Abstract
The title compound, C23H24N2O3, was synthesized from naphthalen-2-ol, 3-nitrobenzaldehyde and 3-methylpiperidine. The dihedral angles between the naphthalene system and the nitrobenzene and methylpiperidine rings are 78.53 (13) and 64.14 (15)°, respectively. The molecular conformation is stabilized by a strong intramolecular O—H⋯N hydrogen bond.
Related literature
For applications of naphthalen-2-ol derivatives in catalytic asymmetric synthesis, see: Szatmari & Fulop (2004 ▶). For related structures, see: Zhao & Sun (2005 ▶); Wang & Zhao (2009 ▶); Xiao & Zhao (2010 ▶);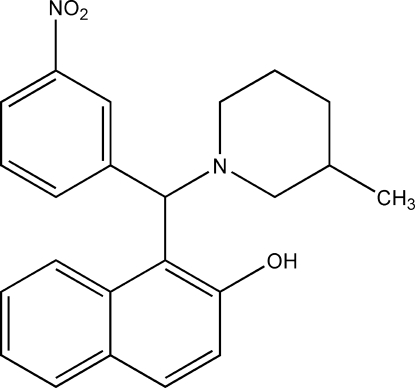
Experimental
Crystal data
C23H24N2O3
M r = 376.44
Orthorhombic,

a = 11.980 (2) Å
b = 10.965 (2) Å
c = 30.30 (3) Å
V = 3980 (4) Å3
Z = 8
Mo Kα radiation
μ = 0.08 mm−1
T = 295 K
0.25 × 0.22 × 0.18 mm
Data collection
Rigaku SCXmini diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2005 ▶) T min = 0.982, T max = 0.992
34080 measured reflections
3877 independent reflections
2409 reflections with I > 2σ(I)
R int = 0.110
Refinement
R[F 2 > 2σ(F 2)] = 0.083
wR(F 2) = 0.174
S = 1.12
3877 reflections
255 parameters
H-atom parameters constrained
Δρmax = 0.14 e Å−3
Δρmin = −0.15 e Å−3
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL/PC (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL/PC.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810045149/bx2327sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810045149/bx2327Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N1 | 0.82 | 1.86 | 2.579 (3) | 147 |
Acknowledgments
This work was supported financially by a Southeast University grant for young researchers (4007041027).
supplementary crystallographic information
Comment
Compounds derived from naphthalen-2-ol have been of great interest in organic chemistry due to their application in catalytic asymmetric synthesis (Szatmari & Fulop, 2004; Zhao & Sun, 2005). As an extension of our work on the structural characterization of naphthol compounds (Wang & Zhao, 2009; Xiao & Zhao, 2010), we report here the structure of (I). In the title compound (Fig. 1) bond lengths and angles have normal values.The dihedral angle between the naphthylen fragment with the nitrobenzene and methyl piperidine rings are 78.53 (13) and 64.14 (15)° respectively. The molecular conformation is stabilized by one strong intramolecular O—H···N hydrogen bonding (Table 1).
Experimental
A dry 50 ml flask was charged with 3-nitrobenzaldehyde (10 mmol), naphthalen-2-ol (10 mmol) and 3-methylpiperidine (10 mmol). The mixture was stirred at 100°C for 12 h and then added ethanol (15 ml), after heated under reflux for 1 h, the precipitate was filtrated out and washed with ethanol for three times to give (I). Colourless crystals suitable for X-ray diffraction were obtained by slow evaporation of a dichloromethane solution.
Refinement
All H atoms were detected in a difference map, but were placed in calculated positions and refined using a riding motion approxmation, with C—H=0.93–0.98 Å, with Uiso(H)=1.2Ueq(C) and Uiso(Hmethyl)=1.2Ueq(Cmethyl); O—H=0.82 Å, with Uiso(H)=1.5Ueq(O).
Figures
Fig. 1.
The molecular structure of the title compound, showing the atomic numbering scheme. The displacement ellipsoids are drawn at the 30% probability level.
Crystal data
| C23H24N2O3 | F(000) = 1600 |
| Mr = 376.44 | Dx = 1.256 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 4541 reflections |
| a = 11.980 (2) Å | θ = 2.3–27.5° |
| b = 10.965 (2) Å | µ = 0.08 mm−1 |
| c = 30.30 (3) Å | T = 295 K |
| V = 3980 (4) Å3 | Prism, colourless |
| Z = 8 | 0.25 × 0.22 × 0.18 mm |
Data collection
| Rigaku SCXmini diffractometer | 3877 independent reflections |
| Radiation source: fine-focus sealed tube | 2409 reflections with I > 2σ(I) |
| graphite | Rint = 0.110 |
| Detector resolution: 13.6612 pixels mm-1 | θmax = 26.0°, θmin = 2.6° |
| CCD_Profile_fitting scans | h = −14→14 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2005) | k = −13→13 |
| Tmin = 0.982, Tmax = 0.992 | l = −37→37 |
| 34080 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.083 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.174 | H-atom parameters constrained |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.0563P)2 + 1.4193P] where P = (Fo2 + 2Fc2)/3 |
| 3877 reflections | (Δ/σ)max < 0.001 |
| 255 parameters | Δρmax = 0.14 e Å−3 |
| 0 restraints | Δρmin = −0.15 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7000 (2) | 0.9558 (2) | 0.65000 (10) | 0.0433 (7) | |
| C2 | 0.6194 (2) | 1.0425 (3) | 0.64171 (11) | 0.0523 (8) | |
| C3 | 0.5315 (3) | 1.0634 (3) | 0.67144 (13) | 0.0659 (9) | |
| H3 | 0.4769 | 1.1206 | 0.6646 | 0.079* | |
| C4 | 0.5255 (3) | 1.0015 (4) | 0.70975 (13) | 0.0707 (10) | |
| H4 | 0.4661 | 1.0161 | 0.7288 | 0.085* | |
| C5 | 0.6075 (3) | 0.9147 (3) | 0.72156 (11) | 0.0584 (9) | |
| C6 | 0.6961 (2) | 0.8920 (3) | 0.69134 (10) | 0.0478 (7) | |
| C7 | 0.7785 (3) | 0.8061 (3) | 0.70421 (10) | 0.0552 (8) | |
| H7 | 0.8381 | 0.7905 | 0.6854 | 0.066* | |
| C8 | 0.7725 (3) | 0.7459 (3) | 0.74356 (11) | 0.0708 (10) | |
| H8 | 0.8279 | 0.6904 | 0.7513 | 0.085* | |
| C9 | 0.6840 (3) | 0.7671 (4) | 0.77209 (12) | 0.0820 (12) | |
| H9 | 0.6796 | 0.7246 | 0.7986 | 0.098* | |
| C10 | 0.6041 (3) | 0.8493 (4) | 0.76161 (12) | 0.0774 (11) | |
| H10 | 0.5457 | 0.8630 | 0.7812 | 0.093* | |
| C11 | 0.7905 (2) | 0.9244 (2) | 0.61686 (9) | 0.0422 (7) | |
| H11 | 0.8033 | 0.8363 | 0.6189 | 0.051* | |
| C12 | 0.6680 (3) | 0.8576 (3) | 0.55808 (11) | 0.0599 (9) | |
| H12A | 0.6106 | 0.8529 | 0.5806 | 0.072* | |
| H12B | 0.7044 | 0.7787 | 0.5564 | 0.072* | |
| C13 | 0.6143 (3) | 0.8861 (3) | 0.51400 (13) | 0.0728 (11) | |
| H13 | 0.5776 | 0.9657 | 0.5166 | 0.087* | |
| C14 | 0.7021 (4) | 0.8968 (4) | 0.47882 (13) | 0.0945 (14) | |
| H14A | 0.6680 | 0.9242 | 0.4515 | 0.113* | |
| H14B | 0.7353 | 0.8175 | 0.4736 | 0.113* | |
| C15 | 0.7918 (3) | 0.9860 (4) | 0.49260 (11) | 0.0828 (12) | |
| H15A | 0.8513 | 0.9851 | 0.4709 | 0.099* | |
| H15B | 0.7605 | 1.0676 | 0.4933 | 0.099* | |
| C16 | 0.8395 (3) | 0.9559 (3) | 0.53738 (10) | 0.0633 (9) | |
| H16A | 0.8776 | 0.8780 | 0.5361 | 0.076* | |
| H16B | 0.8936 | 1.0176 | 0.5457 | 0.076* | |
| C17 | 0.5250 (4) | 0.7925 (3) | 0.50331 (16) | 0.1061 (16) | |
| H17A | 0.4876 | 0.8153 | 0.4765 | 0.159* | |
| H17B | 0.4719 | 0.7892 | 0.5270 | 0.159* | |
| H17C | 0.5590 | 0.7139 | 0.4996 | 0.159* | |
| C18 | 0.9014 (2) | 0.9868 (3) | 0.62676 (9) | 0.0428 (7) | |
| C19 | 1.0000 (2) | 0.9224 (3) | 0.62142 (9) | 0.0472 (7) | |
| H19 | 0.9988 | 0.8410 | 0.6128 | 0.057* | |
| C20 | 1.0999 (3) | 0.9808 (3) | 0.62900 (10) | 0.0553 (8) | |
| C21 | 1.1060 (3) | 1.1004 (4) | 0.64166 (12) | 0.0710 (10) | |
| H21 | 1.1746 | 1.1379 | 0.6463 | 0.085* | |
| C22 | 1.0079 (3) | 1.1635 (3) | 0.64731 (13) | 0.0748 (10) | |
| H22 | 1.0099 | 1.2448 | 0.6560 | 0.090* | |
| C23 | 0.9067 (3) | 1.1075 (3) | 0.64020 (11) | 0.0584 (8) | |
| H23 | 0.8410 | 1.1512 | 0.6445 | 0.070* | |
| N1 | 0.7505 (2) | 0.9505 (2) | 0.57076 (7) | 0.0469 (6) | |
| N2 | 1.2039 (3) | 0.9098 (4) | 0.62304 (11) | 0.0788 (9) | |
| O1 | 0.61885 (19) | 1.11174 (19) | 0.60442 (8) | 0.0645 (6) | |
| H1 | 0.6631 | 1.0833 | 0.5865 | 0.097* | |
| O2 | 1.1967 (2) | 0.8052 (3) | 0.61087 (11) | 0.1051 (10) | |
| O3 | 1.2920 (2) | 0.9589 (3) | 0.63113 (13) | 0.1305 (14) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0363 (15) | 0.0400 (15) | 0.0536 (18) | −0.0022 (13) | −0.0002 (13) | −0.0087 (13) |
| C2 | 0.0470 (18) | 0.0457 (17) | 0.064 (2) | −0.0034 (15) | −0.0002 (15) | −0.0073 (15) |
| C3 | 0.047 (2) | 0.066 (2) | 0.085 (3) | 0.0061 (17) | 0.0059 (18) | −0.010 (2) |
| C4 | 0.048 (2) | 0.084 (3) | 0.079 (3) | −0.0049 (19) | 0.0190 (18) | −0.019 (2) |
| C5 | 0.0454 (19) | 0.068 (2) | 0.062 (2) | −0.0110 (17) | 0.0083 (16) | −0.0132 (17) |
| C6 | 0.0440 (17) | 0.0445 (16) | 0.0550 (19) | −0.0103 (14) | −0.0010 (14) | −0.0083 (14) |
| C7 | 0.057 (2) | 0.0570 (19) | 0.0515 (19) | −0.0080 (16) | 0.0011 (15) | −0.0019 (15) |
| C8 | 0.072 (2) | 0.086 (3) | 0.054 (2) | −0.001 (2) | 0.0013 (18) | 0.0116 (19) |
| C9 | 0.080 (3) | 0.109 (3) | 0.057 (2) | −0.012 (3) | 0.004 (2) | 0.020 (2) |
| C10 | 0.064 (2) | 0.110 (3) | 0.058 (2) | −0.017 (2) | 0.0178 (19) | −0.006 (2) |
| C11 | 0.0437 (17) | 0.0387 (15) | 0.0443 (16) | 0.0001 (13) | −0.0026 (13) | −0.0012 (12) |
| C12 | 0.067 (2) | 0.0439 (17) | 0.069 (2) | −0.0014 (16) | −0.0200 (17) | −0.0041 (15) |
| C13 | 0.086 (3) | 0.0478 (19) | 0.085 (3) | 0.0037 (19) | −0.040 (2) | −0.0030 (18) |
| C14 | 0.134 (4) | 0.089 (3) | 0.060 (3) | 0.004 (3) | −0.028 (3) | −0.002 (2) |
| C15 | 0.105 (3) | 0.090 (3) | 0.053 (2) | −0.001 (2) | −0.009 (2) | 0.006 (2) |
| C16 | 0.072 (2) | 0.067 (2) | 0.051 (2) | 0.0067 (19) | −0.0002 (17) | −0.0001 (16) |
| C17 | 0.115 (4) | 0.070 (3) | 0.134 (4) | −0.009 (2) | −0.071 (3) | −0.004 (2) |
| C18 | 0.0425 (17) | 0.0462 (17) | 0.0397 (16) | −0.0016 (14) | −0.0008 (13) | −0.0007 (12) |
| C19 | 0.0503 (18) | 0.0472 (17) | 0.0441 (17) | 0.0015 (15) | −0.0022 (14) | 0.0004 (13) |
| C20 | 0.0444 (19) | 0.069 (2) | 0.0522 (19) | −0.0005 (17) | −0.0015 (15) | 0.0060 (16) |
| C21 | 0.054 (2) | 0.076 (2) | 0.083 (3) | −0.022 (2) | −0.0081 (19) | −0.005 (2) |
| C22 | 0.071 (3) | 0.058 (2) | 0.095 (3) | −0.013 (2) | −0.002 (2) | −0.0178 (19) |
| C23 | 0.053 (2) | 0.0474 (18) | 0.075 (2) | −0.0015 (16) | 0.0026 (17) | −0.0117 (16) |
| N1 | 0.0488 (14) | 0.0447 (13) | 0.0472 (14) | 0.0003 (12) | −0.0068 (12) | −0.0026 (11) |
| N2 | 0.0454 (19) | 0.101 (3) | 0.090 (2) | 0.009 (2) | −0.0059 (16) | 0.011 (2) |
| O1 | 0.0670 (16) | 0.0489 (13) | 0.0775 (17) | 0.0158 (11) | 0.0021 (12) | 0.0042 (12) |
| O2 | 0.0703 (19) | 0.106 (2) | 0.139 (3) | 0.0348 (18) | −0.0131 (17) | −0.027 (2) |
| O3 | 0.0440 (17) | 0.134 (3) | 0.214 (4) | −0.0033 (18) | −0.020 (2) | 0.008 (3) |
Geometric parameters (Å, °)
| C1—C2 | 1.379 (4) | C13—H13 | 0.9800 |
| C1—C6 | 1.435 (4) | C14—C15 | 1.511 (5) |
| C1—C11 | 1.517 (4) | C14—H14A | 0.9700 |
| C2—O1 | 1.361 (4) | C14—H14B | 0.9700 |
| C2—C3 | 1.404 (4) | C15—C16 | 1.509 (5) |
| C3—C4 | 1.347 (5) | C15—H15A | 0.9700 |
| C3—H3 | 0.9300 | C15—H15B | 0.9700 |
| C4—C5 | 1.413 (5) | C16—N1 | 1.471 (4) |
| C4—H4 | 0.9300 | C16—H16A | 0.9700 |
| C5—C10 | 1.410 (5) | C16—H16B | 0.9700 |
| C5—C6 | 1.424 (4) | C17—H17A | 0.9600 |
| C6—C7 | 1.419 (4) | C17—H17B | 0.9600 |
| C7—C8 | 1.365 (4) | C17—H17C | 0.9600 |
| C7—H7 | 0.9300 | C18—C19 | 1.386 (4) |
| C8—C9 | 1.387 (5) | C18—C23 | 1.387 (4) |
| C8—H8 | 0.9300 | C19—C20 | 1.376 (4) |
| C9—C10 | 1.353 (5) | C19—H19 | 0.9300 |
| C9—H9 | 0.9300 | C20—C21 | 1.368 (5) |
| C10—H10 | 0.9300 | C20—N2 | 1.481 (4) |
| C11—N1 | 1.505 (4) | C21—C22 | 1.375 (5) |
| C11—C18 | 1.524 (4) | C21—H21 | 0.9300 |
| C11—H11 | 0.9800 | C22—C23 | 1.376 (4) |
| C12—N1 | 1.471 (4) | C22—H22 | 0.9300 |
| C12—C13 | 1.515 (5) | C23—H23 | 0.9300 |
| C12—H12A | 0.9700 | N2—O2 | 1.207 (4) |
| C12—H12B | 0.9700 | N2—O3 | 1.210 (4) |
| C13—C14 | 1.502 (5) | O1—H1 | 0.8200 |
| C13—C17 | 1.518 (5) | ||
| C2—C1—C6 | 118.2 (3) | C13—C14—H14A | 109.5 |
| C2—C1—C11 | 122.5 (3) | C15—C14—H14A | 109.5 |
| C6—C1—C11 | 119.3 (2) | C13—C14—H14B | 109.5 |
| O1—C2—C1 | 122.6 (3) | C15—C14—H14B | 109.5 |
| O1—C2—C3 | 116.0 (3) | H14A—C14—H14B | 108.1 |
| C1—C2—C3 | 121.4 (3) | C16—C15—C14 | 112.1 (3) |
| C4—C3—C2 | 120.7 (3) | C16—C15—H15A | 109.2 |
| C4—C3—H3 | 119.6 | C14—C15—H15A | 109.2 |
| C2—C3—H3 | 119.6 | C16—C15—H15B | 109.2 |
| C3—C4—C5 | 121.3 (3) | C14—C15—H15B | 109.2 |
| C3—C4—H4 | 119.3 | H15A—C15—H15B | 107.9 |
| C5—C4—H4 | 119.3 | N1—C16—C15 | 110.6 (3) |
| C10—C5—C4 | 122.7 (3) | N1—C16—H16A | 109.5 |
| C10—C5—C6 | 119.0 (3) | C15—C16—H16A | 109.5 |
| C4—C5—C6 | 118.3 (3) | N1—C16—H16B | 109.5 |
| C7—C6—C5 | 117.3 (3) | C15—C16—H16B | 109.5 |
| C7—C6—C1 | 122.8 (3) | H16A—C16—H16B | 108.1 |
| C5—C6—C1 | 120.0 (3) | C13—C17—H17A | 109.5 |
| C8—C7—C6 | 121.7 (3) | C13—C17—H17B | 109.5 |
| C8—C7—H7 | 119.2 | H17A—C17—H17B | 109.5 |
| C6—C7—H7 | 119.2 | C13—C17—H17C | 109.5 |
| C7—C8—C9 | 120.2 (4) | H17A—C17—H17C | 109.5 |
| C7—C8—H8 | 119.9 | H17B—C17—H17C | 109.5 |
| C9—C8—H8 | 119.9 | C19—C18—C23 | 118.8 (3) |
| C10—C9—C8 | 120.4 (3) | C19—C18—C11 | 119.5 (3) |
| C10—C9—H9 | 119.8 | C23—C18—C11 | 121.7 (3) |
| C8—C9—H9 | 119.8 | C20—C19—C18 | 119.0 (3) |
| C9—C10—C5 | 121.4 (3) | C20—C19—H19 | 120.5 |
| C9—C10—H10 | 119.3 | C18—C19—H19 | 120.5 |
| C5—C10—H10 | 119.3 | C21—C20—C19 | 122.6 (3) |
| N1—C11—C1 | 110.1 (2) | C21—C20—N2 | 119.6 (3) |
| N1—C11—C18 | 112.0 (2) | C19—C20—N2 | 117.8 (3) |
| C1—C11—C18 | 113.0 (2) | C20—C21—C22 | 118.1 (3) |
| N1—C11—H11 | 107.1 | C20—C21—H21 | 120.9 |
| C1—C11—H11 | 107.1 | C22—C21—H21 | 120.9 |
| C18—C11—H11 | 107.1 | C21—C22—C23 | 120.6 (3) |
| N1—C12—C13 | 111.9 (3) | C21—C22—H22 | 119.7 |
| N1—C12—H12A | 109.2 | C23—C22—H22 | 119.7 |
| C13—C12—H12A | 109.2 | C22—C23—C18 | 120.8 (3) |
| N1—C12—H12B | 109.2 | C22—C23—H23 | 119.6 |
| C13—C12—H12B | 109.2 | C18—C23—H23 | 119.6 |
| H12A—C12—H12B | 107.9 | C12—N1—C16 | 109.6 (2) |
| C14—C13—C12 | 110.1 (3) | C12—N1—C11 | 109.0 (2) |
| C14—C13—C17 | 113.3 (4) | C16—N1—C11 | 114.5 (2) |
| C12—C13—C17 | 110.3 (3) | O2—N2—O3 | 123.2 (4) |
| C14—C13—H13 | 107.6 | O2—N2—C20 | 118.5 (3) |
| C12—C13—H13 | 107.6 | O3—N2—C20 | 118.3 (4) |
| C17—C13—H13 | 107.6 | C2—O1—H1 | 109.5 |
| C13—C14—C15 | 110.6 (3) | ||
| C6—C1—C2—O1 | 176.9 (3) | C12—C13—C14—C15 | −52.7 (4) |
| C11—C1—C2—O1 | −4.2 (4) | C17—C13—C14—C15 | −176.8 (3) |
| C6—C1—C2—C3 | −3.8 (4) | C13—C14—C15—C16 | 53.0 (4) |
| C11—C1—C2—C3 | 175.1 (3) | C14—C15—C16—N1 | −56.1 (4) |
| O1—C2—C3—C4 | −178.7 (3) | N1—C11—C18—C19 | −95.4 (3) |
| C1—C2—C3—C4 | 2.0 (5) | C1—C11—C18—C19 | 139.5 (3) |
| C2—C3—C4—C5 | 0.7 (5) | N1—C11—C18—C23 | 83.3 (3) |
| C3—C4—C5—C10 | 179.3 (3) | C1—C11—C18—C23 | −41.7 (4) |
| C3—C4—C5—C6 | −1.4 (5) | C23—C18—C19—C20 | −0.9 (4) |
| C10—C5—C6—C7 | −1.8 (4) | C11—C18—C19—C20 | 177.8 (3) |
| C4—C5—C6—C7 | 178.9 (3) | C18—C19—C20—C21 | 0.0 (5) |
| C10—C5—C6—C1 | 178.8 (3) | C18—C19—C20—N2 | 180.0 (3) |
| C4—C5—C6—C1 | −0.5 (4) | C19—C20—C21—C22 | 0.6 (5) |
| C2—C1—C6—C7 | −176.4 (3) | N2—C20—C21—C22 | −179.4 (3) |
| C11—C1—C6—C7 | 4.7 (4) | C20—C21—C22—C23 | −0.2 (6) |
| C2—C1—C6—C5 | 3.0 (4) | C21—C22—C23—C18 | −0.7 (6) |
| C11—C1—C6—C5 | −175.9 (2) | C19—C18—C23—C22 | 1.3 (5) |
| C5—C6—C7—C8 | 1.1 (4) | C11—C18—C23—C22 | −177.5 (3) |
| C1—C6—C7—C8 | −179.5 (3) | C13—C12—N1—C16 | −60.4 (3) |
| C6—C7—C8—C9 | 0.4 (5) | C13—C12—N1—C11 | 173.6 (3) |
| C7—C8—C9—C10 | −1.3 (6) | C15—C16—N1—C12 | 58.8 (3) |
| C8—C9—C10—C5 | 0.6 (6) | C15—C16—N1—C11 | −178.4 (3) |
| C4—C5—C10—C9 | −179.8 (4) | C1—C11—N1—C12 | −72.9 (3) |
| C6—C5—C10—C9 | 1.0 (5) | C18—C11—N1—C12 | 160.5 (2) |
| C2—C1—C11—N1 | −26.7 (3) | C1—C11—N1—C16 | 164.0 (2) |
| C6—C1—C11—N1 | 152.2 (2) | C18—C11—N1—C16 | 37.3 (3) |
| C2—C1—C11—C18 | 99.5 (3) | C21—C20—N2—O2 | −178.3 (4) |
| C6—C1—C11—C18 | −81.7 (3) | C19—C20—N2—O2 | 1.7 (5) |
| N1—C12—C13—C14 | 57.6 (4) | C21—C20—N2—O3 | 2.6 (5) |
| N1—C12—C13—C17 | −176.7 (3) | C19—C20—N2—O3 | −177.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N1 | 0.82 | 1.86 | 2.579 (3) | 147 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BX2327).
References
- Rigaku (2005). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Szatmari, I. & Fulop, F. (2004). Curr. Org. Synth.1, 155–165.
- Wang, W. X. & Zhao, H. (2009). Acta Cryst. E65, o1277. [DOI] [PMC free article] [PubMed]
- Xiao, J. & Zhao, H. (2010). Acta Cryst. E66, o2938. [DOI] [PMC free article] [PubMed]
- Zhao, B. & Sun, Y.-X. (2005). Acta Cryst. E61, m652–m653.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810045149/bx2327sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810045149/bx2327Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



