) | 0.0194 (18) | 0.0237 (19) | 0.0025 (14) | 0.0054 (16) | 0.0037 (14) |
| C23 | 0.030 (2) | 0.042 (2) | 0.028 (2) | −0.0047 (17) | 0.0056 (17) | −0.0043 (17) |
| C24 | 0.026 (2) | 0.049 (2) | 0.037 (2) | −0.0064 (18) | 0.0085 (18) | −0.0029 (19) |
| C25 | 0.032 (2) | 0.047 (2) | 0.035 (2) | −0.0018 (18) | 0.0132 (18) | −0.0056 (19) |
| C26 | 0.038 (3) | 0.058 (3) | 0.026 (2) | −0.010 (2) | 0.0104 (18) | −0.0021 (19) |
| C27 | 0.030 (2) | 0.043 (2) | 0.023 (2) | −0.0064 (17) | 0.0038 (17) | −0.0043 (17) |
Geometric parameters (Å, °)
| Sn—C10 | 2.121 (3) | C13—C14 | 1.368 (6) |
| Sn—C4 | 2.132 (3) | C13—H13 | 0.9500 |
| Sn—O1 | 2.189 (2) | C14—C15 | 1.388 (5) |
| Sn—O2 | 2.229 (2) | C14—H14 | 0.9500 |
| Sn—O5 | 2.269 (2) | C15—H15 | 0.9500 |
| Sn—O7 | 2.416 (2) | C16—C17 | 1.522 (4) |
| Sn—O6 | 2.430 (2) | C16—C21 | 1.527 (4) |
| O1—C1 | 1.273 (4) | C16—H16 | 1.0000 |
| O2—C2 | 1.273 (4) | C17—C18 | 1.517 (5) |
| O3—C2 | 1.225 (4) | C17—H17A | 0.9900 |
| O4—C1 | 1.247 (4) | C17—H17B | 0.9900 |
| O5—C3 | 1.259 (4) | C18—C19 | 1.518 (5) |
| O6—C3i | 1.256 (4) | C18—H18A | 0.9900 |
| O7—H7A | 0.886 (19) | C18—H18B | 0.9900 |
| O7—H7B | 0.896 (19) | C19—C20 | 1.527 (5) |
| N—C16 | 1.500 (4) | C19—H19A | 0.9900 |
| N—C22 | 1.507 (4) | C19—H19B | 0.9900 |
| N—H1A | 0.85 (4) | C20—C21 | 1.514 (5) |
| N—H1B | 0.91 (4) | C20—H20A | 0.9900 |
| C1—C2 | 1.548 (5) | C20—H20B | 0.9900 |
| C3—O6i | 1.256 (4) | C21—H21A | 0.9900 |
| C3—C3i | 1.537 (7) | C21—H21B | 0.9900 |
| C4—C5 | 1.394 (5) | C22—C23 | 1.516 (5) |
| C4—C9 | 1.397 (5) | C22—C27 | 1.522 (5) |
| C5—C6 | 1.389 (5) | C22—H22 | 1.0000 |
| C5—H5 | 0.9500 | C23—C24 | 1.540 (5) |
| C6—C7 | 1.373 (5) | C23—H23A | 0.9900 |
| C6—H6 | 0.9500 | C23—H23B | 0.9900 |
| C7—C8 | 1.390 (5) | C24—C25 | 1.514 (5) |
| C7—H7 | 0.9500 | C24—H24A | 0.9900 |
| C8—C9 | 1.382 (5) | C24—H24B | 0.9900 |
| C8—H8 | 0.9500 | C25—C26 | 1.518 (5) |
| C9—H9 | 0.9500 | C25—H25A | 0.9900 |
| C10—C11 | 1.385 (5) | C25—H25B | 0.9900 |
| C10—C15 | 1.394 (5) | C26—C27 | 1.534 (5) |
| C11—C12 | 1.398 (6) | C26—H26A | 0.9900 |
| C11—H11 | 0.9500 | C26—H26B | 0.9900 |
| C12—C13 | 1.366 (7) | C27—H27A | 0.9900 |
| C12—H12 | 0.9500 | C27—H27B | 0.9900 |
| C10—Sn—C4 | 168.75 (13) | C14—C15—C10 | 121.4 (4) |
| C10—Sn—O1 | 97.50 (11) | C14—C15—H15 | 119.3 |
| C4—Sn—O1 | 93.06 (10) | C10—C15—H15 | 119.3 |
| C10—Sn—O2 | 92.53 (12) | N—C16—C17 | 109.4 (3) |
| C4—Sn—O2 | 94.18 (11) | N—C16—C21 | 111.8 (3) |
| O1—Sn—O2 | 73.55 (8) | C17—C16—C21 | 110.9 (3) |
| C10—Sn—O5 | 93.06 (12) | N—C16—H16 | 108.2 |
| C4—Sn—O5 | 86.04 (10) | C17—C16—H16 | 108.2 |
| O1—Sn—O5 | 74.35 (8) | C21—C16—H16 | 108.2 |
| O2—Sn—O5 | 147.87 (8) | C18—C17—C16 | 109.9 (3) |
| C10—Sn—O7 | 86.30 (11) | C18—C17—H17A | 109.7 |
| C4—Sn—O7 | 88.01 (10) | C16—C17—H17A | 109.7 |
| O1—Sn—O7 | 140.57 (9) | C18—C17—H17B | 109.7 |
| O2—Sn—O7 | 67.06 (8) | C16—C17—H17B | 109.7 |
| O5—Sn—O7 | 144.90 (8) | H17A—C17—H17B | 108.2 |
| C10—Sn—O6 | 85.62 (11) | C17—C18—C19 | 110.2 (3) |
| C4—Sn—O6 | 83.54 (10) | C17—C18—H18A | 109.6 |
| O1—Sn—O6 | 144.21 (8) | C19—C18—H18A | 109.6 |
| O2—Sn—O6 | 142.15 (7) | C17—C18—H18B | 109.6 |
| O5—Sn—O6 | 69.87 (8) | C19—C18—H18B | 109.6 |
| O7—Sn—O6 | 75.09 (8) | H18A—C18—H18B | 108.1 |
| C1—O1—Sn | 117.4 (2) | C18—C19—C20 | 111.1 (3) |
| C2—O2—Sn | 117.3 (2) | C18—C19—H19A | 109.4 |
| C3—O5—Sn | 119.8 (2) | C20—C19—H19A | 109.4 |
| C3i—O6—Sn | 114.2 (2) | C18—C19—H19B | 109.4 |
| Sn—O7—H7A | 104 (3) | C20—C19—H19B | 109.4 |
| Sn—O7—H7B | 127 (3) | H19A—C19—H19B | 108.0 |
| H7A—O7—H7B | 103 (4) | C21—C20—C19 | 112.4 (3) |
| C16—N—C22 | 118.5 (3) | C21—C20—H20A | 109.1 |
| C16—N—H1A | 108 (2) | C19—C20—H20A | 109.1 |
| C22—N—H1A | 105 (2) | C21—C20—H20B | 109.1 |
| C16—N—H1B | 108 (2) | C19—C20—H20B | 109.1 |
| C22—N—H1B | 109 (2) | H20A—C20—H20B | 107.9 |
| H1A—N—H1B | 108 (3) | C20—C21—C16 | 109.6 (3) |
| O4—C1—O1 | 125.1 (3) | C20—C21—H21A | 109.8 |
| O4—C1—C2 | 118.2 (3) | C16—C21—H21A | 109.8 |
| O1—C1—C2 | 116.6 (3) | C20—C21—H21B | 109.8 |
| O3—C2—O2 | 126.6 (3) | C16—C21—H21B | 109.8 |
| O3—C2—C1 | 118.9 (3) | H21A—C21—H21B | 108.2 |
| O2—C2—C1 | 114.4 (3) | N—C22—C23 | 107.8 (3) |
| O6i—C3—O5 | 125.2 (3) | N—C22—C27 | 110.8 (3) |
| O6i—C3—C3i | 117.5 (4) | C23—C22—C27 | 111.5 (3) |
| O5—C3—C3i | 117.2 (4) | N—C22—H22 | 108.9 |
| C5—C4—C9 | 117.9 (3) | C23—C22—H22 | 108.9 |
| C5—C4—Sn | 121.4 (2) | C27—C22—H22 | 108.9 |
| C9—C4—Sn | 120.6 (2) | C22—C23—C24 | 110.6 (3) |
| C6—C5—C4 | 121.3 (3) | C22—C23—H23A | 109.5 |
| C6—C5—H5 | 119.4 | C24—C23—H23A | 109.5 |
| C4—C5—H5 | 119.4 | C22—C23—H23B | 109.5 |
| C7—C6—C5 | 120.2 (3) | C24—C23—H23B | 109.5 |
| C7—C6—H6 | 119.9 | H23A—C23—H23B | 108.1 |
| C5—C6—H6 | 119.9 | C25—C24—C23 | 110.9 (3) |
| C6—C7—C8 | 119.4 (3) | C25—C24—H24A | 109.5 |
| C6—C7—H7 | 120.3 | C23—C24—H24A | 109.5 |
| C8—C7—H7 | 120.3 | C25—C24—H24B | 109.5 |
| C9—C8—C7 | 120.7 (3) | C23—C24—H24B | 109.5 |
| C9—C8—H8 | 119.7 | H24A—C24—H24B | 108.1 |
| C7—C8—H8 | 119.7 | C24—C25—C26 | 110.5 (3) |
| C8—C9—C4 | 120.6 (3) | C24—C25—H25A | 109.5 |
| C8—C9—H9 | 119.7 | C26—C25—H25A | 109.5 |
| C4—C9—H9 | 119.7 | C24—C25—H25B | 109.5 |
| C11—C10—C15 | 117.5 (3) | C26—C25—H25B | 109.5 |
| C11—C10—Sn | 120.6 (3) | H25A—C25—H25B | 108.1 |
| C15—C10—Sn | 121.9 (3) | C25—C26—C27 | 111.5 (3) |
| C10—C11—C12 | 120.7 (4) | C25—C26—H26A | 109.3 |
| C10—C11—H11 | 119.6 | C27—C26—H26A | 109.3 |
| C12—C11—H11 | 119.6 | C25—C26—H26B | 109.3 |
| C13—C12—C11 | 120.5 (4) | C27—C26—H26B | 109.3 |
| C13—C12—H12 | 119.7 | H26A—C26—H26B | 108.0 |
| C11—C12—H12 | 119.7 | C22—C27—C26 | 110.0 (3) |
| C12—C13—C14 | 119.8 (4) | C22—C27—H27A | 109.7 |
| C12—C13—H13 | 120.1 | C26—C27—H27A | 109.7 |
| C14—C13—H13 | 120.1 | C22—C27—H27B | 109.7 |
| C13—C14—C15 | 120.0 (4) | C26—C27—H27B | 109.7 |
| C13—C14—H14 | 120.0 | H27A—C27—H27B | 108.2 |
| C15—C14—H14 | 120.0 |
Symmetry codes: (i) −x, −y+1, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O7—H7B···O4ii | 0.90 (4) | 1.77 (4) | 2.663 (3) | 175 (4) |
| N—H1A···O3iii | 0.84 (4) | 2.12 (3) | 2.910 (4) | 155 (3) |
| N—H1A···O4iii | 0.84 (4) | 2.37 (4) | 2.986 (4) | 130 (3) |
| N—H1B···O6i | 0.91 (4) | 2.08 (4) | 2.960 (4) | 164 (4) |
Symmetry codes: (ii) −x+1/2, y+1/2, −z+1/2; (iii) x−1/2, −y+1/2, z−1/2; (i) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2310).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Ballmann, J., Fuchs, M. G. G., Dechert, S., John, M. & Meyer, F. (2009). Inorg. Chem.48, 90–99. [DOI] [PubMed]
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Diallo, W., Diop, C. A. K., Diop, L., Mahon, M. F., Molloy, K. C., Russo, U., Biesemans, M. & Willem, R. (2007). J. Organomet. Chem.692, 2187–2192.
- Diassé-Sarr, A., Diop, L., Mahon, M. F. & Molloy, K. C. (1997). Main Group Met. Chem.20, 223–229.
- Diop, C. A. K., Diop, L. & Toscano, A. R. (2002). Main Group Met. Chem.25, 327–328.
- Diop, L., Mahieu, B., Mahon, M. F., Molloy, K. C. & Okio, K. Y. A. (2003). Appl. Organomet. Chem.17, 881–882.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Ng, S. W., Das, V. G. K., Gielen, M. & Tiekink, E. R. T. (1992). Appl. Organomet. Chem.6, 19–25.
- Nonius (2000). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singh, N., Kumar, A., Molloy, K. C. & Kociok-Köhn, G. (2008). Acta Cryst. E64, m115. [DOI] [PMC free article] [PubMed]
- Sousa, G. F. de, Deflon, V. M., Manso, L. C. C., Ellena, J. E., Mascarenhas, Y. P., Lang, E. S., Gatto, C. C. & Mahieu, B. (2007). Transition Met. Chem.32, 649–655.
- Wang, Z., Zhao, G. & Tian, L. (2009). Acta Cryst. E65, m528. [DOI] [PMC free article] [PubMed]
- Xanthopoulou, M. N., Hadjikakou, S. K., Hadjiliadis, N., Kubicki, M., Skoulika, S., Bakas, T., baril, M. & Butler, I. S. (2007). Inorg. Chem.46, 1187–1195. [DOI] [PubMed]
- Xanthopoulou, M. N., Kourkoumelis, N., Hadjikakou, S. K., Hadjiliadis, N., Kubicki, M., Karkabounas, S. & Bakas, T. (2008). Polyhedron, 27, 3318–3324.
- Zia-ur-Rahman, Ali, S., Muhammed, N. & Meetsma, A. (2007). Acta Cryst. E63, m89–m90.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810046738/bh2310sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810046738/bh2310Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



