Abstract
In the centrosymmetric dinuclear title complex, [Zn2(C13H19N4O)2(C2H5OH)2](ClO4)2, the ZnII atom is in a distorted trigonal-bipyramidal coordination geometry. The equatorial plane is constructed by one N atom and one O atom from two 1,3-bis(3,5-dimethylpyrazol-1-yl)propan-2-olate (bppo) ligands and one O atom from an ethanol molecule. One N atom and one O atom from the two bppo ligands occupy the axial positions. Intermolecular O—H⋯O hydrogen bonds between the ethanol molecules and perchlorate anions, and O⋯π interactions between the perchlorate anions and pyrazole rings [O⋯centroid distances = 3.494 (3) and 3.413 (3) Å], lead to a chain structure along [010].
Related literature
For related structures, see: Montoya et al. (2007 ▶).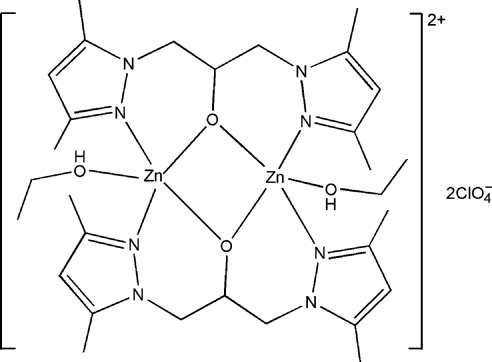
Experimental
Crystal data
[Zn2(C13H19N4O)2(C2H6O)2](ClO4)2
M r = 916.42
Triclinic,

a = 8.8570 (18) Å
b = 11.148 (2) Å
c = 11.300 (2) Å
α = 111.13 (3)°
β = 100.40 (3)°
γ = 100.11 (3)°
V = 987.8 (5) Å3
Z = 1
Mo Kα radiation
μ = 1.42 mm−1
T = 293 K
0.22 × 0.20 × 0.20 mm
Data collection
Bruker APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.765, T max = 0.765
7788 measured reflections
3482 independent reflections
3172 reflections with I > 2σ(I)
R int = 0.017
Refinement
R[F 2 > 2σ(F 2)] = 0.030
wR(F 2) = 0.083
S = 1.07
3482 reflections
249 parameters
2 restraints
H-atom parameters constrained
Δρmax = 0.50 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶) and DIAMOND (Brandenburg, 1999 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681004479X/hy2370sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681004479X/hy2370Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Zn1—N1 | 2.076 (2) |
| Zn1—N3i | 2.042 (2) |
| Zn1—O6 | 1.9908 (16) |
| Zn1—O6i | 2.0428 (16) |
| Zn1—O7 | 2.1292 (18) |
Symmetry code: (i)  .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O7—H7A⋯O3 | 0.85 | 2.03 | 2.860 (3) | 165 |
Acknowledgments
We are grateful for support from Henan University of Urban Construction.
supplementary crystallographic information
Comment
Pyrazole-derived ligands have been extensively studied in recent years. These ligands are known as anionic or neutral groups to coordinate to metal centers through N atoms in monodentate and exobidentate modes. It is essential to study the syntheses and crystal structures of the complexes formed by pyrazole systematically, and to inquire into the factors that influence the formation and structure of such complexes. Such studies may lead to the design and synthesis of functional materials, and also provide a theoretical foundation for supramolecular chemistry and crystal engineering (Montoya et al., 2007). As part of our studies on the synthesis and characterization of these compounds, we report here the synthesis and crystal structure of the title compound.
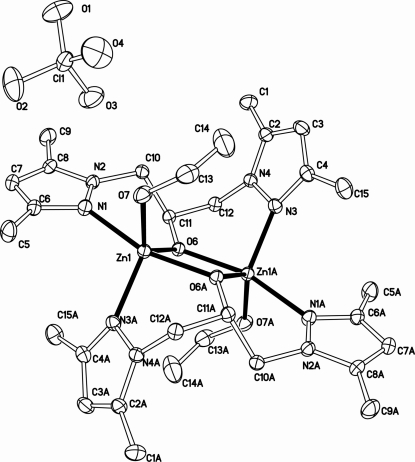
In the title compound (Fig. 1), the ZnII atom is five-coordinated by two O atoms and two N atoms from two 1,3-bis(3,5-dimethyl-pyrazol-1-yl)propan-2-olate (bppo) ligands and one O atom from an ethanol molecule in a distorted trigonal–bipyramidal geometry (Table 1). The equatorial plane is constructed by N3i and O6 from the two bppo ligands and O7 from the ethanol molecule. The N1 and O6i atoms occupy the axial positions [symmetry code: (i) 1 - x, -y, -z]. Two hydroxyl O atoms bridge the Zn atoms, forming a dinuclear complex. Intermolecular O—H···O hydrogen bonds between the ethanol molecules and perchlorate anions (Table 2) and O···π interactions between the perchlorate anions and pyrazole rings, O2···Cg1ii and O3···Cg2, [Cg1 and Cg2 are the centroids of C2/C3/C4/N3/N4 ring and C6/C7/C8/N1/N2 ring; symmetry code: (ii) x, -1 + y, z; O—centroid distances = 3.494 (3) and 3.413 (3) Å, respectively], lead to a chain structure along [010] (Fig. 2).
Experimental
1,3-Bis(3,5-dimethyl-pyrazol-1-yl)propan-2-ol and ZnCl2.6H2O were available commercially and were used without further purification. 1,3-Bis(3,5-dimethyl-pyrazol-1-yl)propan-2-ol (124 mg, 0.5 mmol) were dissolved in anhydrous alcohol (15 ml). To this solution was added ZuCl2.6H2O (122 mg, 0.5 mmol) in anhydrous alcohol (10 ml). After keeping the resulting solution in air to evaporate about half of the solvent, blue prismatic crystals of the title compound were formed. The crystals were isolated, washed with alcohol three times and dried in a vacuum desiccator using silica gel (yield: 75%). Analysis, calculated for C30H50Cl2N8O12Zn2: C 39.32, H 5.50, N, 12.23%; found: C 39.42, H 5.28, N 12.35%.
Refinement
H atoms on C atoms were positioned geometrically and refined as riding atoms, with C—H = 0.93–0.98 Å and Uiso(H) = 1.2(1.5 for methyl)Ueq(C). Hydroxy H atom was located in a difference Fourier map and refined as a riding atom, with O—H = 0.85 Å and Uiso(H) = 1.5Ueq(O).
Figures
Fig. 1.
Molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity. [Symmetry code: (A) 1 - x, -y, -z.]
Fig. 2.
The chain structure in the title compound. Dashed lines denote O···π interactions.
Crystal data
| [Zn2(C13H19N4O)2(C2H6O)2](ClO4)2 | Z = 1 |
| Mr = 916.42 | F(000) = 476 |
| Triclinic, P1 | Dx = 1.541 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.8570 (18) Å | Cell parameters from 2230 reflections |
| b = 11.148 (2) Å | θ = 2.3–25.7° |
| c = 11.300 (2) Å | µ = 1.42 mm−1 |
| α = 111.13 (3)° | T = 293 K |
| β = 100.40 (3)° | Block, colourless |
| γ = 100.11 (3)° | 0.22 × 0.20 × 0.20 mm |
| V = 987.8 (5) Å3 |
Data collection
| Bruker APEX CCD diffractometer | 3482 independent reflections |
| Radiation source: fine-focus sealed tube | 3172 reflections with I > 2σ(I) |
| graphite | Rint = 0.017 |
| φ and ω scans | θmax = 25.0°, θmin = 3.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −10→10 |
| Tmin = 0.765, Tmax = 0.765 | k = −13→13 |
| 7788 measured reflections | l = −13→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.030 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.083 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0486P)2 + 0.4173P] where P = (Fo2 + 2Fc2)/3 |
| 3482 reflections | (Δ/σ)max < 0.001 |
| 249 parameters | Δρmax = 0.50 e Å−3 |
| 2 restraints | Δρmin = −0.22 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Zn1 | 0.44360 (3) | −0.13668 (2) | 0.00434 (2) | 0.03250 (11) | |
| N1 | 0.3373 (2) | −0.18995 (19) | 0.13387 (19) | 0.0385 (4) | |
| N2 | 0.3198 (2) | −0.09350 (19) | 0.24246 (19) | 0.0364 (4) | |
| O6 | 0.40890 (17) | 0.04471 (14) | 0.07488 (15) | 0.0331 (3) | |
| O7 | 0.6260 (2) | −0.21497 (19) | 0.07459 (19) | 0.0528 (5) | |
| H7A | 0.6208 | −0.2359 | 0.1393 | 0.079* | |
| C5 | 0.2447 (4) | −0.4353 (3) | 0.0179 (3) | 0.0597 (7) | |
| H5A | 0.2756 | −0.4202 | −0.0538 | 0.089* | |
| H5B | 0.1397 | −0.4941 | −0.0135 | 0.089* | |
| H5C | 0.3182 | −0.4748 | 0.0544 | 0.089* | |
| C6 | 0.2460 (3) | −0.3054 (2) | 0.1218 (2) | 0.0413 (5) | |
| C7 | 0.1694 (3) | −0.2813 (3) | 0.2213 (3) | 0.0453 (6) | |
| H7 | 0.0970 | −0.3442 | 0.2334 | 0.054* | |
| C8 | 0.2202 (3) | −0.1478 (3) | 0.2983 (2) | 0.0413 (5) | |
| C9 | 0.1855 (4) | −0.0683 (3) | 0.4239 (3) | 0.0606 (8) | |
| H9A | 0.2773 | −0.0431 | 0.4961 | 0.091* | |
| H9B | 0.0971 | −0.1210 | 0.4368 | 0.091* | |
| H9C | 0.1599 | 0.0104 | 0.4193 | 0.091* | |
| C10 | 0.4166 (3) | 0.0433 (2) | 0.2899 (2) | 0.0381 (5) | |
| H10A | 0.5279 | 0.0435 | 0.3030 | 0.046* | |
| H10B | 0.4016 | 0.0957 | 0.3743 | 0.046* | |
| C11 | 0.3753 (3) | 0.1084 (2) | 0.1951 (2) | 0.0331 (5) | |
| H11 | 0.2605 | 0.0997 | 0.1770 | 0.040* | |
| C13 | 0.7924 (3) | −0.1751 (4) | 0.0899 (3) | 0.0641 (8) | |
| H13A | 0.8108 | −0.1760 | 0.0076 | 0.077* | |
| H13B | 0.8423 | −0.2383 | 0.1110 | 0.077* | |
| C14 | 0.8653 (5) | −0.0412 (4) | 0.1945 (6) | 0.1128 (18) | |
| H14A | 0.8059 | 0.0190 | 0.1808 | 0.169* | |
| H14B | 0.9729 | −0.0113 | 0.1926 | 0.169* | |
| H14C | 0.8647 | −0.0439 | 0.2783 | 0.169* | |
| C12 | 0.4601 (3) | 0.2576 (2) | 0.2567 (2) | 0.0367 (5) | |
| H12A | 0.4222 | 0.2976 | 0.1977 | 0.044* | |
| H12B | 0.4328 | 0.2998 | 0.3385 | 0.044* | |
| N3 | 0.7009 (2) | 0.25422 (18) | 0.17989 (19) | 0.0366 (4) | |
| N4 | 0.6328 (2) | 0.28347 (18) | 0.28297 (18) | 0.0359 (4) | |
| C1 | 0.7043 (4) | 0.3867 (3) | 0.5278 (3) | 0.0630 (8) | |
| H1A | 0.6439 | 0.4506 | 0.5295 | 0.094* | |
| H1B | 0.8007 | 0.4283 | 0.5977 | 0.094* | |
| H1C | 0.6428 | 0.3136 | 0.5389 | 0.094* | |
| C2 | 0.7442 (3) | 0.3366 (2) | 0.3990 (2) | 0.0437 (6) | |
| C3 | 0.8885 (3) | 0.3381 (3) | 0.3704 (3) | 0.0501 (6) | |
| H3 | 0.9877 | 0.3674 | 0.4306 | 0.060* | |
| C15 | 0.9736 (3) | 0.2689 (3) | 0.1524 (3) | 0.0614 (8) | |
| H15A | 0.9241 | 0.1944 | 0.0696 | 0.092* | |
| H15B | 1.0653 | 0.2525 | 0.1974 | 0.092* | |
| H15C | 1.0055 | 0.3479 | 0.1373 | 0.092* | |
| C4 | 0.8579 (3) | 0.2875 (2) | 0.2346 (3) | 0.0433 (6) | |
| O1 | 0.7169 (4) | −0.2927 (3) | 0.4897 (3) | 0.1003 (9) | |
| O2 | 0.5741 (4) | −0.4532 (3) | 0.2842 (4) | 0.1233 (12) | |
| O3 | 0.6271 (4) | −0.2330 (3) | 0.3209 (3) | 0.0955 (9) | |
| O4 | 0.8318 (4) | −0.3383 (4) | 0.3177 (3) | 0.1242 (12) | |
| Cl1 | 0.68858 (8) | −0.33052 (6) | 0.35237 (7) | 0.05214 (18) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.03703 (16) | 0.02905 (16) | 0.03013 (16) | 0.00679 (10) | 0.01162 (11) | 0.01013 (11) |
| N1 | 0.0468 (11) | 0.0325 (10) | 0.0367 (11) | 0.0091 (8) | 0.0174 (9) | 0.0121 (8) |
| N2 | 0.0422 (10) | 0.0341 (10) | 0.0325 (10) | 0.0070 (8) | 0.0130 (8) | 0.0131 (8) |
| O6 | 0.0396 (8) | 0.0292 (8) | 0.0296 (8) | 0.0076 (6) | 0.0132 (6) | 0.0099 (6) |
| O7 | 0.0477 (10) | 0.0682 (12) | 0.0590 (12) | 0.0215 (9) | 0.0158 (9) | 0.0403 (10) |
| C5 | 0.078 (2) | 0.0344 (14) | 0.0638 (19) | 0.0087 (13) | 0.0274 (16) | 0.0154 (13) |
| C6 | 0.0462 (13) | 0.0354 (13) | 0.0418 (14) | 0.0060 (10) | 0.0114 (11) | 0.0175 (11) |
| C7 | 0.0459 (13) | 0.0442 (14) | 0.0467 (15) | 0.0021 (11) | 0.0137 (11) | 0.0231 (12) |
| C8 | 0.0449 (13) | 0.0474 (14) | 0.0365 (13) | 0.0088 (10) | 0.0155 (10) | 0.0218 (11) |
| C9 | 0.0747 (19) | 0.0637 (18) | 0.0458 (16) | 0.0113 (15) | 0.0313 (15) | 0.0207 (14) |
| C10 | 0.0417 (12) | 0.0356 (12) | 0.0318 (12) | 0.0043 (9) | 0.0096 (10) | 0.0104 (10) |
| C11 | 0.0325 (11) | 0.0335 (11) | 0.0330 (12) | 0.0086 (9) | 0.0139 (9) | 0.0105 (9) |
| C13 | 0.0497 (16) | 0.099 (3) | 0.066 (2) | 0.0353 (16) | 0.0243 (15) | 0.0470 (19) |
| C14 | 0.068 (2) | 0.086 (3) | 0.177 (5) | 0.002 (2) | −0.007 (3) | 0.072 (3) |
| C12 | 0.0416 (12) | 0.0318 (12) | 0.0366 (13) | 0.0108 (9) | 0.0159 (10) | 0.0107 (10) |
| N3 | 0.0364 (10) | 0.0339 (10) | 0.0340 (10) | 0.0066 (8) | 0.0112 (8) | 0.0079 (8) |
| N4 | 0.0401 (10) | 0.0319 (10) | 0.0304 (10) | 0.0054 (8) | 0.0095 (8) | 0.0082 (8) |
| C1 | 0.080 (2) | 0.0609 (18) | 0.0336 (15) | 0.0046 (15) | 0.0084 (14) | 0.0131 (13) |
| C2 | 0.0550 (15) | 0.0314 (12) | 0.0344 (13) | 0.0021 (10) | 0.0037 (11) | 0.0098 (10) |
| C3 | 0.0450 (14) | 0.0431 (14) | 0.0467 (16) | 0.0014 (11) | −0.0047 (12) | 0.0134 (12) |
| C15 | 0.0406 (14) | 0.0656 (19) | 0.075 (2) | 0.0121 (13) | 0.0207 (14) | 0.0229 (16) |
| C4 | 0.0392 (12) | 0.0352 (13) | 0.0489 (15) | 0.0062 (10) | 0.0078 (11) | 0.0133 (11) |
| O1 | 0.135 (2) | 0.135 (3) | 0.0666 (17) | 0.065 (2) | 0.0451 (17) | 0.0586 (18) |
| O2 | 0.125 (2) | 0.0526 (16) | 0.163 (3) | −0.0057 (15) | 0.031 (2) | 0.0271 (18) |
| O3 | 0.140 (2) | 0.0671 (16) | 0.0824 (18) | 0.0311 (16) | 0.0106 (17) | 0.0403 (14) |
| O4 | 0.088 (2) | 0.178 (3) | 0.116 (3) | 0.040 (2) | 0.0609 (19) | 0.051 (2) |
| Cl1 | 0.0664 (4) | 0.0457 (4) | 0.0509 (4) | 0.0129 (3) | 0.0249 (3) | 0.0234 (3) |
Geometric parameters (Å, °)
| Zn1—N1 | 2.076 (2) | C11—H11 | 0.9800 |
| Zn1—N3i | 2.042 (2) | C13—C14 | 1.468 (6) |
| Zn1—O6 | 1.9908 (16) | C13—H13A | 0.9700 |
| Zn1—O6i | 2.0428 (16) | C13—H13B | 0.9700 |
| Zn1—O7 | 2.1292 (18) | C14—H14A | 0.9600 |
| Zn1—Zn1i | 3.0784 (9) | C14—H14B | 0.9600 |
| N1—C6 | 1.340 (3) | C14—H14C | 0.9600 |
| N1—N2 | 1.364 (3) | C12—N4 | 1.460 (3) |
| N2—C8 | 1.353 (3) | C12—H12A | 0.9700 |
| N2—C10 | 1.461 (3) | C12—H12B | 0.9700 |
| O6—C11 | 1.401 (3) | N3—C4 | 1.341 (3) |
| O6—Zn1i | 2.0428 (16) | N3—N4 | 1.370 (3) |
| O7—C13 | 1.422 (3) | N3—Zn1i | 2.042 (2) |
| O7—H7A | 0.8500 | N4—C2 | 1.346 (3) |
| C5—C6 | 1.496 (4) | C1—C2 | 1.491 (4) |
| C5—H5A | 0.9600 | C1—H1A | 0.9600 |
| C5—H5B | 0.9600 | C1—H1B | 0.9600 |
| C5—H5C | 0.9600 | C1—H1C | 0.9600 |
| C6—C7 | 1.385 (4) | C2—C3 | 1.372 (4) |
| C7—C8 | 1.366 (4) | C3—C4 | 1.385 (4) |
| C7—H7 | 0.9300 | C3—H3 | 0.9300 |
| C8—C9 | 1.497 (4) | C15—C4 | 1.495 (4) |
| C9—H9A | 0.9600 | C15—H15A | 0.9600 |
| C9—H9B | 0.9600 | C15—H15B | 0.9600 |
| C9—H9C | 0.9600 | C15—H15C | 0.9600 |
| C10—C11 | 1.522 (3) | O1—Cl1 | 1.414 (3) |
| C10—H10A | 0.9700 | O2—Cl1 | 1.401 (3) |
| C10—H10B | 0.9700 | O3—Cl1 | 1.422 (3) |
| C11—C12 | 1.534 (3) | O4—Cl1 | 1.402 (3) |
| O6—Zn1—N3i | 112.71 (8) | C10—C11—C12 | 111.32 (19) |
| O6—Zn1—O6i | 80.52 (7) | O6—C11—H11 | 107.6 |
| N3i—Zn1—O6i | 89.87 (7) | C10—C11—H11 | 107.6 |
| O6—Zn1—N1 | 91.69 (7) | C12—C11—H11 | 107.6 |
| N3i—Zn1—N1 | 106.21 (8) | O7—C13—C14 | 111.5 (3) |
| O6i—Zn1—N1 | 163.86 (7) | O7—C13—H13A | 109.3 |
| O6—Zn1—O7 | 130.72 (8) | C14—C13—H13A | 109.3 |
| N3i—Zn1—O7 | 115.77 (8) | O7—C13—H13B | 109.3 |
| O6i—Zn1—O7 | 91.14 (7) | C14—C13—H13B | 109.3 |
| N1—Zn1—O7 | 83.23 (8) | H13A—C13—H13B | 108.0 |
| O6—Zn1—Zn1i | 40.88 (4) | C13—C14—H14A | 109.5 |
| N3i—Zn1—Zn1i | 104.37 (6) | C13—C14—H14B | 109.5 |
| O6i—Zn1—Zn1i | 39.63 (4) | H14A—C14—H14B | 109.5 |
| N1—Zn1—Zn1i | 131.04 (6) | C13—C14—H14C | 109.5 |
| O7—Zn1—Zn1i | 115.79 (6) | H14A—C14—H14C | 109.5 |
| C6—N1—N2 | 106.23 (19) | H14B—C14—H14C | 109.5 |
| C6—N1—Zn1 | 132.19 (16) | N4—C12—C11 | 112.81 (18) |
| N2—N1—Zn1 | 119.85 (14) | N4—C12—H12A | 109.0 |
| C8—N2—N1 | 110.49 (19) | C11—C12—H12A | 109.0 |
| C8—N2—C10 | 129.5 (2) | N4—C12—H12B | 109.0 |
| N1—N2—C10 | 119.68 (18) | C11—C12—H12B | 109.0 |
| C11—O6—Zn1 | 126.98 (13) | H12A—C12—H12B | 107.8 |
| C11—O6—Zn1i | 124.67 (13) | C4—N3—N4 | 105.54 (19) |
| Zn1—O6—Zn1i | 99.48 (7) | C4—N3—Zn1i | 134.36 (17) |
| C13—O7—Zn1 | 128.78 (17) | N4—N3—Zn1i | 117.88 (14) |
| C13—O7—H7A | 103.2 | C2—N4—N3 | 111.10 (19) |
| Zn1—O7—H7A | 118.4 | C2—N4—C12 | 129.4 (2) |
| C6—C5—H5A | 109.5 | N3—N4—C12 | 119.52 (18) |
| C6—C5—H5B | 109.5 | C2—C1—H1A | 109.5 |
| H5A—C5—H5B | 109.5 | C2—C1—H1B | 109.5 |
| C6—C5—H5C | 109.5 | H1A—C1—H1B | 109.5 |
| H5A—C5—H5C | 109.5 | C2—C1—H1C | 109.5 |
| H5B—C5—H5C | 109.5 | H1A—C1—H1C | 109.5 |
| N1—C6—C7 | 109.4 (2) | H1B—C1—H1C | 109.5 |
| N1—C6—C5 | 121.1 (2) | N4—C2—C3 | 106.6 (2) |
| C7—C6—C5 | 129.4 (2) | N4—C2—C1 | 122.5 (2) |
| C8—C7—C6 | 107.2 (2) | C3—C2—C1 | 130.8 (2) |
| C8—C7—H7 | 126.4 | C2—C3—C4 | 106.8 (2) |
| C6—C7—H7 | 126.4 | C2—C3—H3 | 126.6 |
| N2—C8—C7 | 106.7 (2) | C4—C3—H3 | 126.6 |
| N2—C8—C9 | 123.0 (2) | C4—C15—H15A | 109.5 |
| C7—C8—C9 | 130.2 (2) | C4—C15—H15B | 109.5 |
| C8—C9—H9A | 109.5 | H15A—C15—H15B | 109.5 |
| C8—C9—H9B | 109.5 | C4—C15—H15C | 109.5 |
| H9A—C9—H9B | 109.5 | H15A—C15—H15C | 109.5 |
| C8—C9—H9C | 109.5 | H15B—C15—H15C | 109.5 |
| H9A—C9—H9C | 109.5 | N3—C4—C3 | 109.9 (2) |
| H9B—C9—H9C | 109.5 | N3—C4—C15 | 121.5 (2) |
| N2—C10—C11 | 112.67 (19) | C3—C4—C15 | 128.6 (2) |
| N2—C10—H10A | 109.1 | O2—Cl1—O4 | 110.6 (2) |
| C11—C10—H10A | 109.1 | O2—Cl1—O1 | 110.3 (2) |
| N2—C10—H10B | 109.1 | O4—Cl1—O1 | 109.0 (2) |
| C11—C10—H10B | 109.1 | O2—Cl1—O3 | 107.9 (2) |
| H10A—C10—H10B | 107.8 | O4—Cl1—O3 | 110.9 (2) |
| O6—C11—C10 | 111.91 (18) | O1—Cl1—O3 | 108.07 (18) |
| O6—C11—C12 | 110.58 (18) | ||
| O6—Zn1—N1—C6 | 144.8 (2) | C10—N2—C8—C7 | −174.6 (2) |
| N3i—Zn1—N1—C6 | 30.5 (2) | N1—N2—C8—C9 | 176.0 (2) |
| O6i—Zn1—N1—C6 | −154.6 (2) | C10—N2—C8—C9 | 3.1 (4) |
| O7—Zn1—N1—C6 | −84.4 (2) | C6—C7—C8—N2 | 2.3 (3) |
| Zn1i—Zn1—N1—C6 | 157.08 (19) | C6—C7—C8—C9 | −175.3 (3) |
| O6—Zn1—N1—N2 | −18.00 (17) | C8—N2—C10—C11 | −120.3 (3) |
| N3i—Zn1—N1—N2 | −132.25 (17) | N1—N2—C10—C11 | 67.4 (3) |
| O6i—Zn1—N1—N2 | 42.6 (3) | Zn1—O6—C11—C10 | 17.7 (2) |
| O7—Zn1—N1—N2 | 112.83 (18) | Zn1i—O6—C11—C10 | −122.81 (16) |
| Zn1i—Zn1—N1—N2 | −5.7 (2) | Zn1—O6—C11—C12 | 142.41 (15) |
| C6—N1—N2—C8 | 0.5 (3) | Zn1i—O6—C11—C12 | 1.9 (2) |
| Zn1—N1—N2—C8 | 167.30 (16) | N2—C10—C11—O6 | −64.4 (2) |
| C6—N1—N2—C10 | 174.2 (2) | N2—C10—C11—C12 | 171.27 (17) |
| Zn1—N1—N2—C10 | −19.0 (3) | Zn1—O7—C13—C14 | 67.8 (4) |
| N3i—Zn1—O6—C11 | 126.18 (16) | O6—C11—C12—N4 | −60.2 (2) |
| O6i—Zn1—O6—C11 | −147.97 (19) | C10—C11—C12—N4 | 64.9 (2) |
| N1—Zn1—O6—C11 | 17.82 (17) | C4—N3—N4—C2 | 1.5 (2) |
| O7—Zn1—O6—C11 | −64.70 (18) | Zn1i—N3—N4—C2 | 166.94 (15) |
| Zn1i—Zn1—O6—C11 | −147.97 (19) | C4—N3—N4—C12 | −179.55 (19) |
| N3i—Zn1—O6—Zn1i | −85.85 (8) | Zn1i—N3—N4—C12 | −14.1 (2) |
| O6i—Zn1—O6—Zn1i | 0.0 | C11—C12—N4—C2 | −111.0 (3) |
| N1—Zn1—O6—Zn1i | 165.79 (8) | C11—C12—N4—N3 | 70.2 (3) |
| O7—Zn1—O6—Zn1i | 83.27 (10) | N3—N4—C2—C3 | −1.9 (3) |
| O6—Zn1—O7—C13 | −62.0 (3) | C12—N4—C2—C3 | 179.3 (2) |
| N3i—Zn1—O7—C13 | 106.8 (2) | N3—N4—C2—C1 | 175.6 (2) |
| O6i—Zn1—O7—C13 | 16.4 (2) | C12—N4—C2—C1 | −3.3 (4) |
| N1—Zn1—O7—C13 | −148.4 (2) | N4—C2—C3—C4 | 1.5 (3) |
| Zn1i—Zn1—O7—C13 | −15.8 (2) | C1—C2—C3—C4 | −175.6 (3) |
| N2—N1—C6—C7 | 1.0 (3) | N4—N3—C4—C3 | −0.5 (3) |
| Zn1—N1—C6—C7 | −163.53 (18) | Zn1i—N3—C4—C3 | −162.39 (18) |
| N2—N1—C6—C5 | −175.6 (2) | N4—N3—C4—C15 | 179.5 (2) |
| Zn1—N1—C6—C5 | 20.0 (4) | Zn1i—N3—C4—C15 | 17.6 (4) |
| N1—C6—C7—C8 | −2.0 (3) | C2—C3—C4—N3 | −0.7 (3) |
| C5—C6—C7—C8 | 174.1 (3) | C2—C3—C4—C15 | 179.4 (3) |
| N1—N2—C8—C7 | −1.8 (3) |
Symmetry codes: (i) −x+1, −y, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O7—H7A···O3 | 0.85 | 2.03 | 2.860 (3) | 165 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2370).
References
- Brandenburg, K. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2007). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Montoya, V., Pons, J., Garcia-Antón, J., Solans, X., Font-Bardia, M. & Ros, J. (2007). Inorg. Chim. Acta, 360, 625–637.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681004479X/hy2370sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681004479X/hy2370Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report