Abstract
The dicyclohexylamine salt of RG108 (N-phthalyl-l-tryptophan) co-crystallizes with a water molecule and a disordered molecule of dimethylformamide (DMF), viz. dicyclohexylaminium (S)-2-(1,3-dioxoisoindolin-2-yl)-3-(1H-indol-3-yl)propanoate dimethylformamide solvate monohydrate, C12H24N+·C19H13N2O4 −·C3H7NO·H2O. The conformation of the deprotonated compound is constrained by charge-assisted strong hydrogen bonds with the dicyclohexylaminium ion and a dense hydrogen-bond network involving co-crystallized solvent molecules. The dihedral angle between the fused ring systems in the anion is 58.35 (4)°.
Related literature
For the synthesis and biological evaluation, see: Brueckner et al. (2005 ▶).
Experimental
Crystal data
C12H24N+·C19H13N2O4 −·C3H7NO·H2O
M r = 606.75
Orthorhombic,
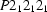
a = 9.0884 (1) Å
b = 15.0206 (3) Å
c = 24.4749 (5) Å
V = 3341.15 (10) Å3
Z = 4
Cu Kα radiation
μ = 0.67 mm−1
T = 293 K
0.55 × 0.04 × 0.03 mm
Data collection
Oxford Diffraction Xcalibur diffractometer with a Ruby (Gemini ultra Cu) detector
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009 ▶) T min = 0.967, T max = 0.981
13362 measured reflections
5552 independent reflections
4936 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.094
S = 1.01
5552 reflections
409 parameters
8 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.30 e Å−3
Δρmin = −0.27 e Å−3
Absolute structure: Flack (1983 ▶), 2160 Friedel pairs
Flack parameter: 0.02 (18)
Data collection: CrysAlis PRO (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681004626X/vm2050sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681004626X/vm2050Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—HN3B⋯O4i | 0.86 (3) | 1.88 (3) | 2.7309 (19) | 169 (2) |
| N2—H2⋯O5ii | 0.86 | 1.97 | 2.813 (3) | 165 |
| N3—HN3A⋯O3iii | 0.99 (3) | 1.79 (3) | 2.7740 (19) | 173 (2) |
| O5—H5B⋯O99 | 0.83 (4) | 1.84 (4) | 2.645 (4) | 164 (4) |
| O5—H5C⋯O1iv | 0.89 (4) | 1.99 (4) | 2.857 (2) | 164 (3) |
Symmetry codes: (i) 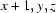 ; (ii)
; (ii) 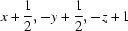 ; (iii)
; (iii) 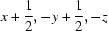 ; (iv)
; (iv) 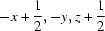 .
.
Acknowledgments
This work was supported in part by the Fonds National de la Recherche Scientifique (FNRS, Belgium). Crystals were obtained during work for the Masters thesis of Miss Isabelle Bouhy. The authors thank Bernadette Norberg for her valuable help during the data collection.
supplementary crystallographic information
Comment
RG108 (N-phthalyl-L-tryptophan) is a DNA methyltransferase (DMNT) inhibitor that was discovered by virtual screening (Brueckner et al., 2005). It reactivates tumor suppressor gene expression in tumor cells by DNA demethylation. RG108 also inhibits human tumor cell line proliferation.
Isomer S (C9) of RG108 is obtained starting from L-tryptophan and phthalic anhydride in DMF.
The unprotonated carboxylate group (both C—O bond lenghts are similar with C19—O3 = 1.246 (2) Å and C19—O4 = 1.247 (2) Å) of RG108 is close to the protonated sp3 nitrogen atom (N3) of the amine (intermolecular O···N distances: O3···N3i = 2.774 (2) Å and O4···N3ii = 2.731 (2) Å; i = -1/2 + x,1/2 - y,-z, ii = -1 + x,y,z, see also Table 1).
A water molecule (O5) has co-crystallized and is involved in the stability of the packing as it forms a network of H-bonds connecting the N—H (N2) of the indole ring of RG108 with a carbonyl function (O1) of the phtalimide ring of a symmetry-related molecule and the oxygen atom (O99) of a molecule of DMF solvent (Table 1).
In addition to H-bonding to the water, the extra (disordered) co-crystallized solvent molecule of DMF is thightly packed in a cavity formed by the aromatic heterocycles of RG108 (the phtalimide and the indole rings).
As a consequence of the dense packing (salt bridge, H-bonds and van der Waals interactions), the two aromatic, planar, heterocycles of RG108 are perpendicular (acute angle between the planes defined by the phtalimide and the indole rings = 58.35 (4)°).
Experimental
Synthesis of the compound was made by micro-ave heating of L-tryptophane and phthalic anhydride in DMF by adapting the procedure described by Brueckner et al. (2005).
Crystals were obtained by evaporation at room temperature of a solution in mixture of methylene chloride and methanol (9/1).
Refinement
The two H atoms of the water molecule and the two H atoms on (protonated) nitrogen N5 were located from ΔF Fourier difference maps and their position refined. All other H atoms were placed at idealized positions and allowed to ride on their parent atoms.
Atoms of a DMF molecule were refined isotropically. Disorder has been taken into account by refining two sets of coordinates (0.7 and 0.3 occupancies respectively) for each atom of the DMF molecule. Bond lengths and valence angles were restrained to be similar in both disordered parts.
Figures
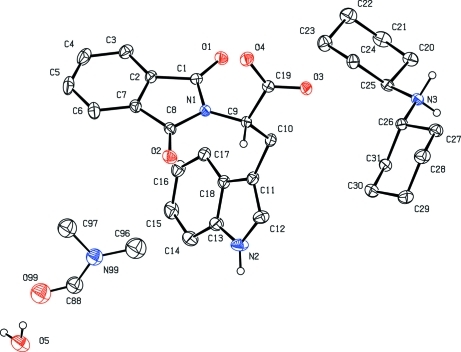
Fig. 1.
ORTEP view (with atom numbering) of the title compound. Only selected H atoms have been retained for clarity (on the chiral carbon, on the protonated nitrogen, and H involved in H-bonds). Displacement ellipsoids for non-H atoms are drawn at the 30% probability level.
Crystal data
| C12H24N+·C19H13N2O4−·C3H7NO·H2O | F(000) = 1304.0 |
| Mr = 606.75 | Dx = 1.206 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 7270 reflections |
| a = 9.0884 (1) Å | θ = 3.5–67.3° |
| b = 15.0206 (3) Å | µ = 0.67 mm−1 |
| c = 24.4749 (5) Å | T = 293 K |
| V = 3341.15 (10) Å3 | Needle, yellow |
| Z = 4 | 0.55 × 0.04 × 0.03 mm |
Data collection
| Oxford Diffraction Xcalibur diffractometer with a Ruby (Gemini ultra Cu) detector | 5552 independent reflections |
| Radiation source: Enhance Ultra (Cu) X-ray Source | 4936 reflections with I > 2σ(I) |
| mirror | Rint = 0.026 |
| Detector resolution: 10.3712 pixels mm-1 | θmax = 67.4°, θmin = 3.5° |
| ω scans | h = −10→9 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009) | k = −15→17 |
| Tmin = 0.967, Tmax = 0.981 | l = −28→29 |
| 13362 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.037 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.094 | [1.00000 + 0.00000exp(0.00(sinθ/λ)2)]/ [σ2(Fo2) + 0.0000 + 0.0000*P + (0.0611P)2 + 0.0400sinθ/λ] where P = 0.33333Fo2 + 0.66667Fc2 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 5552 reflections | Δρmax = 0.30 e Å−3 |
| 409 parameters | Δρmin = −0.27 e Å−3 |
| 8 restraints | Absolute structure: Flack (1983), 2160 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.02 (18) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.28198 (19) | 0.08359 (12) | 0.15901 (7) | 0.0255 (4) | |
| C2 | 0.15124 (19) | 0.04587 (13) | 0.18771 (7) | 0.0281 (4) | |
| C3 | 0.0899 (2) | −0.03769 (15) | 0.18579 (8) | 0.0371 (4) | |
| H3 | 0.1306 | −0.0828 | 0.1646 | 0.044* | |
| C4 | −0.0364 (2) | −0.05175 (17) | 0.21717 (10) | 0.0471 (6) | |
| H4 | −0.0803 | −0.1077 | 0.2171 | 0.056* | |
| C5 | −0.0976 (2) | 0.01558 (18) | 0.24826 (9) | 0.0466 (6) | |
| H5 | −0.1820 | 0.0041 | 0.2686 | 0.056* | |
| C6 | −0.0356 (2) | 0.09991 (17) | 0.24977 (9) | 0.0409 (5) | |
| H6 | −0.0774 | 0.1455 | 0.2703 | 0.049* | |
| C7 | 0.09121 (18) | 0.11355 (14) | 0.21952 (7) | 0.0293 (4) | |
| C8 | 0.18476 (18) | 0.19374 (14) | 0.21386 (7) | 0.0276 (4) | |
| C9 | 0.40737 (17) | 0.23259 (12) | 0.15742 (7) | 0.0245 (4) | |
| H9 | 0.3868 | 0.2900 | 0.1749 | 0.029* | |
| C10 | 0.56294 (17) | 0.20499 (14) | 0.17540 (7) | 0.0283 (4) | |
| H10A | 0.5840 | 0.1463 | 0.1609 | 0.034* | |
| H10B | 0.6334 | 0.2461 | 0.1596 | 0.034* | |
| C11 | 0.58403 (18) | 0.20322 (13) | 0.23613 (8) | 0.0300 (4) | |
| C12 | 0.6295 (2) | 0.27212 (15) | 0.26820 (8) | 0.0382 (5) | |
| H12 | 0.6514 | 0.3289 | 0.2553 | 0.046* | |
| C13 | 0.5969 (2) | 0.15904 (15) | 0.32564 (8) | 0.0365 (4) | |
| C14 | 0.5908 (2) | 0.10309 (17) | 0.37118 (9) | 0.0444 (5) | |
| H14 | 0.6146 | 0.1238 | 0.4059 | 0.053* | |
| C15 | 0.5484 (2) | 0.01611 (17) | 0.36273 (9) | 0.0464 (6) | |
| H15 | 0.5429 | −0.0226 | 0.3923 | 0.056* | |
| C16 | 0.5136 (2) | −0.01489 (15) | 0.31049 (9) | 0.0407 (5) | |
| H16 | 0.4854 | −0.0739 | 0.3059 | 0.049* | |
| C17 | 0.52024 (19) | 0.04037 (14) | 0.26543 (8) | 0.0329 (4) | |
| H17 | 0.4971 | 0.0188 | 0.2309 | 0.039* | |
| C18 | 0.56222 (18) | 0.12900 (14) | 0.27259 (7) | 0.0297 (4) | |
| C19 | 0.39345 (18) | 0.24740 (12) | 0.09508 (7) | 0.0251 (4) | |
| C20 | 1.0315 (2) | 0.06650 (15) | 0.02751 (8) | 0.0362 (4) | |
| H20A | 1.1333 | 0.0726 | 0.0389 | 0.043* | |
| H20B | 1.0264 | 0.0797 | −0.0112 | 0.043* | |
| C21 | 0.9818 (3) | −0.02937 (15) | 0.03696 (10) | 0.0466 (5) | |
| H21A | 1.0377 | −0.0688 | 0.0134 | 0.056* | |
| H21B | 1.0017 | −0.0460 | 0.0745 | 0.056* | |
| C22 | 0.8190 (3) | −0.04061 (16) | 0.02532 (10) | 0.0460 (5) | |
| H22A | 0.7894 | −0.1011 | 0.0338 | 0.055* | |
| H22B | 0.8004 | −0.0301 | −0.0132 | 0.055* | |
| C23 | 0.7290 (2) | 0.02426 (15) | 0.05937 (9) | 0.0417 (5) | |
| H23A | 0.6254 | 0.0169 | 0.0510 | 0.050* | |
| H23B | 0.7431 | 0.0114 | 0.0979 | 0.050* | |
| C24 | 0.7748 (2) | 0.11973 (14) | 0.04766 (8) | 0.0325 (4) | |
| H24A | 0.7539 | 0.1338 | 0.0098 | 0.039* | |
| H24B | 0.7179 | 0.1598 | 0.0704 | 0.039* | |
| C25 | 0.93764 (19) | 0.13371 (13) | 0.05872 (7) | 0.0277 (4) | |
| H25 | 0.9559 | 0.1267 | 0.0979 | 0.033* | |
| C26 | 0.90346 (18) | 0.30325 (13) | 0.06517 (7) | 0.0265 (4) | |
| H26 | 0.7980 | 0.2957 | 0.0582 | 0.032* | |
| C27 | 0.9557 (2) | 0.38741 (14) | 0.03688 (8) | 0.0350 (4) | |
| H27A | 0.9352 | 0.3831 | −0.0019 | 0.042* | |
| H27B | 1.0613 | 0.3931 | 0.0414 | 0.042* | |
| C28 | 0.8803 (3) | 0.46978 (15) | 0.06000 (9) | 0.0430 (5) | |
| H28A | 0.9198 | 0.5225 | 0.0425 | 0.052* | |
| H28B | 0.7758 | 0.4671 | 0.0521 | 0.052* | |
| C29 | 0.9029 (2) | 0.47659 (15) | 0.12145 (9) | 0.0424 (5) | |
| H29A | 1.0063 | 0.4862 | 0.1293 | 0.051* | |
| H29B | 0.8480 | 0.5270 | 0.1356 | 0.051* | |
| C30 | 0.8515 (2) | 0.39192 (16) | 0.14926 (9) | 0.0409 (5) | |
| H30A | 0.7461 | 0.3857 | 0.1443 | 0.049* | |
| H30B | 0.8706 | 0.3963 | 0.1882 | 0.049* | |
| C31 | 0.92799 (19) | 0.30959 (14) | 0.12677 (7) | 0.0317 (4) | |
| H31A | 0.8892 | 0.2568 | 0.1445 | 0.038* | |
| H31B | 1.0326 | 0.3129 | 0.1344 | 0.038* | |
| N1 | 0.29621 (15) | 0.17046 (10) | 0.17706 (6) | 0.0251 (3) | |
| N2 | 0.6388 (2) | 0.24668 (12) | 0.32209 (7) | 0.0429 (4) | |
| H2 | 0.6661 | 0.2799 | 0.3489 | 0.051* | |
| N3 | 0.98457 (16) | 0.22521 (11) | 0.04155 (6) | 0.0269 (3) | |
| O1 | 0.36330 (14) | 0.04770 (9) | 0.12646 (6) | 0.0342 (3) | |
| O2 | 0.17295 (15) | 0.26576 (10) | 0.23543 (6) | 0.0374 (3) | |
| O3 | 0.50423 (13) | 0.27765 (10) | 0.07154 (5) | 0.0331 (3) | |
| O4 | 0.27256 (13) | 0.23090 (11) | 0.07320 (6) | 0.0383 (3) | |
| O5 | 0.1772 (2) | 0.13211 (12) | 0.59148 (8) | 0.0539 (4) | |
| O99 | 0.0737 (4) | 0.1983 (3) | 0.49899 (14) | 0.0750 (9)* | 0.70 |
| N99 | 0.1552 (5) | 0.2310 (3) | 0.41554 (16) | 0.0510 (11)* | 0.70 |
| C88 | 0.1620 (4) | 0.2142 (3) | 0.46712 (15) | 0.0517 (8)* | 0.70 |
| H88 | 0.2571 | 0.2153 | 0.4812 | 0.062* | 0.70 |
| C96 | 0.2644 (6) | 0.2521 (4) | 0.3781 (2) | 0.0796 (13)* | 0.70 |
| H96A | 0.2211 | 0.2610 | 0.3428 | 0.119* | 0.70 |
| H96B | 0.3342 | 0.2042 | 0.3762 | 0.119* | 0.70 |
| H96C | 0.3136 | 0.3056 | 0.3894 | 0.119* | 0.70 |
| C97 | −0.0037 (4) | 0.2278 (3) | 0.39149 (17) | 0.0656 (10)* | 0.70 |
| H97A | −0.0004 | 0.2411 | 0.3531 | 0.098* | 0.70 |
| H97B | −0.0642 | 0.2709 | 0.4098 | 0.098* | 0.70 |
| H97C | −0.0443 | 0.1694 | 0.3968 | 0.098* | 0.70 |
| O99B | 0.0109 (6) | 0.2045 (4) | 0.5007 (2) | 0.0357 (11)* | 0.30 |
| N99B | 0.1149 (8) | 0.2235 (5) | 0.4188 (3) | 0.0317 (18)* | 0.30 |
| C88B | 0.0171 (12) | 0.2275 (8) | 0.4510 (4) | 0.071 (3)* | 0.30 |
| H88B | −0.0696 | 0.2516 | 0.4373 | 0.085* | 0.30 |
| C96B | 0.2691 (11) | 0.2064 (7) | 0.4520 (4) | 0.067 (2)* | 0.30 |
| H96D | 0.3491 | 0.2029 | 0.4265 | 0.100* | 0.30 |
| H96E | 0.2624 | 0.1517 | 0.4721 | 0.100* | 0.30 |
| H96F | 0.2860 | 0.2548 | 0.4769 | 0.100* | 0.30 |
| C97B | 0.1649 (19) | 0.2484 (12) | 0.3692 (7) | 0.112 (5)* | 0.30 |
| H97D | 0.2612 | 0.2238 | 0.3633 | 0.168* | 0.30 |
| H97E | 0.1701 | 0.3122 | 0.3674 | 0.168* | 0.30 |
| H97F | 0.0992 | 0.2270 | 0.3414 | 0.168* | 0.30 |
| H5B | 0.133 (4) | 0.145 (3) | 0.5631 (15) | 0.072 (11)* | |
| H5C | 0.150 (4) | 0.076 (3) | 0.5979 (13) | 0.072 (10)* | |
| HN3B | 1.077 (3) | 0.2319 (16) | 0.0481 (9) | 0.033 (6)* | |
| HN3A | 0.984 (3) | 0.2272 (17) | 0.0012 (11) | 0.046 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0274 (8) | 0.0247 (9) | 0.0245 (8) | −0.0008 (8) | −0.0019 (7) | −0.0009 (7) |
| C2 | 0.0286 (8) | 0.0303 (10) | 0.0254 (8) | −0.0058 (8) | −0.0033 (7) | 0.0066 (7) |
| C3 | 0.0407 (10) | 0.0347 (11) | 0.0358 (10) | −0.0087 (9) | −0.0072 (8) | 0.0085 (8) |
| C4 | 0.0443 (11) | 0.0470 (14) | 0.0499 (12) | −0.0198 (11) | −0.0082 (10) | 0.0180 (11) |
| C5 | 0.0300 (9) | 0.0640 (16) | 0.0456 (12) | −0.0128 (11) | 0.0021 (8) | 0.0228 (12) |
| C6 | 0.0299 (9) | 0.0547 (14) | 0.0382 (11) | −0.0004 (10) | 0.0050 (8) | 0.0111 (10) |
| C7 | 0.0259 (8) | 0.0376 (11) | 0.0242 (9) | −0.0010 (8) | −0.0007 (7) | 0.0052 (8) |
| C8 | 0.0257 (8) | 0.0340 (10) | 0.0231 (8) | 0.0020 (8) | 0.0004 (6) | 0.0010 (7) |
| C9 | 0.0256 (8) | 0.0243 (9) | 0.0235 (8) | −0.0044 (7) | 0.0028 (6) | 0.0002 (7) |
| C10 | 0.0239 (7) | 0.0330 (10) | 0.0279 (8) | −0.0048 (8) | −0.0010 (7) | 0.0047 (8) |
| C11 | 0.0274 (8) | 0.0323 (10) | 0.0303 (9) | −0.0040 (8) | −0.0033 (7) | 0.0067 (8) |
| C12 | 0.0496 (11) | 0.0329 (11) | 0.0320 (10) | −0.0066 (9) | −0.0117 (9) | 0.0063 (8) |
| C13 | 0.0405 (9) | 0.0386 (12) | 0.0305 (9) | −0.0040 (9) | −0.0032 (8) | 0.0047 (8) |
| C14 | 0.0525 (11) | 0.0517 (14) | 0.0291 (10) | −0.0029 (11) | −0.0046 (9) | 0.0090 (10) |
| C15 | 0.0509 (12) | 0.0486 (14) | 0.0397 (11) | −0.0048 (11) | −0.0007 (9) | 0.0212 (10) |
| C16 | 0.0394 (10) | 0.0349 (12) | 0.0479 (12) | −0.0061 (9) | −0.0029 (9) | 0.0128 (9) |
| C17 | 0.0296 (8) | 0.0345 (11) | 0.0345 (10) | −0.0036 (8) | −0.0009 (7) | 0.0025 (8) |
| C18 | 0.0266 (8) | 0.0331 (11) | 0.0293 (9) | −0.0016 (8) | −0.0010 (7) | 0.0037 (8) |
| C19 | 0.0260 (8) | 0.0242 (9) | 0.0251 (8) | 0.0023 (7) | −0.0001 (6) | 0.0000 (7) |
| C20 | 0.0339 (9) | 0.0391 (12) | 0.0355 (10) | 0.0120 (9) | −0.0027 (8) | −0.0033 (9) |
| C21 | 0.0586 (13) | 0.0366 (13) | 0.0445 (12) | 0.0161 (11) | −0.0031 (10) | −0.0029 (10) |
| C22 | 0.0613 (13) | 0.0301 (11) | 0.0465 (12) | −0.0004 (11) | 0.0006 (10) | 0.0005 (9) |
| C23 | 0.0467 (11) | 0.0334 (12) | 0.0450 (12) | −0.0015 (10) | 0.0041 (9) | 0.0059 (9) |
| C24 | 0.0297 (8) | 0.0311 (11) | 0.0368 (10) | 0.0033 (8) | 0.0038 (7) | 0.0022 (8) |
| C25 | 0.0309 (9) | 0.0303 (10) | 0.0219 (8) | 0.0057 (8) | −0.0011 (7) | 0.0008 (7) |
| C26 | 0.0237 (7) | 0.0289 (9) | 0.0268 (8) | 0.0011 (7) | −0.0005 (6) | −0.0033 (7) |
| C27 | 0.0409 (10) | 0.0352 (11) | 0.0290 (9) | −0.0018 (9) | −0.0006 (8) | 0.0002 (8) |
| C28 | 0.0516 (12) | 0.0326 (11) | 0.0448 (11) | −0.0035 (10) | −0.0019 (9) | −0.0012 (9) |
| C29 | 0.0456 (10) | 0.0364 (12) | 0.0452 (11) | −0.0059 (10) | 0.0020 (9) | −0.0125 (10) |
| C30 | 0.0449 (10) | 0.0424 (12) | 0.0355 (11) | −0.0055 (10) | 0.0090 (9) | −0.0106 (9) |
| C31 | 0.0295 (8) | 0.0382 (11) | 0.0274 (9) | −0.0010 (8) | 0.0012 (7) | −0.0028 (8) |
| N1 | 0.0267 (7) | 0.0236 (8) | 0.0251 (7) | −0.0033 (6) | 0.0035 (6) | −0.0021 (6) |
| N2 | 0.0647 (11) | 0.0360 (10) | 0.0280 (8) | −0.0077 (9) | −0.0123 (8) | 0.0022 (7) |
| N3 | 0.0224 (7) | 0.0340 (9) | 0.0244 (8) | 0.0040 (6) | −0.0023 (6) | −0.0013 (6) |
| O1 | 0.0384 (6) | 0.0295 (7) | 0.0346 (7) | −0.0011 (6) | 0.0070 (6) | −0.0068 (6) |
| O2 | 0.0414 (7) | 0.0334 (8) | 0.0374 (7) | 0.0041 (6) | 0.0071 (6) | −0.0081 (6) |
| O3 | 0.0316 (6) | 0.0428 (8) | 0.0247 (6) | −0.0066 (6) | 0.0013 (5) | 0.0054 (6) |
| O4 | 0.0276 (6) | 0.0523 (9) | 0.0349 (7) | −0.0029 (6) | −0.0065 (5) | 0.0039 (6) |
| O5 | 0.0860 (13) | 0.0317 (9) | 0.0442 (10) | 0.0013 (9) | 0.0080 (9) | 0.0005 (7) |
Geometric parameters (Å, °)
| C1—O1 | 1.213 (2) | C23—H23B | 0.9700 |
| C1—N1 | 1.384 (2) | C24—C25 | 1.519 (3) |
| C1—C2 | 1.492 (3) | C24—H24A | 0.9700 |
| C2—C3 | 1.374 (3) | C24—H24B | 0.9700 |
| C2—C7 | 1.392 (3) | C25—N3 | 1.499 (3) |
| C3—C4 | 1.397 (3) | C25—H25 | 0.9800 |
| C3—H3 | 0.9300 | C26—N3 | 1.500 (2) |
| C4—C5 | 1.383 (4) | C26—C27 | 1.517 (3) |
| C4—H4 | 0.9300 | C26—C31 | 1.527 (2) |
| C5—C6 | 1.387 (4) | C26—H26 | 0.9800 |
| C5—H5 | 0.9300 | C27—C28 | 1.524 (3) |
| C6—C7 | 1.385 (3) | C27—H27A | 0.9700 |
| C6—H6 | 0.9300 | C27—H27B | 0.9700 |
| C7—C8 | 1.481 (3) | C28—C29 | 1.522 (3) |
| C8—O2 | 1.209 (2) | C28—H28A | 0.9700 |
| C8—N1 | 1.400 (2) | C28—H28B | 0.9700 |
| C9—N1 | 1.457 (2) | C29—C30 | 1.516 (3) |
| C9—C10 | 1.538 (2) | C29—H29A | 0.9700 |
| C9—C19 | 1.547 (2) | C29—H29B | 0.9700 |
| C9—H9 | 0.9800 | C30—C31 | 1.522 (3) |
| C10—C11 | 1.499 (3) | C30—H30A | 0.9700 |
| C10—H10A | 0.9700 | C30—H30B | 0.9700 |
| C10—H10B | 0.9700 | C31—H31A | 0.9700 |
| C11—C12 | 1.363 (3) | C31—H31B | 0.9700 |
| C11—C18 | 1.442 (3) | N2—H2 | 0.8600 |
| C12—N2 | 1.376 (3) | N3—HN3B | 0.86 (2) |
| C12—H12 | 0.9300 | N3—HN3A | 0.99 (3) |
| C13—N2 | 1.373 (3) | O5—H5B | 0.82 (4) |
| C13—C14 | 1.397 (3) | O5—H5C | 0.89 (4) |
| C13—C18 | 1.410 (3) | O99—C88 | 1.145 (5) |
| C14—C15 | 1.378 (4) | N99—C88 | 1.289 (5) |
| C14—H14 | 0.9300 | N99—C96 | 1.387 (7) |
| C15—C16 | 1.397 (3) | N99—C97 | 1.561 (6) |
| C15—H15 | 0.9300 | C88—H88 | 0.9300 |
| C16—C17 | 1.381 (3) | C96—H96A | 0.9600 |
| C16—H16 | 0.9300 | C96—H96B | 0.9600 |
| C17—C18 | 1.396 (3) | C96—H96C | 0.9600 |
| C17—H17 | 0.9300 | C97—H97A | 0.9600 |
| C19—O3 | 1.246 (2) | C97—H97B | 0.9600 |
| C19—O4 | 1.247 (2) | C97—H97C | 0.9600 |
| C20—C25 | 1.527 (3) | O99B—C88B | 1.266 (12) |
| C20—C21 | 1.527 (3) | N99B—C88B | 1.189 (13) |
| C20—H20A | 0.9700 | N99B—C97B | 1.350 (18) |
| C20—H20B | 0.9700 | N99B—C96B | 1.640 (12) |
| C21—C22 | 1.517 (3) | C88B—H88B | 0.9300 |
| C21—H21A | 0.9700 | C96B—H96D | 0.9600 |
| C21—H21B | 0.9700 | C96B—H96E | 0.9600 |
| C22—C23 | 1.521 (3) | C96B—H96F | 0.9600 |
| C22—H22A | 0.9700 | C97B—H97D | 0.9600 |
| C22—H22B | 0.9700 | C97B—H97E | 0.9600 |
| C23—C24 | 1.521 (3) | C97B—H97F | 0.9600 |
| C23—H23A | 0.9700 | ||
| O1—C1—N1 | 124.88 (17) | H24A—C24—H24B | 108.0 |
| O1—C1—C2 | 128.72 (18) | N3—C25—C24 | 110.73 (15) |
| N1—C1—C2 | 106.39 (15) | N3—C25—C20 | 107.89 (15) |
| C3—C2—C7 | 121.81 (17) | C24—C25—C20 | 111.34 (16) |
| C3—C2—C1 | 130.83 (19) | N3—C25—H25 | 108.9 |
| C7—C2—C1 | 107.35 (16) | C24—C25—H25 | 108.9 |
| C2—C3—C4 | 116.9 (2) | C20—C25—H25 | 108.9 |
| C2—C3—H3 | 121.5 | N3—C26—C27 | 108.74 (14) |
| C4—C3—H3 | 121.5 | N3—C26—C31 | 110.93 (15) |
| C5—C4—C3 | 121.5 (2) | C27—C26—C31 | 110.66 (16) |
| C5—C4—H4 | 119.2 | N3—C26—H26 | 108.8 |
| C3—C4—H4 | 119.2 | C27—C26—H26 | 108.8 |
| C4—C5—C6 | 121.27 (19) | C31—C26—H26 | 108.8 |
| C4—C5—H5 | 119.4 | C26—C27—C28 | 111.49 (16) |
| C6—C5—H5 | 119.4 | C26—C27—H27A | 109.3 |
| C7—C6—C5 | 117.3 (2) | C28—C27—H27A | 109.3 |
| C7—C6—H6 | 121.3 | C26—C27—H27B | 109.3 |
| C5—C6—H6 | 121.3 | C28—C27—H27B | 109.3 |
| C6—C7—C2 | 121.15 (19) | H27A—C27—H27B | 108.0 |
| C6—C7—C8 | 130.40 (19) | C29—C28—C27 | 111.16 (18) |
| C2—C7—C8 | 108.46 (15) | C29—C28—H28A | 109.4 |
| O2—C8—N1 | 124.68 (17) | C27—C28—H28A | 109.4 |
| O2—C8—C7 | 129.50 (17) | C29—C28—H28B | 109.4 |
| N1—C8—C7 | 105.82 (16) | C27—C28—H28B | 109.4 |
| N1—C9—C10 | 111.75 (15) | H28A—C28—H28B | 108.0 |
| N1—C9—C19 | 111.15 (14) | C30—C29—C28 | 110.23 (18) |
| C10—C9—C19 | 113.34 (13) | C30—C29—H29A | 109.6 |
| N1—C9—H9 | 106.7 | C28—C29—H29A | 109.6 |
| C10—C9—H9 | 106.7 | C30—C29—H29B | 109.6 |
| C19—C9—H9 | 106.7 | C28—C29—H29B | 109.6 |
| C11—C10—C9 | 113.96 (14) | H29A—C29—H29B | 108.1 |
| C11—C10—H10A | 108.8 | C29—C30—C31 | 112.23 (16) |
| C9—C10—H10A | 108.8 | C29—C30—H30A | 109.2 |
| C11—C10—H10B | 108.8 | C31—C30—H30A | 109.2 |
| C9—C10—H10B | 108.8 | C29—C30—H30B | 109.2 |
| H10A—C10—H10B | 107.7 | C31—C30—H30B | 109.2 |
| C12—C11—C18 | 105.80 (16) | H30A—C30—H30B | 107.9 |
| C12—C11—C10 | 126.61 (18) | C30—C31—C26 | 109.94 (16) |
| C18—C11—C10 | 127.58 (18) | C30—C31—H31A | 109.7 |
| C11—C12—N2 | 111.09 (18) | C26—C31—H31A | 109.7 |
| C11—C12—H12 | 124.5 | C30—C31—H31B | 109.7 |
| N2—C12—H12 | 124.5 | C26—C31—H31B | 109.7 |
| N2—C13—C14 | 129.6 (2) | H31A—C31—H31B | 108.2 |
| N2—C13—C18 | 108.08 (17) | C1—N1—C8 | 111.92 (15) |
| C14—C13—C18 | 122.2 (2) | C1—N1—C9 | 124.30 (14) |
| C15—C14—C13 | 117.5 (2) | C8—N1—C9 | 123.65 (15) |
| C15—C14—H14 | 121.3 | C13—N2—C12 | 108.05 (17) |
| C13—C14—H14 | 121.3 | C13—N2—H2 | 126.0 |
| C14—C15—C16 | 121.1 (2) | C12—N2—H2 | 126.0 |
| C14—C15—H15 | 119.4 | C25—N3—C26 | 117.94 (14) |
| C16—C15—H15 | 119.4 | C25—N3—HN3B | 109.4 (16) |
| C17—C16—C15 | 121.4 (2) | C26—N3—HN3B | 108.4 (16) |
| C17—C16—H16 | 119.3 | C25—N3—HN3A | 107.8 (15) |
| C15—C16—H16 | 119.3 | C26—N3—HN3A | 110.9 (15) |
| C16—C17—C18 | 118.99 (19) | HN3B—N3—HN3A | 101 (2) |
| C16—C17—H17 | 120.5 | H5B—O5—H5C | 103 (3) |
| C18—C17—H17 | 120.5 | C88—N99—C96 | 131.1 (4) |
| C17—C18—C13 | 118.79 (18) | C88—N99—C97 | 114.1 (4) |
| C17—C18—C11 | 134.22 (18) | C96—N99—C97 | 114.8 (4) |
| C13—C18—C11 | 106.97 (17) | O99—C88—N99 | 132.4 (4) |
| O3—C19—O4 | 125.87 (17) | O99—C88—H88 | 113.8 |
| O3—C19—C9 | 116.25 (15) | N99—C88—H88 | 113.8 |
| O4—C19—C9 | 117.84 (15) | N99—C96—H96A | 109.5 |
| C25—C20—C21 | 112.50 (17) | N99—C96—H96B | 109.5 |
| C25—C20—H20A | 109.1 | H96A—C96—H96B | 109.5 |
| C21—C20—H20A | 109.1 | N99—C96—H96C | 109.5 |
| C25—C20—H20B | 109.1 | H96A—C96—H96C | 109.5 |
| C21—C20—H20B | 109.1 | H96B—C96—H96C | 109.5 |
| H20A—C20—H20B | 107.8 | N99—C97—H97A | 109.5 |
| C22—C21—C20 | 111.41 (18) | N99—C97—H97B | 109.5 |
| C22—C21—H21A | 109.3 | H97A—C97—H97B | 109.5 |
| C20—C21—H21A | 109.3 | N99—C97—H97C | 109.5 |
| C22—C21—H21B | 109.3 | H97A—C97—H97C | 109.5 |
| C20—C21—H21B | 109.3 | H97B—C97—H97C | 109.5 |
| H21A—C21—H21B | 108.0 | C88B—N99B—C97B | 146.5 (11) |
| C21—C22—C23 | 110.53 (19) | C88B—N99B—C96B | 108.5 (8) |
| C21—C22—H22A | 109.5 | C97B—N99B—C96B | 101.6 (10) |
| C23—C22—H22A | 109.5 | N99B—C88B—O99B | 131.1 (10) |
| C21—C22—H22B | 109.5 | N99B—C88B—H88B | 114.5 |
| C23—C22—H22B | 109.5 | O99B—C88B—H88B | 114.5 |
| H22A—C22—H22B | 108.1 | N99B—C96B—H96D | 109.5 |
| C24—C23—C22 | 110.70 (17) | N99B—C96B—H96E | 109.5 |
| C24—C23—H23A | 109.5 | H96D—C96B—H96E | 109.5 |
| C22—C23—H23A | 109.5 | N99B—C96B—H96F | 109.5 |
| C24—C23—H23B | 109.5 | H96D—C96B—H96F | 109.5 |
| C22—C23—H23B | 109.5 | H96E—C96B—H96F | 109.5 |
| H23A—C23—H23B | 108.1 | N99B—C97B—H97D | 109.5 |
| C25—C24—C23 | 111.33 (16) | N99B—C97B—H97E | 109.5 |
| C25—C24—H24A | 109.4 | H97D—C97B—H97E | 109.5 |
| C23—C24—H24A | 109.4 | N99B—C97B—H97F | 109.5 |
| C25—C24—H24B | 109.4 | H97D—C97B—H97F | 109.5 |
| C23—C24—H24B | 109.4 | H97E—C97B—H97F | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—HN3B···O4i | 0.86 (3) | 1.88 (3) | 2.7309 (19) | 169 (2) |
| N2—H2···O5ii | 0.86 | 1.97 | 2.813 (3) | 165 |
| N3—HN3A···O3iii | 0.99 (3) | 1.79 (3) | 2.7740 (19) | 173 (2) |
| O5—H5B···O99 | 0.83 (4) | 1.84 (4) | 2.645 (4) | 164 (4) |
| O5—H5C···O1iv | 0.89 (4) | 1.99 (4) | 2.857 (2) | 164 (3) |
Symmetry codes: (i) x+1, y, z; (ii) x+1/2, −y+1/2, −z+1; (iii) x+1/2, −y+1/2, −z; (iv) −x+1/2, −y, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: VM2050).
References
- Brueckner, B., Boy, R. G., Siedlecki, P., Munsch, T., Kliem, H. C., Zielenkiewicz, P., Suhai, S., Wiessler, M. & Lyko, F. (2005). Cancer Res.65, 6305–6311. [DOI] [PubMed]
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Oxford Diffraction (2009). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681004626X/vm2050sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681004626X/vm2050Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report