Abstract
In the title compound, C14H12N2O2S, the aminophenol and the benzoyl groups adopt a syn–anti configuration with respect to the thiono C=S group across the thiourea C—N. The dihedral angle between the mean planes of the benzoyl and hydroxyphenyl rings is 36.77 (8)°. The molecules are stabilized by intramolecular N—H⋯O hydrogen bonds. In the crystal, weak intermolecular C—H⋯O, O—H⋯S and N—H⋯O hydrogen bonds link the molecules into a chain along the c axis.
Related literature
For the preparation and chemical properties of related compounds, see: Zhang et al. (2001 ▶). For related structures, see: Abosadiya et al. (2007 ▶); Hung et al. (2010 ▶); Yamin & Yusof (2003 ▶). For bond-length data, see: Allen et al. (1987 ▶).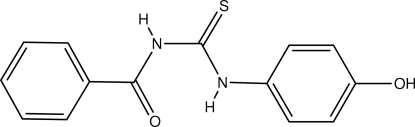
Experimental
Crystal data
C14H12N2O2S
M r = 272.33
Orthorhombic,

a = 5.5865 (10) Å
b = 14.451 (2) Å
c = 16.462 (3) Å
V = 1329.0 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.24 mm−1
T = 298 K
0.50 × 0.48 × 0.35 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.886, T max = 0.919
7608 measured reflections
2351 independent reflections
2143 reflections with I > 2σ(I)
R int = 0.018
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.085
S = 1.05
2351 reflections
173 parameters
H-atom parameters constrained
Δρmax = 0.13 e Å−3
Δρmin = −0.14 e Å−3
Absolute structure: Flack (1983 ▶), 958 Friedel pairs
Flack parameter: 0.09 (9)
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL, PARST (Nardelli, 1995 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810045988/jj2068sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810045988/jj2068Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2B⋯O1 | 0.86 | 1.95 | 2.631 (2) | 135 |
| N1—H1B⋯O2i | 0.86 | 2.29 | 3.109 (2) | 158 |
| O2—H2C⋯S1ii | 0.82 | 2.53 | 3.1533 (18) | 134 |
| C1—H1A⋯O2i | 0.93 | 2.51 | 3.429 (3) | 172 |
| C11—H11A⋯O1iii | 0.93 | 2.43 | 3.262 (2) | 149 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The authors thank Universiti Kebangsaan Malaysia for providing facilities and grants UKM-GUP-BTT-07–30-190 and UKM-OUP-TK-16–73/2010, the Libyan Government for providing a scholarship for AA and J.-C. Daran for his advice.
supplementary crystallographic information
Comment
The title compound, I, is closely related to the previously reported N-benzoyl-N'-(3-hydroxyphenyl)thiourea (II) (Abosadiya et al., 2007) compound. As in most benzoylthiourea derivatives of the type R1C(O)NHC(S)NHR2, the title compound has a syn–anti configuration for the hydroxyphenyl and benzoyl groups with respect to the thiono C=S bond across the thiourea C—N bond (Fig 1). The bond lengths and angles in the molecules are in normal ranges (Allen et al., 1987) and comparable to those in (II). All C—N bond lengths are shorter than the normal value for C—N single bond, and the bond lengths C7—O1 and C8—S1 become longer than normal values for a double bond, which suggests the presence of delocalized π-electrons. For example, the C=S bond (1.671 (2) Å) and C—N bond lengths (C8—N1 = 1.388 (2) Å, C8—N2 = 1.328 (2) Å and C9—N2 = 1.428 (2) Å) in the title compound are longer than that observed in an unsubsituted phenyl ring (III - Yamin et al., 2003) in which the C=S bond length (1.6567 (15) Å) and C—N bond lengths are 1.393 (2), 1.326 (2) and 1.408 (2) Å, respectively. This is due to donating effect of OH group in the para position, which contributes to an increase in the bond length.
The benzoyl ring [C1/C2/C3/C4/C5/C6/C7/O1] (A), hydroxyphenyl ring [N2/C9/C10/C11/C12/C13/C14/O2] (B) and thiourea [(S1/N1/N2/C8/] (C) fragments are essentially planar with maximum deviations from their mean planes of 0.045 (2) Å for atom O1 (A), 0.010 (2) Å for atom C10 (B) and 0.008 (2)Å for atom C8 (C), respectively. The dihedral angle between the mean planes of A and B is 36.77 (8)°. The crytal structure is stabilized by an intramolecular N2—H2B···O1 hydrogen bond which forms a six-membered ring (N2/H2B/O1/C7/N1/C8) commonly observed in this class of ligands. In addition, the molecules are linked by weak intermolecular C11—H···O1, C1—H···O2, O2—H···S1 and N1—H···O2 hydrogen bonds (Table 2), resulting in a one-dimensional chain along the c-axis (Fig 2).
Experimental
The title compound was first synthesized by (Zhang et al., 2001) under the condition of solid-liquid phase transfer catalysis using polyethylene glycol as catalyst, however, a much simpler method was used to synthesize the title compound. The reaction scheme involved a reaction of benzoyl chloride (10 mmol) with ammonium thiocyanate (10 mmol) in dry acetone. The product, benzoyl isothiocyanate was reacted with 4-hydroxy aniline (10 mmol) to give the title compound with a 56% yield. A slow evaporation of ethanolic solution of the compound gave light brawn crystals suitable for X-ray diffraction.
Refinement
All the non hydrogen atom were refined anisotropically. the hydrogen positions were calculated to give an idealized geometry fixed to ride on their respective atoms, with Uiso=1.2Ueq (C) for aromatic (CH = 0.93 Å), Uiso=1.2Ueq (N) for N (NH = 0.86 Å) and Uiso=1.5Ueq (O) for OH (OH = 0.82 Å).
Figures
Fig. 1.
The molecular structure of (I), with displacement ellipsods drawn at the 50% probability level. Dashed lines indicate intramolecular N—H···O hydrogen bonds.
Fig. 2.
Crystal packing of (I) viewed down the a axis. Dashed lines indicate weak C—H···O, C—H···O, O—H···S and N—H···O hydrogen bonds.
Crystal data
| C14H12N2O2S | F(000) = 568 |
| Mr = 272.33 | Dx = 1.361 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 1024 reflections |
| a = 5.5865 (10) Å | θ = 1.9–25.0° |
| b = 14.451 (2) Å | µ = 0.24 mm−1 |
| c = 16.462 (3) Å | T = 298 K |
| V = 1329.0 (4) Å3 | Block, brown |
| Z = 4 | 0.50 × 0.48 × 0.35 mm |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 2351 independent reflections |
| Radiation source: fine-focus sealed tube | 2143 reflections with I > 2σ(I) |
| graphite | Rint = 0.018 |
| ω scans | θmax = 25.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −6→6 |
| Tmin = 0.886, Tmax = 0.919 | k = −15→17 |
| 7608 measured reflections | l = −19→17 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.031 | H-atom parameters constrained |
| wR(F2) = 0.085 | w = 1/[σ2(Fo2) + (0.0494P)2 + 0.1346P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 2351 reflections | Δρmax = 0.13 e Å−3 |
| 173 parameters | Δρmin = −0.14 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 958 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.09 (9) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 1.13932 (14) | −0.04354 (4) | 0.71251 (4) | 0.0812 (2) | |
| O1 | 0.7785 (3) | 0.23667 (10) | 0.72476 (9) | 0.0719 (4) | |
| N1 | 0.8692 (3) | 0.09206 (10) | 0.76731 (8) | 0.0518 (4) | |
| H1B | 0.8429 | 0.0530 | 0.8057 | 0.062* | |
| N2 | 1.0996 (3) | 0.12902 (11) | 0.65596 (9) | 0.0565 (4) | |
| H2B | 1.0469 | 0.1841 | 0.6646 | 0.068* | |
| C1 | 0.5090 (4) | 0.10914 (14) | 0.89160 (13) | 0.0632 (5) | |
| H1A | 0.6077 | 0.0573 | 0.8928 | 0.076* | |
| C2 | 0.3268 (5) | 0.11715 (16) | 0.94751 (14) | 0.0712 (6) | |
| H2A | 0.3032 | 0.0712 | 0.9862 | 0.085* | |
| C3 | 0.1812 (4) | 0.19276 (18) | 0.94584 (14) | 0.0726 (6) | |
| H3A | 0.0581 | 0.1983 | 0.9836 | 0.087* | |
| C4 | 0.2149 (4) | 0.26068 (17) | 0.88890 (14) | 0.0734 (6) | |
| H4A | 0.1140 | 0.3119 | 0.8879 | 0.088* | |
| C5 | 0.3978 (4) | 0.25336 (14) | 0.83317 (12) | 0.0632 (5) | |
| H5A | 0.4208 | 0.3000 | 0.7950 | 0.076* | |
| C6 | 0.5475 (3) | 0.17701 (12) | 0.83367 (10) | 0.0487 (4) | |
| C7 | 0.7395 (4) | 0.17240 (12) | 0.77127 (10) | 0.0512 (4) | |
| C8 | 1.0373 (4) | 0.06508 (13) | 0.70994 (11) | 0.0527 (4) | |
| C9 | 1.2439 (4) | 0.11670 (13) | 0.58521 (11) | 0.0504 (4) | |
| C10 | 1.1577 (4) | 0.15191 (12) | 0.51275 (11) | 0.0520 (5) | |
| H10A | 1.0118 | 0.1829 | 0.5119 | 0.062* | |
| C11 | 1.2858 (4) | 0.14146 (12) | 0.44198 (11) | 0.0518 (5) | |
| H11A | 1.2257 | 0.1643 | 0.3933 | 0.062* | |
| C12 | 1.5042 (3) | 0.09692 (12) | 0.44377 (12) | 0.0515 (5) | |
| C13 | 1.5928 (4) | 0.06247 (16) | 0.51603 (11) | 0.0596 (5) | |
| H13A | 1.7399 | 0.0323 | 0.5170 | 0.072* | |
| C14 | 1.4626 (4) | 0.07290 (16) | 0.58682 (12) | 0.0594 (5) | |
| H14A | 1.5229 | 0.0503 | 0.6356 | 0.071* | |
| O2 | 1.6406 (3) | 0.08472 (10) | 0.37487 (9) | 0.0682 (4) | |
| H2C | 1.5717 | 0.1079 | 0.3359 | 0.102* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.1110 (5) | 0.0497 (3) | 0.0828 (4) | 0.0182 (3) | 0.0277 (4) | 0.0010 (3) |
| O1 | 0.1018 (11) | 0.0536 (8) | 0.0603 (8) | 0.0184 (8) | 0.0223 (8) | 0.0179 (6) |
| N1 | 0.0712 (10) | 0.0432 (8) | 0.0410 (7) | 0.0061 (8) | 0.0053 (7) | 0.0030 (6) |
| N2 | 0.0712 (10) | 0.0480 (8) | 0.0504 (8) | 0.0014 (8) | 0.0101 (8) | −0.0027 (7) |
| C1 | 0.0732 (14) | 0.0513 (11) | 0.0649 (12) | 0.0051 (10) | 0.0142 (10) | 0.0055 (9) |
| C2 | 0.0783 (15) | 0.0630 (13) | 0.0723 (13) | −0.0108 (12) | 0.0191 (12) | 0.0028 (11) |
| C3 | 0.0575 (13) | 0.0890 (17) | 0.0713 (13) | −0.0058 (12) | 0.0105 (11) | −0.0166 (13) |
| C4 | 0.0649 (14) | 0.0824 (16) | 0.0728 (14) | 0.0219 (12) | 0.0005 (12) | −0.0090 (12) |
| C5 | 0.0755 (14) | 0.0596 (12) | 0.0546 (11) | 0.0119 (11) | −0.0026 (11) | 0.0023 (9) |
| C6 | 0.0573 (11) | 0.0472 (10) | 0.0416 (8) | 0.0009 (8) | −0.0045 (8) | −0.0040 (7) |
| C7 | 0.0669 (11) | 0.0461 (10) | 0.0406 (9) | 0.0026 (9) | −0.0032 (8) | 0.0018 (7) |
| C8 | 0.0627 (11) | 0.0508 (10) | 0.0446 (9) | −0.0008 (8) | −0.0022 (8) | −0.0056 (8) |
| C9 | 0.0560 (11) | 0.0481 (9) | 0.0471 (9) | −0.0079 (9) | 0.0045 (8) | −0.0071 (8) |
| C10 | 0.0548 (11) | 0.0411 (9) | 0.0600 (11) | 0.0009 (9) | 0.0083 (9) | 0.0050 (8) |
| C11 | 0.0618 (12) | 0.0426 (10) | 0.0509 (10) | −0.0032 (8) | 0.0049 (9) | 0.0051 (8) |
| C12 | 0.0543 (11) | 0.0470 (10) | 0.0531 (10) | −0.0119 (9) | 0.0061 (8) | −0.0105 (8) |
| C13 | 0.0464 (10) | 0.0724 (13) | 0.0602 (11) | −0.0001 (10) | −0.0044 (9) | −0.0115 (10) |
| C14 | 0.0522 (11) | 0.0780 (13) | 0.0480 (10) | −0.0026 (10) | −0.0083 (8) | −0.0081 (9) |
| O2 | 0.0727 (10) | 0.0735 (9) | 0.0583 (8) | 0.0002 (8) | 0.0169 (7) | −0.0080 (7) |
Geometric parameters (Å, °)
| S1—C8 | 1.670 (2) | C4—H4A | 0.9300 |
| O1—C7 | 1.223 (2) | C5—C6 | 1.385 (3) |
| N1—C7 | 1.370 (2) | C5—H5A | 0.9300 |
| N1—C8 | 1.388 (2) | C6—C7 | 1.486 (3) |
| N1—H1B | 0.8600 | C9—C14 | 1.376 (3) |
| N2—C8 | 1.328 (2) | C9—C10 | 1.384 (3) |
| N2—C9 | 1.428 (2) | C10—C11 | 1.376 (3) |
| N2—H2B | 0.8600 | C10—H10A | 0.9300 |
| C1—C2 | 1.377 (3) | C11—C12 | 1.379 (3) |
| C1—C6 | 1.385 (3) | C11—H11A | 0.9300 |
| C1—H1A | 0.9300 | C12—O2 | 1.378 (2) |
| C2—C3 | 1.362 (3) | C12—C13 | 1.381 (3) |
| C2—H2A | 0.9300 | C13—C14 | 1.382 (3) |
| C3—C4 | 1.370 (3) | C13—H13A | 0.9300 |
| C3—H3A | 0.9300 | C14—H14A | 0.9300 |
| C4—C5 | 1.377 (3) | O2—H2C | 0.8200 |
| C7—N1—C8 | 128.94 (15) | O1—C7—C6 | 121.81 (17) |
| C7—N1—H1B | 115.5 | N1—C7—C6 | 116.91 (15) |
| C8—N1—H1B | 115.5 | N2—C8—N1 | 115.91 (16) |
| C8—N2—C9 | 127.36 (16) | N2—C8—S1 | 125.54 (15) |
| C8—N2—H2B | 116.3 | N1—C8—S1 | 118.53 (13) |
| C9—N2—H2B | 116.3 | C14—C9—C10 | 119.66 (17) |
| C2—C1—C6 | 121.0 (2) | C14—C9—N2 | 122.90 (17) |
| C2—C1—H1A | 119.5 | C10—C9—N2 | 117.43 (17) |
| C6—C1—H1A | 119.5 | C11—C10—C9 | 120.56 (18) |
| C3—C2—C1 | 119.7 (2) | C11—C10—H10A | 119.7 |
| C3—C2—H2A | 120.2 | C9—C10—H10A | 119.7 |
| C1—C2—H2A | 120.2 | C10—C11—C12 | 119.57 (18) |
| C2—C3—C4 | 120.4 (2) | C10—C11—H11A | 120.2 |
| C2—C3—H3A | 119.8 | C12—C11—H11A | 120.2 |
| C4—C3—H3A | 119.8 | O2—C12—C11 | 122.08 (18) |
| C3—C4—C5 | 120.2 (2) | O2—C12—C13 | 117.68 (18) |
| C3—C4—H4A | 119.9 | C11—C12—C13 | 120.24 (18) |
| C5—C4—H4A | 119.9 | C12—C13—C14 | 119.87 (19) |
| C4—C5—C6 | 120.4 (2) | C12—C13—H13A | 120.1 |
| C4—C5—H5A | 119.8 | C14—C13—H13A | 120.1 |
| C6—C5—H5A | 119.8 | C9—C14—C13 | 120.08 (19) |
| C1—C6—C5 | 118.32 (18) | C9—C14—H14A | 120.0 |
| C1—C6—C7 | 123.80 (17) | C13—C14—H14A | 120.0 |
| C5—C6—C7 | 117.87 (17) | C12—O2—H2C | 109.5 |
| O1—C7—N1 | 121.28 (18) | ||
| C6—C1—C2—C3 | 0.2 (3) | C9—N2—C8—S1 | 7.0 (3) |
| C1—C2—C3—C4 | 0.1 (4) | C7—N1—C8—N2 | 7.5 (3) |
| C2—C3—C4—C5 | −0.5 (4) | C7—N1—C8—S1 | −171.15 (16) |
| C3—C4—C5—C6 | 0.6 (3) | C8—N2—C9—C14 | −49.9 (3) |
| C2—C1—C6—C5 | −0.1 (3) | C8—N2—C9—C10 | 130.9 (2) |
| C2—C1—C6—C7 | −179.5 (2) | C14—C9—C10—C11 | 1.6 (3) |
| C4—C5—C6—C1 | −0.3 (3) | N2—C9—C10—C11 | −179.13 (16) |
| C4—C5—C6—C7 | 179.15 (19) | C9—C10—C11—C12 | −1.2 (3) |
| C8—N1—C7—O1 | −7.2 (3) | C10—C11—C12—O2 | −179.80 (17) |
| C8—N1—C7—C6 | 171.87 (17) | C10—C11—C12—C13 | 0.4 (3) |
| C1—C6—C7—O1 | −174.8 (2) | O2—C12—C13—C14 | −179.95 (18) |
| C5—C6—C7—O1 | 5.8 (3) | C11—C12—C13—C14 | −0.2 (3) |
| C1—C6—C7—N1 | 6.1 (3) | C10—C9—C14—C13 | −1.4 (3) |
| C5—C6—C7—N1 | −173.29 (17) | N2—C9—C14—C13 | 179.44 (19) |
| C9—N2—C8—N1 | −171.58 (17) | C12—C13—C14—C9 | 0.7 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2B···O1 | 0.86 | 1.95 | 2.631 (2) | 135 |
| N1—H1B···O2i | 0.86 | 2.29 | 3.109 (2) | 158 |
| O2—H2C···S1ii | 0.82 | 2.53 | 3.1533 (18) | 134 |
| C1—H1A···O2i | 0.93 | 2.51 | 3.429 (3) | 172 |
| C11—H11A···O1iii | 0.93 | 2.43 | 3.262 (2) | 149 |
Symmetry codes: (i) −x+5/2, −y, z+1/2; (ii) −x+5/2, −y, z−1/2; (iii) x+1/2, −y+1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JJ2068).
References
- Abosadiya, H. M., Yamin, B. M. & Ngah, N. (2007). Acta Cryst. E63, o2403–o2404.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bruker (2000). SADABS, SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Hung, W. W., Hassan, I. N., Yamin, B. M. & Kassim, M. B. (2010). Acta Cryst. E66, o314. [DOI] [PMC free article] [PubMed]
- Nardelli, M. (1995). J. Appl. Cryst.28, 659.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst D65, 148–155. [DOI] [PMC free article] [PubMed]
- Yamin, B. M. & Yusof, M. S. M. (2003). Acta Cryst. E59, o340–o341.
- Zhang, Y.-M., Wei, T.-B. & Gao, L.-M. (2001). Synth. Commun.31 3099–3105.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810045988/jj2068sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810045988/jj2068Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




