Abstract
The crystal structure of the title compound, C14H13ClN2O3S, features a three-dimensional network stabilized by intermolecular C—H⋯O hydrogen bonds between the molecules. The 4-methylphenylsulfonyl ring forms a dihedral angle of 30.6 (1)° with the 4-chloropyridine ring.
Related literature
For the biological activity of 2-alkylaminopyridinyl or 2-acylaminopyridinyl imidazole derivatives as p38α MAPK inhibitors, see: Laufer et al. (2008 ▶, 2010 ▶); Ziegler et al. (2009 ▶). For general background to protecting groups, see: Kocieński (2005 ▶). For the preparation of the N-protected 4-chloropyridine, see: Berliner & Belecki (2005 ▶); Sciotti et al. (2005 ▶); Shi & Wang (2002 ▶).
Experimental
Crystal data
C14H13ClN2O3S
M r = 324.77
Orthorhombic,

a = 12.578 (2) Å
b = 7.5460 (8) Å
c = 30.194 (3) Å
V = 2865.7 (7) Å3
Z = 8
Cu Kα radiation
μ = 3.83 mm−1
T = 193 K
0.35 × 0.35 × 0.25 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (CORINC; Dräger & Gattow, 1971 ▶) T min = 0.872, T max = 0.997
5291 measured reflections
2713 independent reflections
2412 reflections with I > 2σ(I)
R int = 0.079
3 standard reflections every 60 min intensity decay: 2%
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.129
S = 1.13
2713 reflections
193 parameters
H-atom parameters constrained
Δρmax = 0.44 e Å−3
Δρmin = −0.33 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 Software; data reduction: CORINC (Dräger & Gattow, 1971 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: PLATON.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810048324/bt5410sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810048324/bt5410Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O13i | 0.95 | 2.46 | 3.404 (3) | 174 |
| C14—H14B⋯O10ii | 0.98 | 2.50 | 3.170 (4) | 126 |
| C18—H18⋯O9iii | 0.95 | 2.46 | 3.334 (3) | 152 |
Symmetry codes: (i)  ; (ii)
; (ii) 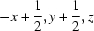 ; (iii)
; (iii)  .
.
Acknowledgments
The authors would like to thank the Federal Ministry of Education and Research, Germany, Merckle GmbH, Ulm, Germany and Fonds der Chemischen Industrie, Germany for their generous support of this work.
supplementary crystallographic information
Comment
In recent years, compounds with the 2-aminopyridine moiety exhibited interesting biological activities like the 2-alkylaminopyridinyl or 2-acylaminopyridinyl imidazole derivatives as p38α mitogen-activated protein kinase (p38α MAPK) inhibitors. The N-protected 4-chloropyridine is an important precursor to block the nucleophilic and basic properties of the amino-group in the C2 position of the pyridine ring. The analysis of the crystal structure shows that the aromatic C18—H-group of the 4-chloropyridine ring of one molecule interacts with the oxygen-atom O9 of the sulfonyl group of another molecule related to the first by centre of symmetry with a distance of H18···O9 2.47 Å. Furthermore, the aromatic C3—H group of the 4-methylphenylsulfonyl ring forms an intermolecular C3—H3···O13_a hydrogen bond (2.46 Å) to the oxygen atom O13 of the acetamide moiety of a third molecule. An additional hydrogen bond was observed between the methyl-group C14—H3 of the acetamide moiety and the oxygen-atom O10 of the sulfonyl group of a further molecule, whereas the O10···H14B distance is 2.50 Å. The dihedral angle between the 4-methylphenylsulfonyl ring and the 4-chloropyridine ring is 30.6 (1)°.
Experimental
Synthesis of chloromethyl methyl ether as a solution of toluene: To a solution of dimethoxymethane (44.3 ml, 0.50 mol, 1 equiv) and Zn(OAc)2 (9.2 mg, 0.01%) in toluene (133 ml) was added acetyl chloride (35.5 ml, 0.50 mol, 1 equiv). During the next 15 min, the reaction mixture warmed slowly at T = 318 K, and then cooled to ambient temperature over 3 h. The progress was again monitored until NMR analysis indicated complete conversion. The solution of MOMCl in toluene prepared using this stoichiometry is approximately 2.1 M.
Synthesis of N-(4-chloropyridin-2-yl)-4-methylbenzenesulfonamide: 2-Amino- 4-chloropyridine (20.1 g, 156 mmol. 1 equiv) and 4-toluenesulfonyl chloride (32.4 g, 168 mmol, 1.1 equiv) were dissolved in dry pyridine (70 ml) and heated at T = 353 K for 5 h. After cooling to room temperature, water was added and the compound N-(4-chloropyridin-2-yl)-4-methylbenzenesulfonamide dropped down as a beige solid with high analytical quality, which was filtered off and washed with water (30.6 g, 70.8%).
Synthesis of N-(4-chloropyridin-2-yl)-N-tosylacetamide: Under a nitrogen atmosphere, N-(4-chloropyridin-2-yl)-4-methylbenzene-sulfonamide (20.0 g, 71 mmol, 1 equiv) was added to a suspension of NaH (4.2 g, 104 mmol, 1.5 equiv) in anhydrous THF (200 ml) with stirring. The resulting reaction mixture was stirred for 20 min, and then the solution of methoxymethyl chloride in toluene (52.1 ml, 1.5 equiv) was slowly added. The mixture was stirred for 3 h and then an aqueous saturated solution of NH4Cl was added. After separation, the aqueous layer was extracted with EtOAc, dried over Na2SO4 and evaporated. After treatment with hexane, the compound N-(4-chloropyridin-2-yl)- N-(methoxymethyl)-4-methylbenzenesulfonamide was obtained as the main product of the reaction (15.8 g, 69.7%) and dropped down as a pale yellow solid, whereas the compound N-(4-chloropyridin-2-yl)-N-tosylacetamide was isolated from the filtrate as the byproduct (15.4%). Suitable crystals of the byproduct N-(4-chloropyridin-2-yl)-N-tosylacetamide for X-ray were obtained by slow evaporation at T = 298 K of a solution mixture of EtOAc/hexane.
Refinement
Hydrogen atoms were placed at calculated positions with C—H = 0.95 Å (aromatic) or 0.98–0.99 Å (sp3 C-atom) and refined in the riding-model approximation with isotropic displacement parameters (set at 1.2–1.5 times of the Ueq of the parent atom).
Figures
Fig. 1.
View of compound I. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C14H13ClN2O3S | F(000) = 1344 |
| Mr = 324.77 | Dx = 1.506 Mg m−3 |
| Orthorhombic, Pbca | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 25 reflections |
| a = 12.578 (2) Å | θ = 65–69° |
| b = 7.5460 (8) Å | µ = 3.83 mm−1 |
| c = 30.194 (3) Å | T = 193 K |
| V = 2865.7 (7) Å3 | Block, colourless |
| Z = 8 | 0.35 × 0.35 × 0.25 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | 2412 reflections with I > 2σ(I) |
| Radiation source: rotating anode | Rint = 0.079 |
| graphite | θmax = 70.0°, θmin = 2.9° |
| ω/2θ scans | h = −15→15 |
| Absorption correction: ψ scan (CORINC; Dräger & Gattow, 1971) | k = 0→9 |
| Tmin = 0.872, Tmax = 0.997 | l = 0→36 |
| 5291 measured reflections | 3 standard reflections every 60 min |
| 2713 independent reflections | intensity decay: 2% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H-atom parameters constrained |
| wR(F2) = 0.129 | w = 1/[σ2(Fo2) + (0.0592P)2 + 0.6955P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.13 | (Δ/σ)max = 0.001 |
| 2713 reflections | Δρmax = 0.44 e Å−3 |
| 193 parameters | Δρmin = −0.33 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.00129 (16) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.26049 (7) | 0.59127 (8) | 0.519895 (18) | 0.0474 (2) | |
| C1 | 0.55234 (19) | 0.0478 (3) | 0.36074 (7) | 0.0279 (5) | |
| C2 | 0.5457 (2) | −0.0380 (3) | 0.31992 (7) | 0.0299 (5) | |
| H2 | 0.4787 | −0.0702 | 0.3079 | 0.036* | |
| C3 | 0.6385 (2) | −0.0751 (3) | 0.29725 (7) | 0.0358 (6) | |
| H3 | 0.6346 | −0.1337 | 0.2694 | 0.043* | |
| C4 | 0.7371 (2) | −0.0290 (3) | 0.31410 (8) | 0.0374 (6) | |
| C5 | 0.7421 (2) | 0.0548 (3) | 0.35535 (8) | 0.0379 (5) | |
| H5 | 0.8093 | 0.0851 | 0.3675 | 0.046* | |
| C6 | 0.6503 (2) | 0.0940 (3) | 0.37862 (7) | 0.0342 (5) | |
| H6 | 0.6542 | 0.1519 | 0.4065 | 0.041* | |
| C7 | 0.8367 (3) | −0.0685 (4) | 0.28847 (11) | 0.0545 (8) | |
| H7A | 0.8945 | −0.0963 | 0.3091 | 0.082* | |
| H7B | 0.8562 | 0.0351 | 0.2707 | 0.082* | |
| H7C | 0.8243 | −0.1700 | 0.2689 | 0.082* | |
| S8 | 0.43683 (5) | 0.09319 (7) | 0.390620 (16) | 0.0289 (2) | |
| O9 | 0.46215 (17) | 0.0989 (2) | 0.43669 (5) | 0.0394 (4) | |
| O10 | 0.35394 (15) | −0.0198 (2) | 0.37556 (5) | 0.0390 (4) | |
| N11 | 0.40466 (17) | 0.3056 (2) | 0.37834 (6) | 0.0295 (4) | |
| C12 | 0.38183 (19) | 0.3544 (3) | 0.33447 (7) | 0.0314 (5) | |
| O13 | 0.37972 (16) | 0.2443 (2) | 0.30578 (5) | 0.0404 (4) | |
| C14 | 0.3638 (3) | 0.5483 (3) | 0.32551 (8) | 0.0451 (6) | |
| H14A | 0.4324 | 0.6097 | 0.3245 | 0.068* | |
| H14B | 0.3201 | 0.5990 | 0.3492 | 0.068* | |
| H14C | 0.3273 | 0.5623 | 0.2971 | 0.068* | |
| C15 | 0.41589 (19) | 0.4361 (3) | 0.41287 (6) | 0.0270 (5) | |
| C16 | 0.33884 (19) | 0.4476 (3) | 0.44486 (6) | 0.0278 (4) | |
| H16 | 0.2787 | 0.3713 | 0.4447 | 0.033* | |
| C17 | 0.3522 (2) | 0.5750 (3) | 0.47748 (7) | 0.0305 (5) | |
| C18 | 0.4390 (2) | 0.6871 (3) | 0.47593 (8) | 0.0373 (6) | |
| H18 | 0.4488 | 0.7773 | 0.4975 | 0.045* | |
| C19 | 0.5105 (2) | 0.6634 (3) | 0.44216 (8) | 0.0389 (6) | |
| H19 | 0.5704 | 0.7400 | 0.4411 | 0.047* | |
| N20 | 0.50149 (17) | 0.5390 (3) | 0.41048 (6) | 0.0343 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0655 (5) | 0.0449 (4) | 0.0320 (3) | 0.0135 (3) | 0.0132 (3) | −0.0045 (2) |
| C1 | 0.0379 (13) | 0.0247 (10) | 0.0211 (9) | 0.0010 (9) | −0.0010 (8) | 0.0012 (8) |
| C2 | 0.0408 (13) | 0.0237 (10) | 0.0251 (10) | −0.0023 (10) | −0.0025 (9) | −0.0026 (8) |
| C3 | 0.0527 (15) | 0.0273 (10) | 0.0274 (10) | 0.0045 (11) | 0.0038 (11) | −0.0014 (8) |
| C4 | 0.0440 (15) | 0.0286 (11) | 0.0396 (11) | 0.0089 (11) | 0.0078 (11) | 0.0102 (9) |
| C5 | 0.0349 (13) | 0.0353 (12) | 0.0436 (12) | 0.0010 (10) | −0.0063 (11) | 0.0064 (10) |
| C6 | 0.0455 (15) | 0.0296 (11) | 0.0275 (10) | 0.0013 (10) | −0.0086 (10) | −0.0012 (8) |
| C7 | 0.0509 (18) | 0.0453 (15) | 0.0675 (18) | 0.0124 (14) | 0.0187 (16) | 0.0125 (13) |
| S8 | 0.0396 (4) | 0.0244 (3) | 0.0225 (3) | 0.0014 (2) | 0.0028 (2) | 0.00126 (17) |
| O9 | 0.0628 (12) | 0.0318 (8) | 0.0236 (8) | 0.0098 (8) | 0.0048 (8) | 0.0027 (6) |
| O10 | 0.0411 (10) | 0.0320 (8) | 0.0438 (9) | −0.0061 (8) | 0.0062 (7) | 0.0011 (7) |
| N11 | 0.0382 (11) | 0.0257 (9) | 0.0244 (8) | 0.0029 (8) | 0.0009 (7) | −0.0017 (7) |
| C12 | 0.0326 (12) | 0.0350 (11) | 0.0265 (10) | 0.0013 (10) | −0.0022 (9) | 0.0019 (9) |
| O13 | 0.0564 (11) | 0.0390 (9) | 0.0257 (7) | 0.0014 (9) | −0.0055 (7) | −0.0002 (7) |
| C14 | 0.0530 (16) | 0.0427 (14) | 0.0397 (12) | 0.0063 (13) | 0.0026 (12) | 0.0058 (11) |
| C15 | 0.0336 (11) | 0.0252 (10) | 0.0221 (9) | 0.0050 (9) | −0.0035 (8) | 0.0013 (7) |
| C16 | 0.0327 (11) | 0.0256 (10) | 0.0252 (9) | 0.0019 (9) | −0.0014 (8) | 0.0014 (8) |
| C17 | 0.0397 (13) | 0.0281 (10) | 0.0235 (9) | 0.0091 (10) | −0.0021 (9) | 0.0020 (8) |
| C18 | 0.0520 (15) | 0.0272 (11) | 0.0327 (11) | 0.0039 (11) | −0.0129 (10) | −0.0039 (9) |
| C19 | 0.0379 (13) | 0.0321 (12) | 0.0467 (13) | −0.0030 (11) | −0.0089 (11) | 0.0011 (10) |
| N20 | 0.0350 (11) | 0.0315 (10) | 0.0364 (10) | 0.0005 (9) | −0.0008 (8) | 0.0025 (8) |
Geometric parameters (Å, °)
| Cl1—C17 | 1.728 (2) | S8—N11 | 1.6942 (18) |
| C1—C6 | 1.390 (3) | N11—C12 | 1.405 (3) |
| C1—C2 | 1.395 (3) | N11—C15 | 1.441 (3) |
| C1—S8 | 1.744 (2) | C12—O13 | 1.201 (3) |
| C2—C3 | 1.382 (4) | C12—C14 | 1.505 (3) |
| C2—H2 | 0.9500 | C14—H14A | 0.9800 |
| C3—C4 | 1.385 (4) | C14—H14B | 0.9800 |
| C3—H3 | 0.9500 | C14—H14C | 0.9800 |
| C4—C5 | 1.398 (4) | C15—N20 | 1.329 (3) |
| C4—C7 | 1.502 (4) | C15—C16 | 1.371 (3) |
| C5—C6 | 1.384 (4) | C16—C17 | 1.387 (3) |
| C5—H5 | 0.9500 | C16—H16 | 0.9500 |
| C6—H6 | 0.9500 | C17—C18 | 1.381 (4) |
| C7—H7A | 0.9800 | C18—C19 | 1.371 (4) |
| C7—H7B | 0.9800 | C18—H18 | 0.9500 |
| C7—H7C | 0.9800 | C19—N20 | 1.345 (3) |
| S8—O10 | 1.4213 (19) | C19—H19 | 0.9500 |
| S8—O9 | 1.4276 (16) | ||
| C6—C1—C2 | 120.8 (2) | N11—S8—C1 | 105.75 (10) |
| C6—C1—S8 | 119.24 (16) | C12—N11—C15 | 121.53 (18) |
| C2—C1—S8 | 119.92 (18) | C12—N11—S8 | 120.24 (15) |
| C3—C2—C1 | 118.8 (2) | C15—N11—S8 | 117.69 (14) |
| C3—C2—H2 | 120.6 | O13—C12—N11 | 120.2 (2) |
| C1—C2—H2 | 120.6 | O13—C12—C14 | 122.7 (2) |
| C2—C3—C4 | 121.5 (2) | N11—C12—C14 | 117.1 (2) |
| C2—C3—H3 | 119.2 | C12—C14—H14A | 109.5 |
| C4—C3—H3 | 119.2 | C12—C14—H14B | 109.5 |
| C3—C4—C5 | 118.8 (2) | H14A—C14—H14B | 109.5 |
| C3—C4—C7 | 120.5 (2) | C12—C14—H14C | 109.5 |
| C5—C4—C7 | 120.7 (3) | H14A—C14—H14C | 109.5 |
| C6—C5—C4 | 120.8 (2) | H14B—C14—H14C | 109.5 |
| C6—C5—H5 | 119.6 | N20—C15—C16 | 125.0 (2) |
| C4—C5—H5 | 119.6 | N20—C15—N11 | 116.06 (19) |
| C5—C6—C1 | 119.3 (2) | C16—C15—N11 | 118.9 (2) |
| C5—C6—H6 | 120.4 | C15—C16—C17 | 117.3 (2) |
| C1—C6—H6 | 120.4 | C15—C16—H16 | 121.4 |
| C4—C7—H7A | 109.5 | C17—C16—H16 | 121.4 |
| C4—C7—H7B | 109.5 | C18—C17—C16 | 119.8 (2) |
| H7A—C7—H7B | 109.5 | C18—C17—Cl1 | 120.60 (17) |
| C4—C7—H7C | 109.5 | C16—C17—Cl1 | 119.64 (19) |
| H7A—C7—H7C | 109.5 | C19—C18—C17 | 117.6 (2) |
| H7B—C7—H7C | 109.5 | C19—C18—H18 | 121.2 |
| O10—S8—O9 | 119.57 (11) | C17—C18—H18 | 121.2 |
| O10—S8—N11 | 108.79 (10) | N20—C19—C18 | 124.4 (2) |
| O9—S8—N11 | 103.77 (9) | N20—C19—H19 | 117.8 |
| O10—S8—C1 | 109.13 (10) | C18—C19—H19 | 117.8 |
| O9—S8—C1 | 108.91 (12) | C15—N20—C19 | 115.9 (2) |
| C6—C1—C2—C3 | −0.5 (3) | O9—S8—N11—C15 | 3.9 (2) |
| S8—C1—C2—C3 | −178.90 (16) | C1—S8—N11—C15 | −110.65 (18) |
| C1—C2—C3—C4 | −0.1 (3) | C15—N11—C12—O13 | 175.1 (2) |
| C2—C3—C4—C5 | 1.0 (3) | S8—N11—C12—O13 | 3.8 (3) |
| C2—C3—C4—C7 | −179.1 (2) | C15—N11—C12—C14 | −3.2 (3) |
| C3—C4—C5—C6 | −1.2 (4) | S8—N11—C12—C14 | −174.53 (19) |
| C7—C4—C5—C6 | 178.9 (2) | C12—N11—C15—N20 | −69.5 (3) |
| C4—C5—C6—C1 | 0.6 (3) | S8—N11—C15—N20 | 102.1 (2) |
| C2—C1—C6—C5 | 0.3 (3) | C12—N11—C15—C16 | 109.6 (2) |
| S8—C1—C6—C5 | 178.69 (17) | S8—N11—C15—C16 | −78.8 (2) |
| C6—C1—S8—O10 | −158.12 (17) | N20—C15—C16—C17 | −0.8 (3) |
| C2—C1—S8—O10 | 20.3 (2) | N11—C15—C16—C17 | −179.85 (18) |
| C6—C1—S8—O9 | −26.0 (2) | C15—C16—C17—C18 | 1.9 (3) |
| C2—C1—S8—O9 | 152.42 (17) | C15—C16—C17—Cl1 | −177.71 (16) |
| C6—C1—S8—N11 | 85.00 (19) | C16—C17—C18—C19 | −1.6 (3) |
| C2—C1—S8—N11 | −96.60 (19) | Cl1—C17—C18—C19 | 178.00 (18) |
| O10—S8—N11—C12 | −56.1 (2) | C17—C18—C19—N20 | 0.2 (4) |
| O9—S8—N11—C12 | 175.60 (19) | C16—C15—N20—C19 | −0.5 (3) |
| C1—S8—N11—C12 | 61.0 (2) | N11—C15—N20—C19 | 178.49 (19) |
| O10—S8—N11—C15 | 132.24 (18) | C18—C19—N20—C15 | 0.9 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O13i | 0.95 | 2.46 | 3.404 (3) | 174 |
| C14—H14B···O10ii | 0.98 | 2.50 | 3.170 (4) | 126 |
| C18—H18···O9iii | 0.95 | 2.46 | 3.334 (3) | 152 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) −x+1/2, y+1/2, z; (iii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5410).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Berliner, M. A. & Belecki, K. (2005). J. Org. Chem.70, 9618–9621. [DOI] [PubMed]
- Dräger, M. & Gattow, G. (1971). Acta Chem. Scand.25, 761–762.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Kocieński, P. J. (2005). Protecting Groups, 3rd ed. Stuttgart: Georg Thieme Verlag.
- Laufer, S. A., Hauser, D. R. J., Domeyer, D. M., Kinkel, K. & Liedtke, A. J. (2008). J. Med. Chem.51, 4122–4149. [DOI] [PubMed]
- Laufer, S., Hauser, D., Stegmiller, T., Bracht, C., Ruff, K., Schattel, V., Albrecht, W. & Koch, P. (2010). Bioorg. Med. Chem. Lett.20, 6671–6675. [DOI] [PubMed]
- Sciotti, R. J., Starr, J. T., Richardson, C., Rewcastle, G. W., Palmer, B. D., Sutherland, H. S., Spicer, J. A. & Chen, H. (2005). PCT Int. Appl. WO 2005089763, 100 pp.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shi, M. & Wang, C.-J. (2002). Tetrahedron Asymmetry, 13, 2161–2166.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Ziegler, K., Hauser, D. R. J., Unger, A., Albrecht, W. & Laufer, S. A. (2009). ChemMedChem, 4, 1939-1948. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810048324/bt5410sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810048324/bt5410Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



