Abstract
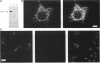
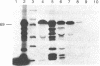
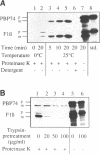
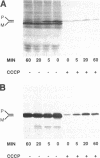
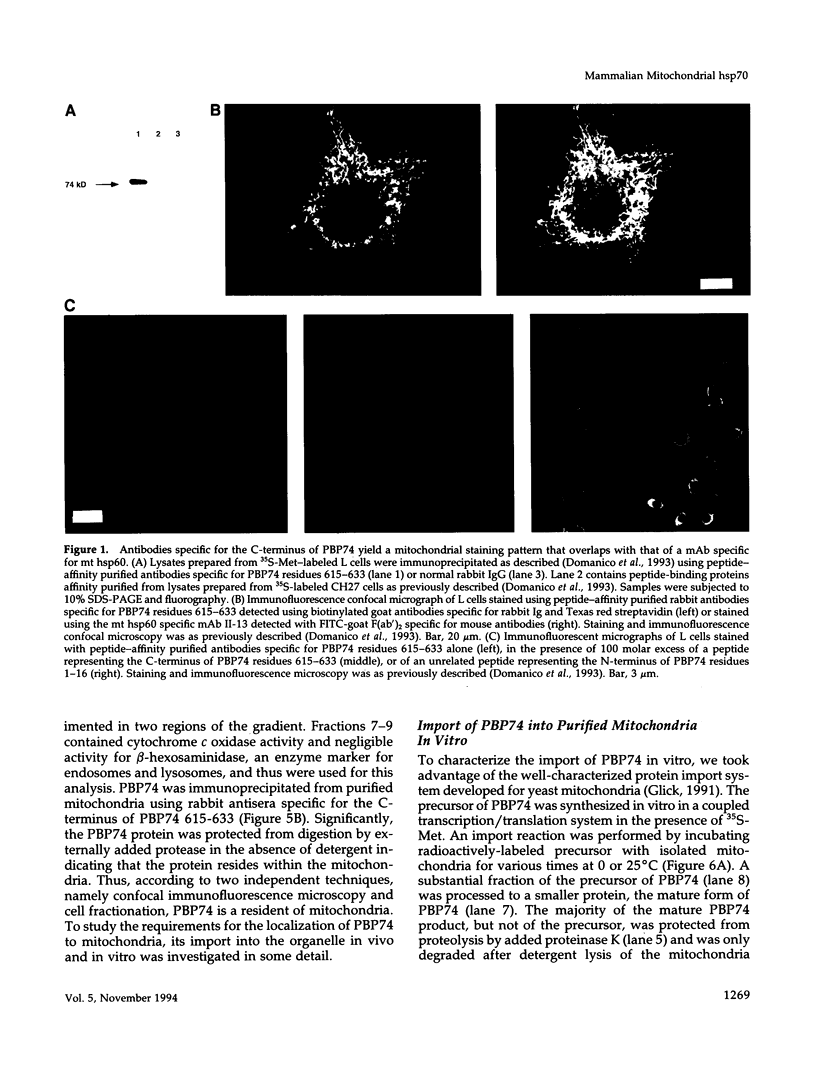
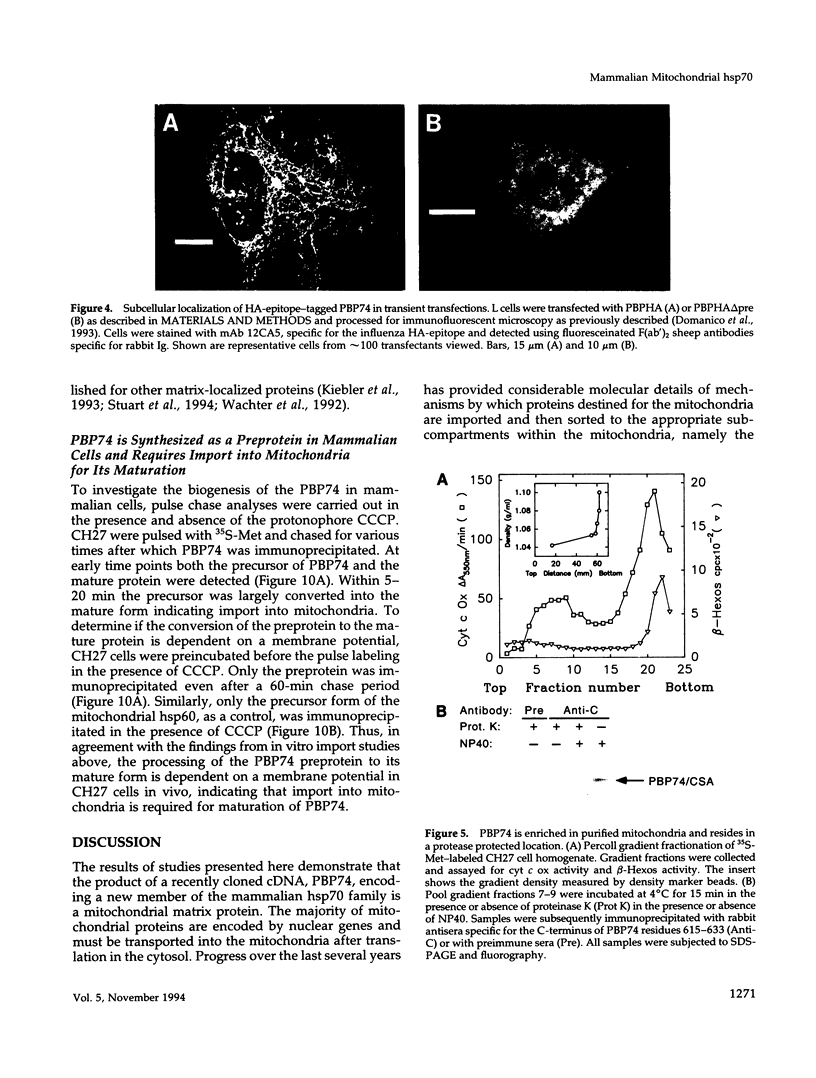
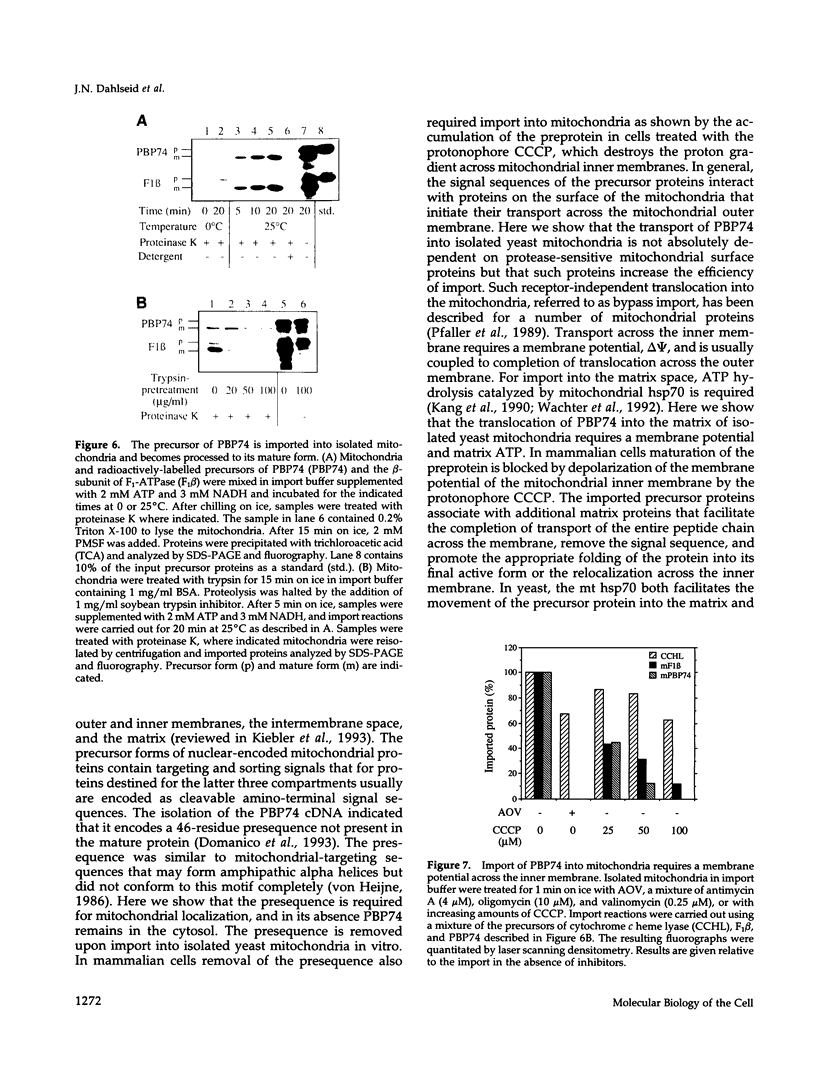
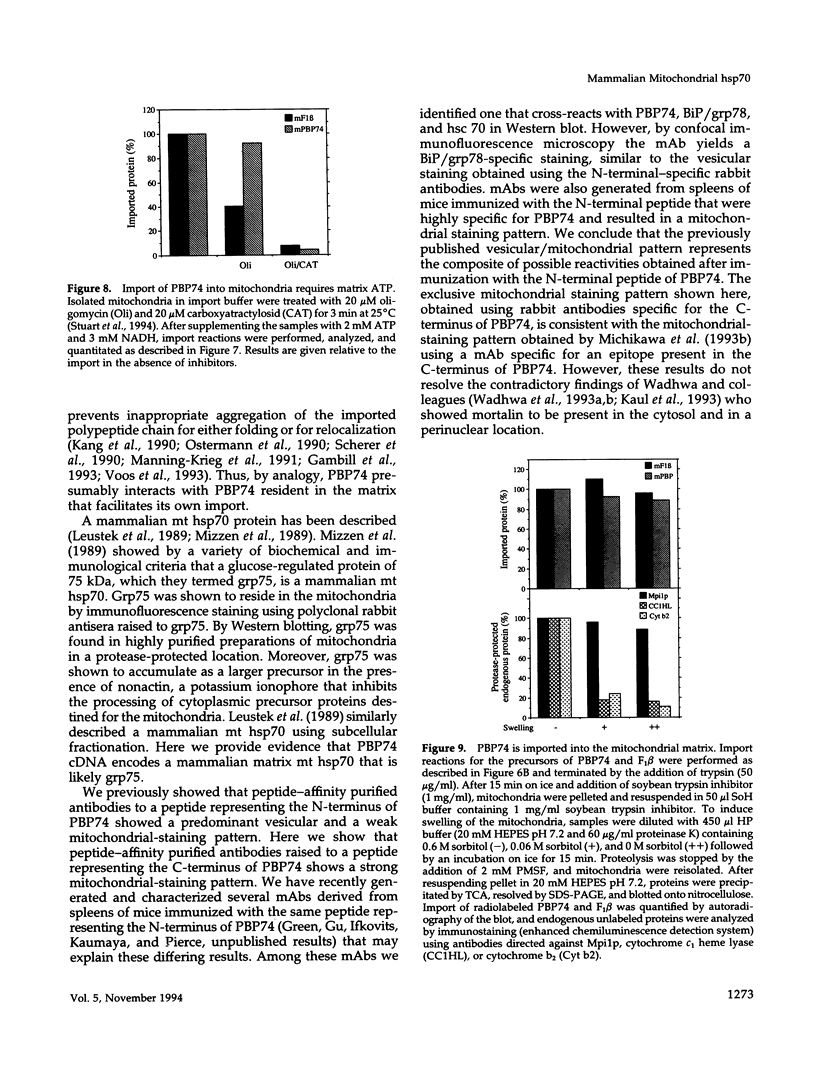
The cloning of a cDNA encoding a new member of the highly conserved mammalian 70-kDa heat shock protein (hsp 70) family termed PBP74 was recently reported. Critical to an understanding of the function of this new hsp 70 is delineating its subcellular localization. Here we use a variety of immunological and biochemical approaches both in vitro and in vivo to demonstrate that PBP74 is imported into and resides in mitochondria. By confocal immunofluorescence microscopy PBP74 is detected in mitochondria, colocalizing with the mitochondrial 60-kDa heat shock protein. To address the inherent problem of serological cross-reactivity among the hsp70 family members, an influenza virus hemagglutinin epitope tag was introduced into the PBP74 cDNA. The epitope-tagged PBP74 protein transiently expressed in L cells localized to mitochondria. Moreover, deletion of the N-terminal 46-amino acid presequence results in a cytosolic localization of the epitope-tagged protein. Cell fractionation studies demonstrated PBP74 in purified mitochondria in a protease-protected location. After coupled transcription-translation the precursor of PBP74 is imported into isolated yeast mitochondria, where it becomes processed to the mature protein. According to a subfractionation of the mitochondria, the imported protein was found to be localized in the matrix space. Import in vitro is time- and temperature-dependent, requires matrix ATP, and is abolished upon depletion of the membrane potential across the mitochondrial inner membrane. Similarly, in mammalian cells PBP74 is synthesized as a pre-protein that requires membrane potential-dependent import into mitochondria for its maturation. Taken together, our data demonstrate that PBP74 is a mammalian mitochondrial hsp70.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNagel D. C., Pierce S. K. A case for chaperones in antigen processing. Immunol Today. 1992 Mar;13(3):86–89. doi: 10.1016/0167-5699(92)90147-Y. [DOI] [PubMed] [Google Scholar]
- Domanico S. Z., DeNagel D. C., Dahlseid J. N., Green J. M., Pierce S. K. Cloning of the gene encoding peptide-binding protein 74 shows that it is a new member of the heat shock protein 70 family. Mol Cell Biol. 1993 Jun;13(6):3598–3610. doi: 10.1128/mcb.13.6.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Glick B. S. Protein import into isolated yeast mitochondria. Methods Cell Biol. 1991;34:389–399. doi: 10.1016/s0091-679x(08)61693-3. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Ho P. C., Mutch D. A., Winkel K. D., Saul A. J., Jones G. L., Doran T. J., Rzepczyk C. M. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990 Mar;20(3):477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- Jelachich M. L., Grusby M. J., Clark D., Tasch D., Margoliash E., Pierce S. K. Synergistic effects of antigen and soluble T-cell factors in B-lymphocyte activation. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5537–5541. doi: 10.1073/pnas.81.17.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kaul S. C., Wadhwa R., Komatsu Y., Sugimoto Y., Mitsui Y. On the cytosolic and perinuclear mortalin: an insight by heat shock. Biochem Biophys Res Commun. 1993 May 28;193(1):348–355. doi: 10.1006/bbrc.1993.1630. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Becker K., Pfanner N., Neupert W. Mitochondrial protein import: specific recognition and membrane translocation of preproteins. J Membr Biol. 1993 Sep;135(3):191–207. doi: 10.1007/BF00211091. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey E. K., Margoliash E., Pierce S. K. Identification of a peptide binding protein that plays a role in antigen presentation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1659–1663. doi: 10.1073/pnas.84.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Rasheed S. A simple procedure for maximum yield of high-quality plasmid DNA. Biotechniques. 1990 Dec;9(6):676–679. [PubMed] [Google Scholar]
- Leustek T., Dalie B., Amir-Shapira D., Brot N., Weissbach H. A member of the Hsp70 family is localized in mitochondria and resembles Escherichia coli DnaK. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7805–7808. doi: 10.1073/pnas.86.20.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Stuart R. A., Drygas M. E., Nargang F. E., Neupert W. Import of cytochrome c heme lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J. 1992 Feb;11(2):449–456. doi: 10.1002/j.1460-2075.1992.tb05074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa Y., Baba T., Arai Y., Sakakura T., Kusakabe M. Structure and organization of the gene encoding a mouse mitochondrial stress-70 protein. FEBS Lett. 1993 Dec 20;336(1):27–33. doi: 10.1016/0014-5793(93)81602-v. [DOI] [PubMed] [Google Scholar]
- Michikawa Y., Baba T., Arai Y., Sakakura T., Tanaka M., Kusakabe M. Antigenic protein specific for C3H strain mouse is a mitochondrial stress-70 protein. Biochem Biophys Res Commun. 1993 Oct 15;196(1):223–232. doi: 10.1006/bbrc.1993.2238. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Chang C., Garrels J. I., Welch W. J. Identification, characterization, and purification of two mammalian stress proteins present in mitochondria, grp 75, a member of the hsp 70 family and hsp 58, a homolog of the bacterial groEL protein. J Biol Chem. 1989 Dec 5;264(34):20664–20675. [PubMed] [Google Scholar]
- Ostermann J., Voos W., Kang P. J., Craig E. A., Neupert W., Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990 Dec 17;277(1-2):281–284. doi: 10.1016/0014-5793(90)80865-g. [DOI] [PubMed] [Google Scholar]
- Pfaller R., Pfanner N., Neupert W. Mitochondrial protein import. Bypass of proteinaceous surface receptors can occur with low specificity and efficiency. J Biol Chem. 1989 Jan 5;264(1):34–39. [PubMed] [Google Scholar]
- Pfaller R., Steger H. F., Rassow J., Pfanner N., Neupert W. Import pathways of precursor proteins into mitochondria: multiple receptor sites are followed by a common membrane insertion site. J Cell Biol. 1988 Dec;107(6 Pt 2):2483–2490. doi: 10.1083/jcb.107.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Xu X., Wandinger-Ness A., Dalke D. P., Pierce S. K. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994 May;125(3):595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter Z. Dual effects of cytokines in regulation of MHC-unrestricted cell mediated cytotoxicity. Crit Rev Immunol. 1993;13(1):1–34. [PubMed] [Google Scholar]
- Rubin D. M., Mehta A. D., Zhu J., Shoham S., Chen X., Wells Q. R., Palter K. B. Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene. 1993 Jun 30;128(2):155–163. doi: 10.1016/0378-1119(93)90558-k. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. A., Gruhler A., van der Klei I., Guiard B., Koll H., Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994 Feb 15;220(1):9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- VanBuskirk A. M., DeNagel D. C., Guagliardi L. E., Brodsky F. M., Pierce S. K. Cellular and subcellular distribution of PBP72/74, a peptide-binding protein that plays a role in antigen processing. J Immunol. 1991 Jan 15;146(2):500–506. [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W., Gambill B. D., Guiard B., Pfanner N., Craig E. A. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol. 1993 Oct;123(1):119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter C., Schatz G., Glick B. S. Role of ATP in the intramitochondrial sorting of cytochrome c1 and the adenine nucleotide translocator. EMBO J. 1992 Dec;11(13):4787–4794. doi: 10.1002/j.1460-2075.1992.tb05584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R., Kaul S. C., Ikawa Y., Sugimoto Y. Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J Biol Chem. 1993 Mar 25;268(9):6615–6621. [PubMed] [Google Scholar]
- Wadhwa R., Kaul S. C., Mitsui Y., Sugimoto Y. Differential subcellular distribution of mortalin in mortal and immortal mouse and human fibroblasts. Exp Cell Res. 1993 Aug;207(2):442–448. doi: 10.1006/excr.1993.1213. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]