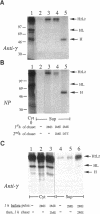
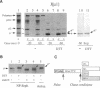
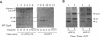Abstract
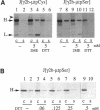
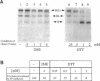
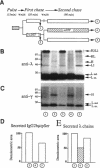
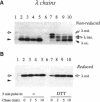
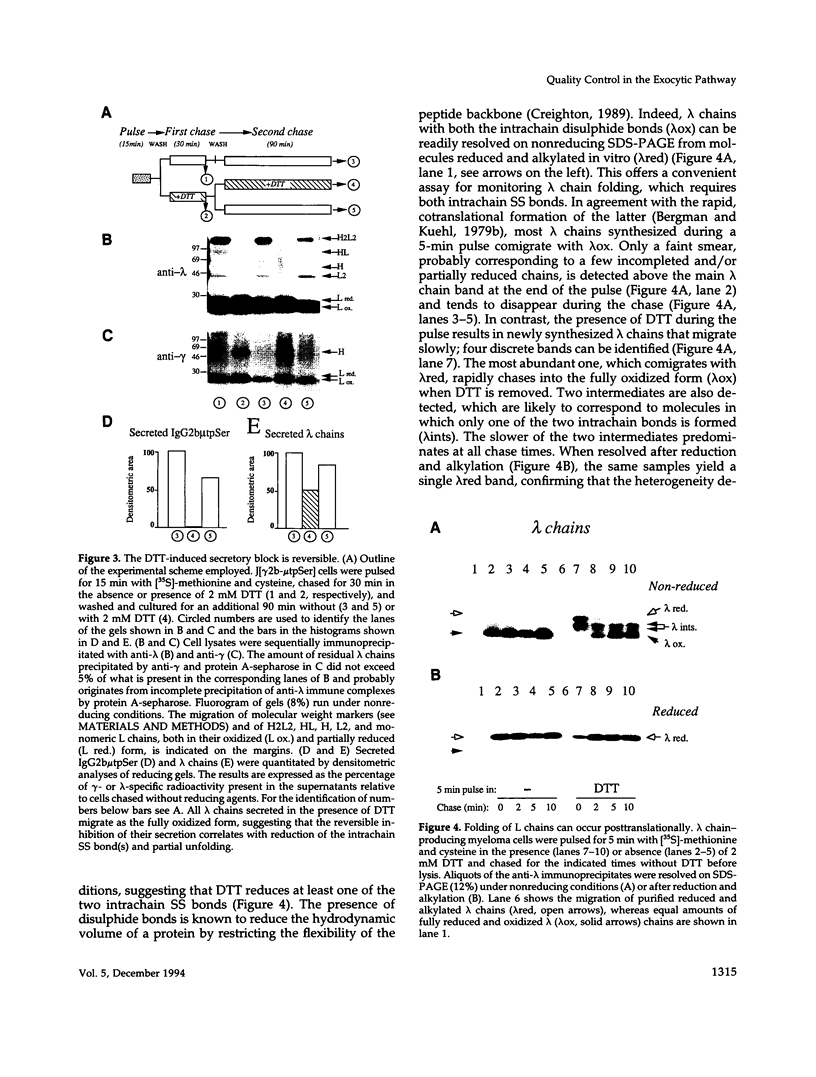
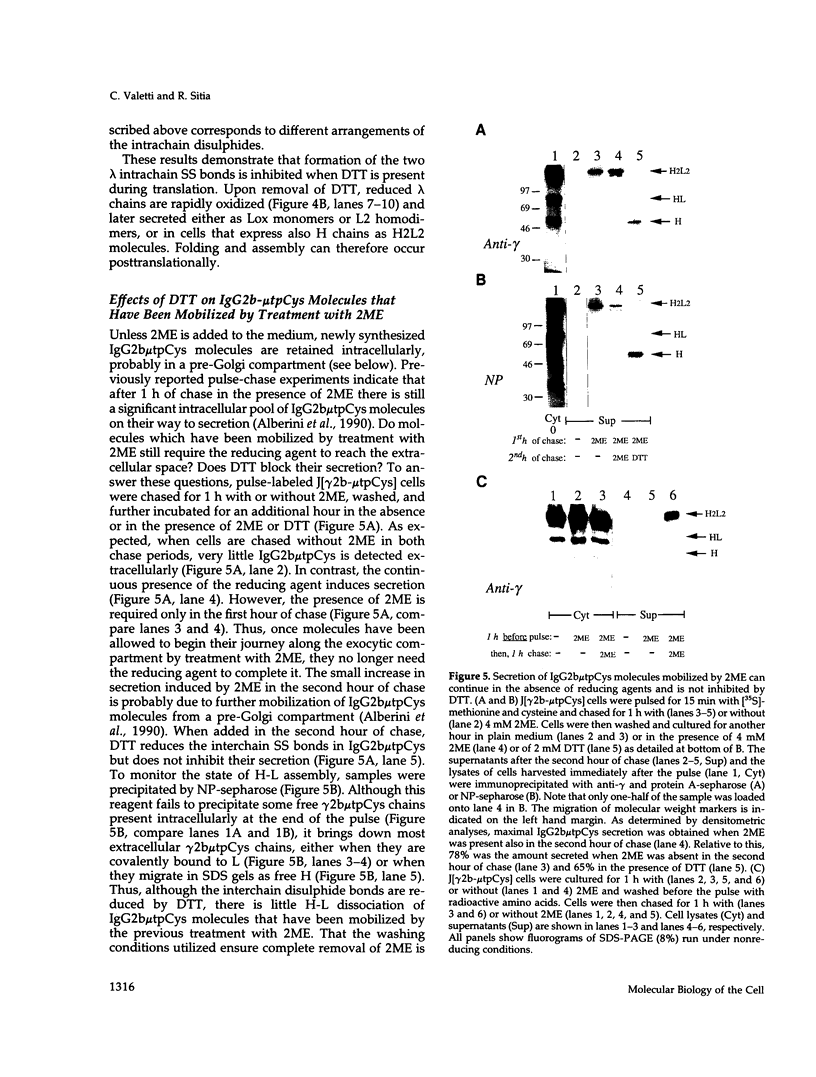
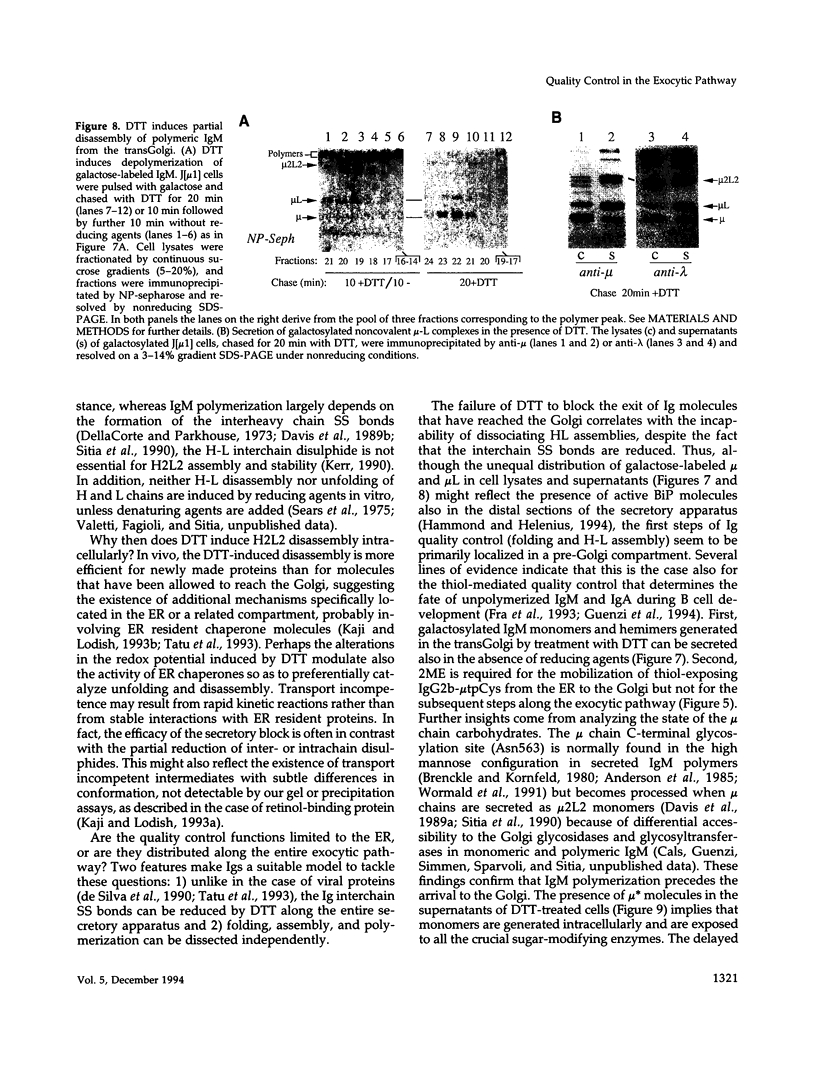
Dithiothreitol (DTT) blocks the endoplasmic reticulum (ER)-Golgi transport of newly synthesized immunoglobulin (Ig) molecules, whereas 2-mercaptoethanol (2ME) allows secretion of unpolymerized Igs otherwise retained intracellularly by disulphide interchange reactions. To understand this dichotomy, we have compared the effects of DTT and 2ME on the assembly, intracellular transport, and secretion of a panel of chimeric Igs that are either constitutively secreted or retained intracellularly. Our results demonstrate that DTT, but not 2ME, reduces some of the inter- and intrachain disulphide bonds and causes partial disassembly of H2L2 complexes and unfolding of individual chains in the ER. Upon DTT removal, heavy (H) and light (L) chains reform hapten-binding H2L2 molecules, which are later secreted. Reduction of the H2L2 interchain disulphide bonds can occur along the entire secretory pathway; however, in or beyond the Golgi this does not result in efficient H-L disassembly or unfolding. As a consequence, DTT does not block the exit from the Golgi. Moreover, unpolymerized Igs--normally retained in a pre-Golgi compartment--no longer require reducing agents to be secreted once they have reached the Golgi. Thus, little if any thiol-mediated retention seems to take place in or beyond the Golgi complex.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C. M., Bet P., Milstein C., Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990 Oct 4;347(6292):485–487. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Atkinson P. H., Grimes W. J. Major carbohydrate structures at five glycosylation sites on murine IgM determined by high resolution 1H-NMR spectroscopy. Arch Biochem Biophys. 1985 Dec;243(2):605–618. doi: 10.1016/0003-9861(85)90538-7. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of an intrachain disulfide bond on nascent immunoglobulin light chains. J Biol Chem. 1979 Sep 25;254(18):8869–8876. [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of intermolecular disulfide bonds on nascent immunoglobulin polypeptides. J Biol Chem. 1979 Jul 10;254(13):5690–5694. [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Curr Opin Cell Biol. 1991 Aug;3(4):592–600. doi: 10.1016/0955-0674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992 Mar 19;356(6366):260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- Brenckle R., Kornfeld R. Structure of the oligosaccharides of mouse immunoglobulin M secreted by the MOPC 104E plasmacytoma. Arch Biochem Biophys. 1980 Apr 15;201(1):160–173. doi: 10.1016/0003-9861(80)90499-3. [DOI] [PubMed] [Google Scholar]
- Brewer J. W., Randall T. D., Parkhouse R. M., Corley R. B. Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem. 1994 Jun 24;269(25):17338–17348. [PubMed] [Google Scholar]
- Chanat E., Weiss U., Huttner W. B., Tooze S. A. Reduction of the disulfide bond of chromogranin B (secretogranin I) in the trans-Golgi network causes its missorting to the constitutive secretory pathways. EMBO J. 1993 May;12(5):2159–2168. doi: 10.1002/j.1460-2075.1993.tb05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley K. J., Baenziger J. U. Post-translational modifications of the core-specific lectin. Relationship to assembly, ligand binding, and secretion. J Biol Chem. 1987 Jul 25;262(21):10296–10303. [PubMed] [Google Scholar]
- Davis A. C., Collins C., Shulman M. J. Differential glycosylation of polymeric and monomeric IgM. Mol Immunol. 1989 Feb;26(2):147–152. doi: 10.1016/0161-5890(89)90096-5. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Roux K. H., Pursey J., Shulman M. J. Intermolecular disulfide bonding in IgM: effects of replacing cysteine residues in the mu heavy chain. EMBO J. 1989 Sep;8(9):2519–2526. doi: 10.1002/j.1460-2075.1989.tb08389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA) and immunoglobulin M (IgM). Requirement for J chain and a disulphide-exchanging enzyme for polymerization. Biochem J. 1973 Nov;136(3):597–606. doi: 10.1042/bj1360597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A. M., Fagioli C., Finazzi D., Sitia R., Alberini C. M. Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J. 1993 Dec;12(12):4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilbert H. F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Guenzi S., Fra A. M., Sparvoli A., Bet P., Rocco M., Sitia R. The efficiency of cysteine-mediated intracellular retention determines the differential fate of secretory IgA and IgM in B and plasma cells. Eur J Immunol. 1994 Oct;24(10):2477–2482. doi: 10.1002/eji.1830241033. [DOI] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994 Jul;126(1):41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J Cell Biol. 1990 Sep;111(3):829–837. doi: 10.1083/jcb.111.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila A. P., Eder A. M., Fuller S. D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992 Sep;118(6):1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jascur T., Matter K., Hauri H. P. Oligomerization and intracellular protein transport: dimerization of intestinal dipeptidylpeptidase IV occurs in the Golgi apparatus. Biochemistry. 1991 Feb 19;30(7):1908–1915. doi: 10.1021/bi00221a025. [DOI] [PubMed] [Google Scholar]
- Kaji E. H., Lodish H. F. In vitro unfolding of retinol-binding protein by dithiothreitol. Endoplasmic reticulum-associated factors. J Biol Chem. 1993 Oct 15;268(29):22195–22202. [PubMed] [Google Scholar]
- Kaji E. H., Lodish H. F. Unfolding of newly made retinol-binding protein by dithiothreitol. Sensitivity to retinoids. J Biol Chem. 1993 Oct 15;268(29):22188–22194. [PubMed] [Google Scholar]
- Kerr M. A. The structure and function of human IgA. Biochem J. 1990 Oct 15;271(2):285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Knittler M. R., Haas I. G. Interaction of BiP with newly synthesized immunoglobulin light chain molecules: cycles of sequential binding and release. EMBO J. 1992 Apr;11(4):1573–1581. doi: 10.1002/j.1460-2075.1992.tb05202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. The secretory pathway is normal in dithiothreitol-treated cells, but disulfide-bonded proteins are reduced and reversibly retained in the endoplasmic reticulum. J Biol Chem. 1993 Sep 25;268(27):20598–20605. [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993 Sep 24;74(6):1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Pypaert M., Hoe M. H., Slusarewicz P., Berger E. G., Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993 Jan;120(1):5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Randall T. D., Brewer J. W., Corley R. B. Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J Biol Chem. 1992 Sep 5;267(25):18002–18007. [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):479–483. doi: 10.1073/pnas.81.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff M. D., Bargellesi A., Baumal R., Buxbaum J., Coffino P., Laskov R. Variations in the synthesis and assembly of immunoglobulins by mouse myeloma cells: a genetic and biochemical analysis. J Cell Physiol. 1970 Dec;76(3):331–348. doi: 10.1002/jcp.1040760311. [DOI] [PubMed] [Google Scholar]
- Sears D. W., Mohrer J., Beychok S. A kinetic study in vitro of the reoxidation of interchain disulfide bonds in a human immunoglobulin IgGLk. Correlation between sulfhydryl disappearance and intermediates in covalent assembly of H2L2. Proc Natl Acad Sci U S A. 1975 Jan;72(1):353–357. doi: 10.1073/pnas.72.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M. S., Milstein C. Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J. 1987 Dec 20;6(13):3969–3977. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990 Mar 9;60(5):781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Sun W. Y., Xiong J., Shulman M. J. Substitution of asparagine for serine-406 of the immunoglobulin mu heavy chain alters glycosylation at asparagine-402. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1627–1634. doi: 10.1016/0006-291x(91)91761-z. [DOI] [PubMed] [Google Scholar]
- Tatu U., Braakman I., Helenius A. Membrane glycoprotein folding, oligomerization and intracellular transport: effects of dithiothreitol in living cells. EMBO J. 1993 May;12(5):2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Grossi C. E., Milstein C., Sitia R. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol. 1991 Nov;115(4):983–994. doi: 10.1083/jcb.115.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Wormald M. R., Wooten E. W., Bazzo R., Edge C. J., Feinstein A., Rademacher T. W., Dwek R. A. The conformational effects of N-glycosylation on the tailpiece from serum IgM. Eur J Biochem. 1991 May 23;198(1):131–139. doi: 10.1111/j.1432-1033.1991.tb15995.x. [DOI] [PubMed] [Google Scholar]
- Young J., Kane L. P., Exley M., Wileman T. Regulation of selective protein degradation in the endoplasmic reticulum by redox potential. J Biol Chem. 1993 Sep 15;268(26):19810–19818. [PubMed] [Google Scholar]
- de Silva A. M., Balch W. E., Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990 Sep;111(3):857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]