Abstract
The study of the effect of programmed cessation of transcription in a large nuclear domain, on the distribution of elements of the pre-mRNA splicing machinery, is the main aim of this paper. To this end, we took advantage of the nuclear partitioning of mouse spermatocytes early in meiosis into autosomal transcribing and XY nontranscribing compartments. This system also allows to extend this study to stages in sperm differentiation that are accompanied by reduction and eventual cessation of transcription. We show by indirect immunofluorescence in spermatogenetic cells that 1) fluorescent signals of the pre-mRNA splicing factors SF53/4 and SC35, of the Sm antigens, and of RNA polymerase II, are largely absent from the nontranscribing, X-inactivated compartment, but are abundantly present in the transcribing autosomal compartment and 2) the presence, gradual reduction, and absence of transcriptive activity in nuclei undergoing the sperm formation sequence are positively correlated with the fluorescence patterns of the antibodies against SF53/4, SC35, and the Sm antigens. These data suggest that cessation of transcription during spermatogenesis is accompanied by exclusion of the splicing machinery from nontranscribing chromatin to its vicinity.
Full text
PDF


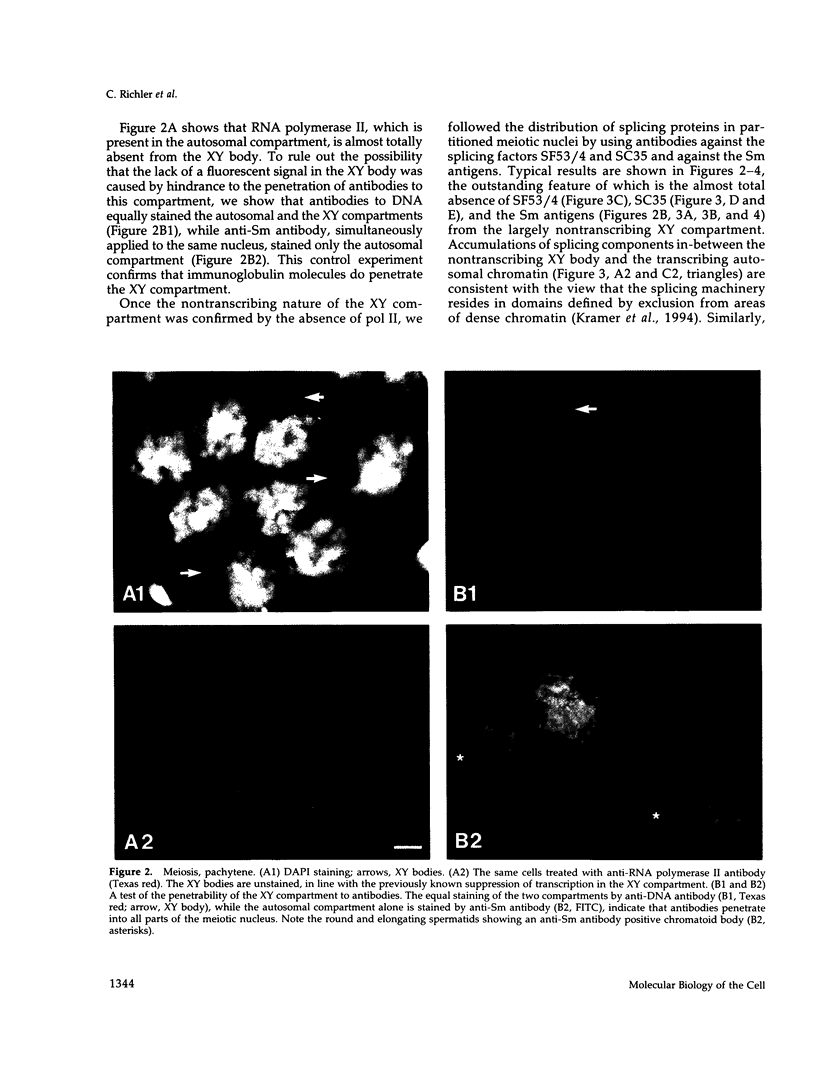
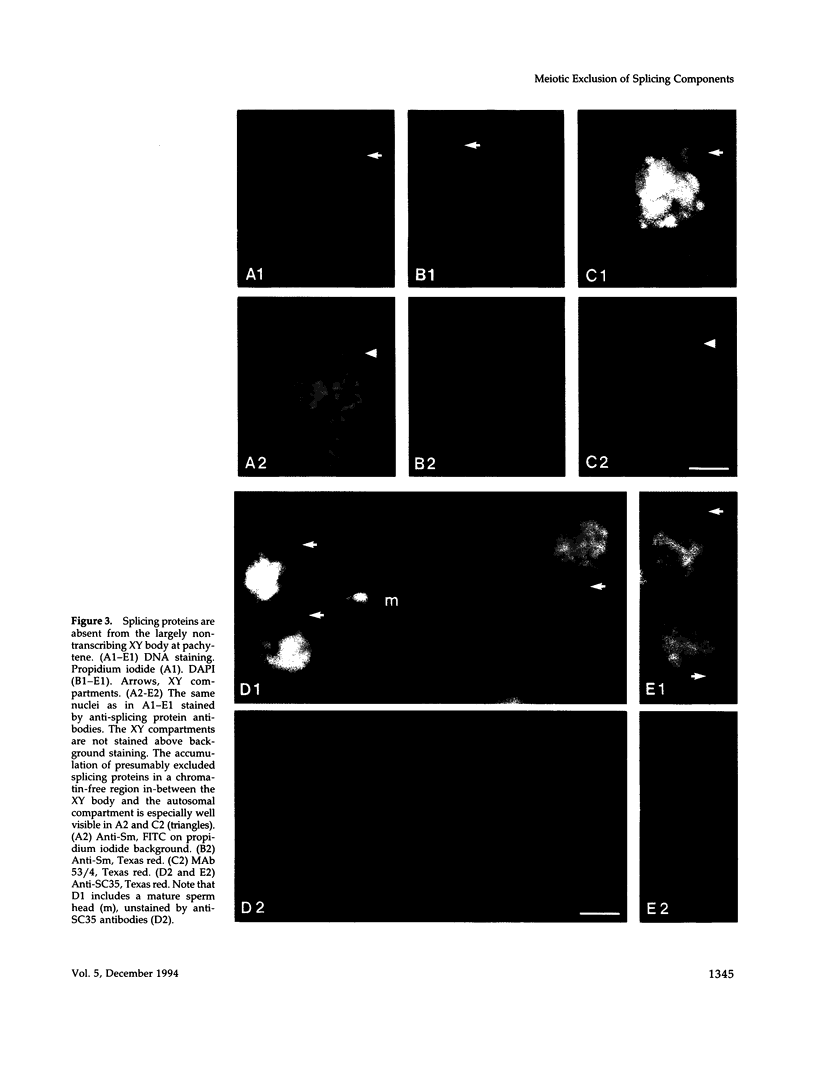







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arad-Dann H., Isenberg D. A., Shoenfeld Y., Offen D., Sperling J., Sperling R. Autoantibodies against a specific nuclear RNP protein in sera of patients with autoimmune rheumatic diseases associated with myositis. J Immunol. 1987 Apr 15;138(8):2463–2468. [PubMed] [Google Scholar]
- Ast G., Goldblatt D., Offen D., Sperling J., Sperling R. A novel splicing factor is an integral component of 200S large nuclear ribonucleoprotein (InRNP) particles. EMBO J. 1991 Feb;10(2):425–432. doi: 10.1002/j.1460-2075.1991.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurén G., Wieslander L. Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell. 1994 Jan 14;76(1):183–192. doi: 10.1016/0092-8674(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Biggiogera M., Fakan S., Leser G., Martin T. E., Gordon J. Immunoelectron microscopical visualization of ribonucleoproteins in the chromatoid body of mouse spermatids. Mol Reprod Dev. 1990 Jun;26(2):150–158. doi: 10.1002/mrd.1080260209. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Taneja K. L., Lawrence J. B. Discrete nuclear domains of poly(A) RNA and their relationship to the functional organization of the nucleus. J Cell Biol. 1991 Dec;115(5):1191–1202. doi: 10.1083/jcb.115.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. The chromatoid body in mouse spermatogenesis: evidence that it may be formed by the extrusion of nucleolar components. J Ultrastruct Res. 1972 Apr;39(1):15–23. doi: 10.1016/s0022-5320(72)80003-0. [DOI] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Eliceiri G. L., Ryerse J. S. Detection of intranuclear clusters of Sm antigens with monoclonal anti-Sm antibodies by immunoelectron microscopy. J Cell Physiol. 1984 Nov;121(2):449–451. doi: 10.1002/jcp.1041210226. [DOI] [PubMed] [Google Scholar]
- Erickson R. P. Post-meiotic gene expression. Trends Genet. 1990 Aug;6(8):264–269. doi: 10.1016/0168-9525(90)90209-o. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. M. Light microscope analysis of meiotic prophase chromosomes by silver staining. Chromosoma. 1979 Apr 30;72(2):241–248. doi: 10.1007/BF00293237. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Callan H. G. The sphere organelle contains small nuclear ribonucleoproteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6635–6639. doi: 10.1073/pnas.86.17.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafar H., Gabriel-Robez O., Rumpler Y. Pattern of ribonucleic acid synthesis in vitro in primary spermatocytes from mouse testis carrying an X-autosome translocation. Chromosoma. 1989 Nov;98(5):330–334. doi: 10.1007/BF00292385. [DOI] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Kramer J., Zachar Z., Bingham P. M. Nuclear pre-mRNA metabolism: channels and tracks. Trends Cell Biol. 1994 Feb;4(2):35–37. doi: 10.1016/0962-8924(94)90001-9. [DOI] [PubMed] [Google Scholar]
- McCarrey J. R., Dilworth D. D. Expression of Xist in mouse germ cells correlates with X-chromosome inactivation. Nat Genet. 1992 Nov;2(3):200–203. doi: 10.1038/ng1192-200. [DOI] [PubMed] [Google Scholar]
- Miriami E., Sperling J., Sperling R. Heat shock affects 5' splice site selection, cleavage and ligation of CAD pre-mRNA in hamster cells, but not its packaging in InRNP particles. Nucleic Acids Res. 1994 Aug 11;22(15):3084–3091. doi: 10.1093/nar/22.15.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monesi V. Differential rate of ribonucleic acid synthesis in the autosomes and sex chromosomes during male meiosis in the mouse. Chromosoma. 1965;17(1):11–21. doi: 10.1007/BF00285153. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Richler C., Soreq H., Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992 Nov;2(3):192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Richler C., Uliel E., Rosenmann A., Wahrman J. Chromosomally derived sterile mice have a 'fertile' active XY chromatin conformation but no XY body. Chromosoma. 1989 May;97(6):465–474. doi: 10.1007/BF00295031. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Singer R. H. RNA travel: tracks from DNA to cytoplasm. Cell. 1993 Nov 5;75(3):399–401. doi: 10.1016/0092-8674(93)90373-x. [DOI] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Mohandas T. K., Shapiro L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nat Genet. 1992 Nov;2(3):196–199. doi: 10.1038/ng1192-196. [DOI] [PubMed] [Google Scholar]
- Saunders P. T., Millar M. R., Maguire S. M., Sharpe R. M. Stage-specific expression of rat transition protein 2 mRNA and possible localization to the chromatoid body of step 7 spermatids by in situ hybridization using a nonradioactive riboprobe. Mol Reprod Dev. 1992 Dec;33(4):385–391. doi: 10.1002/mrd.1080330404. [DOI] [PubMed] [Google Scholar]
- Seraphin B., Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989 Oct 20;59(2):349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- Solari A. J. The behavior of the XY pair in mammals. Int Rev Cytol. 1974;38(0):273–317. doi: 10.1016/s0074-7696(08)60928-6. [DOI] [PubMed] [Google Scholar]
- Spann P., Feinerman M., Sperling J., Sperling R. Isolation and visualization of large compact ribonucleoprotein particles of specific nuclear RNAs. Proc Natl Acad Sci U S A. 1989 Jan;86(2):466–470. doi: 10.1073/pnas.86.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Lark G., Huang S. Differences in snRNP localization between transformed and nontransformed cells. Mol Biol Cell. 1992 May;3(5):555–569. doi: 10.1091/mbc.3.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Sperling R., Spann P., Offen D., Sperling J. U1, U2, and U6 small nuclear ribonucleoproteins (snRNPs) are associated with large nuclear RNP particles containing transcripts of an amplified gene in vivo. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6721–6725. doi: 10.1073/pnas.83.18.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R., Sperling J., Levine A. D., Spann P., Stark G. R., Kornberg R. D. Abundant nuclear ribonucleoprotein form of CAD RNA. Mol Cell Biol. 1985 Mar;5(3):569–575. doi: 10.1128/mcb.5.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström K. O., Parvinen M. Incorporation of (3H)uridine by the chromatoid body during rat spermatogenesis. J Cell Biol. 1976 Jul;70(1):239–246. doi: 10.1083/jcb.70.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. H., Wilkie T. M., Tomashefsky P., Bellvé A. R., Simon M. I. Differential gene expression during mouse spermatogenesis. Biol Reprod. 1989 Oct;41(4):729–739. doi: 10.1095/biolreprod41.4.729. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Aronson D. B., Burgess R. R. Purification of eukaryotic RNA polymerase II by immunoaffinity chromatography. Elution of active enzyme with protein stabilizing agents from a polyol-responsive monoclonal antibody. J Biol Chem. 1990 Apr 25;265(12):7069–7077. [PubMed] [Google Scholar]
- Toppari J., Kangasniemi M., Kaipia A., Mali P., Huhtaniemi I., Parvinen M. Stage- and cell-specific gene expression and hormone regulation of the seminiferous epithelium. J Electron Microsc Tech. 1991 Oct;19(2):203–214. doi: 10.1002/jemt.1060190207. [DOI] [PubMed] [Google Scholar]
- Tsvetkov A., Jantsch M., Wu Z., Murphy C., Gall J. G. Transcription on lampbrush chromosome loops in the absence of U2 snRNA. Mol Biol Cell. 1992 Mar;3(3):249–261. doi: 10.1091/mbc.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. L., Cargile C. B., Floy K. M., Delannoy M., Migeon B. R. The Barr body is a looped X chromosome formed by telomere association. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6191–6195. doi: 10.1073/pnas.88.14.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink D. G., Schul W., van der Kraan I., van Steensel B., van Driel R., de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993 Jul;122(2):283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Gall J. G. U7 small nuclear RNA in C snurposomes of the Xenopus germinal vesicle. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6257–6259. doi: 10.1073/pnas.90.13.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. A., Murphy C., Callan H. G., Gall J. G. Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres, and snurposomes. J Cell Biol. 1991 May;113(3):465–483. doi: 10.1083/jcb.113.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Lawrence J. B. Nuclear RNA tracks: structural basis for transcription and splicing? Trends Cell Biol. 1993 Oct;3(10):346–353. doi: 10.1016/0962-8924(93)90105-a. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Kramer J., Mims I. P., Bingham P. M. Evidence for channeled diffusion of pre-mRNAs during nuclear RNA transport in metazoans. J Cell Biol. 1993 May;121(4):729–742. doi: 10.1083/jcb.121.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler A. M., Lane W. S., Stolk J. A., Roth M. B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zirbel R. M., Mathieu U. R., Kurz A., Cremer T., Lichter P. Evidence for a nuclear compartment of transcription and splicing located at chromosome domain boundaries. Chromosome Res. 1993 Jul;1(2):93–106. doi: 10.1007/BF00710032. [DOI] [PubMed] [Google Scholar]