Abstract
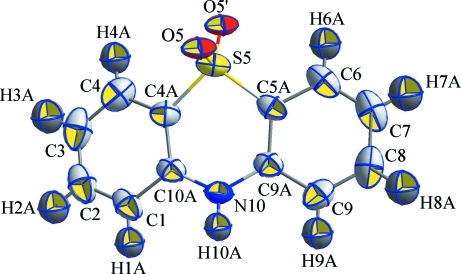
In the title compound, C12H9NOS, the sulfoxide O atom is disordered over two sites with occupancies of 0.907 (4) and 0.093 (4). The dihedral angle betweeen the two aromatic rings is 18.40 (14)°. Different types of supramolecular interactions including intermolecular N—H⋯O hydrogen bonds and π–π contacts [centroid–centroid distances = 3.9096 (16) and 4.1423 (16) Å] between the aromatic rings of symmetry-related molecules are observed in the crystal structure.
Related literature
For N-arylphenothiazine structures, see: Chu & Van der Helm (1974 ▶, 1975 ▶, 1976 ▶) and for N-arylphenothiazine oxide structures, see: Chu et al. (1985 ▶), Wang et al. (2009 ▶). For a dioxophenothiazinium cation co-crystallized with terephthalate trihydrate, see: Zhu et al. (2007 ▶).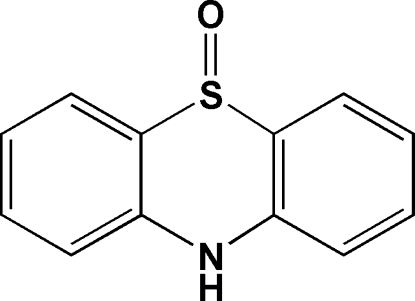
Experimental
Crystal data
C12H9NOS
M r = 215.26
Monoclinic,
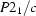
a = 6.4482 (4) Å
b = 7.6610 (5) Å
c = 22.0956 (14) Å
β = 110.466 (2)°
V = 1022.62 (11) Å3
Z = 4
Mo Kα radiation
μ = 0.29 mm−1
T = 297 K
0.50 × 0.50 × 0.40 mm
Data collection
Bruker APEX area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2002 ▶) T min = 0.871, T max = 0.895
7632 measured reflections
2361 independent reflections
1962 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.063
wR(F 2) = 0.175
S = 1.04
2361 reflections
146 parameters
6 restraints
H-atom parameters constrained
Δρmax = 0.44 e Å−3
Δρmin = −0.19 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT (Bruker, 2002 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2010) ▶; software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810047914/si2310sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810047914/si2310Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N10—H10A⋯O5i | 0.86 | 2.10 | 2.856 (3) | 146 |
Symmetry code: (i) 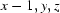 .
.
Acknowledgments
We are grateful for financial support by the National Natural Science Foundation of China (Nos. 20471049, 21071117) and NFFTBS (No. J1030415).
supplementary crystallographic information
Comment
The crystal structures of N-arylphenothiazine (Chu & Van der Helm, 1974, 1975, 1976), N-arylphenothiazine oxides (Chu et al., 1985; Wang et al., 2009) and dioxide (Zhu et al., 2007) have been reported, yet that of phenothiazine or its oxide has not been reported. The title compound (I) was obtained by the oxidation of phenothiazine in THF solution in air.
In the structure of I (Fig. 1), the sulfoxide O atom is disordered over two sites and the occupancy factors are 0.907 (4) (boat-axial S—O) and 0.093 (4) (boat-equatorial S—O). The same disorder in 10-acetyl-10H-phenothiazine 5-oxide was reported recently (Wang et al., 2009). The weighted average S—O distance of 1.471 Å in I is comparable to 1.466 Å in 10-acetyl-10H-phenothiazine 5-oxide, 1.498 (2) Å in 10-methylphenothiazine 5-oxide, and longer than 1.446 Å for dioxophenothiazinium cation (Zhu et al. 2007). The significantly shorter N—C distances in I than those in other N-arylphenothiazines or oxides are due to N—H instead of N-aryl groups (see the following table). For the same reason the dihedral angle betweeen the two benzene rings 18.40 (14) ° in I is smaller than those in the other compounds.
N—C (Å) substituent (reference)
1.365 (3), 1.368 (3) H (this work)
1.402 (2), 1.455 (5) methyl (Chu & Van der Helm, 1974)
1.406 (4), 1.427 (4) ethyl (Chu & Van der Helm, 1975)
1.410 (2), 1.414 (2) isopropyl (Chu & Van der Helm, 1976)
1.428 (2), 1.436 (2) acetyl (Wang et al., 2009)
1.409 (3), 1.409 (3) 2-dimethylammonium-propyl (Zhu et al. 2007)
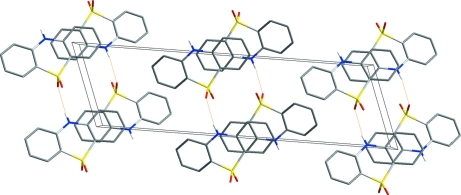
In the crystal structure (Fig. 2), intermolecular interactions N—H···O hydrogen bond and π–π contacts between the aromatic rings [centroid to centroid distances = 3.9096 (16) and 4.1423 (16) Å] of symmetry-related molecules are observed.
Experimental
A mixture of 1,3,5-benzenetricarboxylic acid (0.5 mmol) and phenothiazine (0.5 mmol) was dissolved in 10 ml THF. The solution changed from colorless to red in air in several hours. Brown crystals were obtained by slow evaporation for about 4 days at room temperature.
Refinement
The aromatic H atoms were generated geometrically (C—H 0.93, N—H 0.86 Å) and were allowed to ride on their parent atoms in the riding model approximations, with their temperature factors set to 1.2 times those of the parent atoms. The position of the oxygen atom is refined at two sites, with occupancy factors of 0.907 (4) and 0.093 (4).
Figures
Fig. 1.
Thermal ellipsoid plot of I. Displacement ellipsoids are drawn at the 50% probabability level.
Fig. 2.
A perspective view of the crystal structure of I. Hydrogen atoms have been omitted for clarity.
Crystal data
| C12H9NOS | F(000) = 448 |
| Mr = 215.26 | Dx = 1.398 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3079 reflections |
| a = 6.4482 (4) Å | θ = 2.7–27.3° |
| b = 7.6610 (5) Å | µ = 0.28 mm−1 |
| c = 22.0956 (14) Å | T = 297 K |
| β = 110.466 (2)° | Block, brown |
| V = 1022.62 (11) Å3 | 0.50 × 0.50 × 0.40 mm |
| Z = 4 |
Data collection
| Bruker APEX area-detector diffractometer | 2361 independent reflections |
| Radiation source: fine-focus sealed tube | 1962 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| φ and ω scan | θmax = 28.6°, θmin = 2.0° |
| Absorption correction: multi-scan (SADABS; Bruker, 2002) | h = −8→8 |
| Tmin = 0.871, Tmax = 0.895 | k = −9→9 |
| 7632 measured reflections | l = −28→29 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.063 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.175 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.098P)2 + 0.4384P] where P = (Fo2 + 2Fc2)/3 |
| 2361 reflections | (Δ/σ)max < 0.001 |
| 146 parameters | Δρmax = 0.44 e Å−3 |
| 6 restraints | Δρmin = −0.19 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S5 | 0.42382 (10) | 0.16598 (9) | 0.58407 (3) | 0.0511 (3) | |
| O5 | 0.5476 (3) | 0.3348 (3) | 0.60011 (10) | 0.0515 (6) | 0.907 (4) |
| O5' | 0.537 (2) | 0.0431 (17) | 0.5773 (6) | 0.024 (4) | 0.093 (4) |
| N10 | −0.0212 (3) | 0.2763 (3) | 0.59516 (10) | 0.0465 (5) | |
| H10A | −0.1256 | 0.3363 | 0.6009 | 0.056* | |
| C1 | −0.1959 (5) | 0.3230 (3) | 0.48155 (14) | 0.0563 (7) | |
| H1A | −0.3197 | 0.3677 | 0.4885 | 0.068* | |
| C2 | −0.1951 (6) | 0.3105 (4) | 0.42027 (16) | 0.0695 (9) | |
| H2A | −0.3182 | 0.3472 | 0.3859 | 0.083* | |
| C3 | −0.0141 (7) | 0.2438 (4) | 0.40843 (15) | 0.0746 (10) | |
| H3A | −0.0169 | 0.2332 | 0.3662 | 0.090* | |
| C4A | 0.1721 (4) | 0.2076 (3) | 0.52193 (12) | 0.0459 (6) | |
| C4 | 0.1689 (6) | 0.1936 (4) | 0.45877 (15) | 0.0624 (8) | |
| H4A | 0.2917 | 0.1500 | 0.4509 | 0.075* | |
| C5A | 0.3231 (4) | 0.1236 (3) | 0.64649 (12) | 0.0458 (6) | |
| C6 | 0.4605 (5) | 0.0291 (4) | 0.69942 (15) | 0.0621 (8) | |
| H6A | 0.5904 | −0.0202 | 0.6979 | 0.075* | |
| C7 | 0.4058 (6) | 0.0086 (4) | 0.75312 (16) | 0.0750 (9) | |
| H7A | 0.4974 | −0.0554 | 0.7880 | 0.090* | |
| C8 | 0.2155 (7) | 0.0822 (4) | 0.75575 (15) | 0.0725 (9) | |
| H8A | 0.1807 | 0.0699 | 0.7930 | 0.087* | |
| C9A | 0.1255 (4) | 0.1936 (3) | 0.64756 (12) | 0.0433 (5) | |
| C9 | 0.0752 (5) | 0.1739 (4) | 0.70411 (15) | 0.0592 (7) | |
| H9A | −0.0533 | 0.2232 | 0.7067 | 0.071* | |
| C10A | −0.0122 (4) | 0.2694 (3) | 0.53439 (12) | 0.0429 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S5 | 0.0328 (4) | 0.0552 (4) | 0.0649 (5) | 0.0046 (2) | 0.0165 (3) | −0.0057 (3) |
| O5 | 0.0265 (9) | 0.0631 (13) | 0.0647 (13) | −0.0072 (8) | 0.0156 (9) | −0.0056 (9) |
| O5' | 0.024 (4) | 0.025 (4) | 0.025 (4) | 0.0011 (10) | 0.0089 (16) | −0.0008 (10) |
| N10 | 0.0303 (9) | 0.0531 (12) | 0.0573 (13) | 0.0056 (9) | 0.0166 (9) | 0.0031 (10) |
| C1 | 0.0421 (14) | 0.0503 (14) | 0.0633 (17) | −0.0057 (11) | 0.0018 (12) | 0.0081 (12) |
| C2 | 0.067 (2) | 0.0634 (18) | 0.0589 (18) | −0.0144 (15) | −0.0016 (15) | 0.0080 (14) |
| C3 | 0.096 (3) | 0.073 (2) | 0.0481 (17) | −0.026 (2) | 0.0172 (17) | −0.0066 (15) |
| C4A | 0.0401 (13) | 0.0447 (12) | 0.0511 (14) | −0.0055 (10) | 0.0139 (11) | −0.0057 (10) |
| C4 | 0.0675 (19) | 0.0626 (17) | 0.0614 (17) | −0.0154 (14) | 0.0279 (15) | −0.0152 (14) |
| C5A | 0.0364 (12) | 0.0416 (12) | 0.0532 (14) | −0.0010 (10) | 0.0077 (10) | −0.0025 (10) |
| C6 | 0.0524 (16) | 0.0517 (15) | 0.0674 (18) | 0.0074 (12) | 0.0024 (13) | 0.0060 (13) |
| C7 | 0.080 (2) | 0.0612 (19) | 0.063 (2) | −0.0008 (17) | −0.0001 (17) | 0.0118 (15) |
| C8 | 0.095 (3) | 0.0701 (19) | 0.0504 (17) | −0.0125 (18) | 0.0227 (17) | 0.0062 (14) |
| C9A | 0.0352 (12) | 0.0415 (12) | 0.0502 (14) | −0.0050 (9) | 0.0113 (10) | −0.0005 (10) |
| C9 | 0.0559 (17) | 0.0644 (17) | 0.0630 (17) | −0.0101 (13) | 0.0278 (14) | −0.0038 (13) |
| C10A | 0.0333 (11) | 0.0391 (11) | 0.0526 (14) | −0.0056 (9) | 0.0101 (10) | 0.0009 (10) |
Geometric parameters (Å, °)
| S5—O5' | 1.233 (13) | C4A—C10A | 1.393 (3) |
| S5—O5 | 1.496 (2) | C4A—C4 | 1.393 (4) |
| S5—C5A | 1.748 (3) | C4—H4A | 0.9300 |
| S5—C4A | 1.750 (3) | C5A—C9A | 1.390 (3) |
| N10—C10A | 1.365 (3) | C5A—C6 | 1.397 (4) |
| N10—C9A | 1.368 (3) | C6—C7 | 1.360 (5) |
| N10—H10A | 0.8600 | C6—H6A | 0.9300 |
| C1—C2 | 1.359 (5) | C7—C8 | 1.370 (5) |
| C1—C10A | 1.403 (3) | C7—H7A | 0.9300 |
| C1—H1A | 0.9300 | C8—C9 | 1.376 (5) |
| C2—C3 | 1.380 (5) | C8—H8A | 0.9300 |
| C2—H2A | 0.9300 | C9A—C9 | 1.404 (4) |
| C3—C4 | 1.364 (5) | C9—H9A | 0.9300 |
| C3—H3A | 0.9300 | ||
| O5'—S5—O5 | 113.5 (6) | C4A—C4—H4A | 120.0 |
| O5'—S5—C5A | 110.7 (6) | C9A—C5A—C6 | 120.1 (3) |
| O5—S5—C5A | 106.75 (12) | C9A—C5A—S5 | 122.5 (2) |
| O5'—S5—C4A | 118.1 (6) | C6—C5A—S5 | 117.0 (2) |
| O5—S5—C4A | 107.46 (12) | C7—C6—C5A | 120.5 (3) |
| C5A—S5—C4A | 98.86 (12) | C7—C6—H6A | 119.7 |
| C10A—N10—C9A | 124.1 (2) | C5A—C6—H6A | 119.7 |
| C10A—N10—H10A | 118.0 | C6—C7—C8 | 119.9 (3) |
| C9A—N10—H10A | 118.0 | C6—C7—H7A | 120.0 |
| C2—C1—C10A | 120.8 (3) | C8—C7—H7A | 120.0 |
| C2—C1—H1A | 119.6 | C7—C8—C9 | 120.9 (3) |
| C10A—C1—H1A | 119.6 | C7—C8—H8A | 119.5 |
| C1—C2—C3 | 120.8 (3) | C9—C8—H8A | 119.5 |
| C1—C2—H2A | 119.6 | N10—C9A—C5A | 122.1 (2) |
| C3—C2—H2A | 119.6 | N10—C9A—C9 | 119.8 (2) |
| C4—C3—C2 | 119.9 (3) | C5A—C9A—C9 | 118.2 (2) |
| C4—C3—H3A | 120.1 | C8—C9—C9A | 120.2 (3) |
| C2—C3—H3A | 120.1 | C8—C9—H9A | 119.9 |
| C10A—C4A—C4 | 120.6 (3) | C9A—C9—H9A | 119.9 |
| C10A—C4A—S5 | 121.9 (2) | N10—C10A—C4A | 122.7 (2) |
| C4—C4A—S5 | 117.2 (2) | N10—C10A—C1 | 119.6 (2) |
| C3—C4—C4A | 120.1 (3) | C4A—C10A—C1 | 117.7 (3) |
| C3—C4—H4A | 120.0 | ||
| C10A—C1—C2—C3 | 0.3 (4) | C5A—C6—C7—C8 | −0.6 (5) |
| C1—C2—C3—C4 | −1.6 (5) | C6—C7—C8—C9 | 1.5 (5) |
| O5'—S5—C4A—C10A | 145.5 (7) | C10A—N10—C9A—C5A | 13.3 (4) |
| O5—S5—C4A—C10A | −84.5 (2) | C10A—N10—C9A—C9 | −165.2 (2) |
| C5A—S5—C4A—C10A | 26.3 (2) | C6—C5A—C9A—N10 | −175.3 (2) |
| O5'—S5—C4A—C4 | −40.9 (7) | S5—C5A—C9A—N10 | 11.0 (3) |
| O5—S5—C4A—C4 | 89.1 (2) | C6—C5A—C9A—C9 | 3.2 (4) |
| C5A—S5—C4A—C4 | −160.1 (2) | S5—C5A—C9A—C9 | −170.48 (19) |
| C2—C3—C4—C4A | 0.8 (5) | C7—C8—C9—C9A | 0.0 (5) |
| C10A—C4A—C4—C3 | 1.3 (4) | N10—C9A—C9—C8 | 176.2 (3) |
| S5—C4A—C4—C3 | −172.4 (2) | C5A—C9A—C9—C8 | −2.3 (4) |
| O5'—S5—C5A—C9A | −151.4 (7) | C9A—N10—C10A—C4A | −13.6 (4) |
| O5—S5—C5A—C9A | 84.6 (2) | C9A—N10—C10A—C1 | 165.2 (2) |
| C4A—S5—C5A—C9A | −26.7 (2) | C4—C4A—C10A—N10 | 176.3 (2) |
| O5'—S5—C5A—C6 | 34.7 (7) | S5—C4A—C10A—N10 | −10.4 (3) |
| O5—S5—C5A—C6 | −89.3 (2) | C4—C4A—C10A—C1 | −2.5 (4) |
| C4A—S5—C5A—C6 | 159.4 (2) | S5—C4A—C10A—C1 | 170.87 (18) |
| C9A—C5A—C6—C7 | −1.8 (4) | C2—C1—C10A—N10 | −177.1 (2) |
| S5—C5A—C6—C7 | 172.2 (2) | C2—C1—C10A—C4A | 1.7 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N10—H10A···O5i | 0.86 | 2.10 | 2.856 (3) | 146 |
Symmetry codes: (i) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SI2310).
References
- Brandenburg, K. (2010). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2002). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chu, S. S. C., de Meester, P., Jovanovic, M. V. & Biehl, E. R. (1985). Acta Cryst. C41, 1111–1114.
- Chu, S. S. C. & Van der Helm, D. (1974). Acta Cryst. B30, 2489–2490.
- Chu, S. S. C. & Van der Helm, D. (1975). Acta Cryst. B31, 1179–1183.
- Chu, S. S. C. & Van der Helm, D. (1976). Acta Cryst. B32, 1012–1016.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, Q., Yang, L., Xu, Z. & Sun, Y. (2009). Acta Cryst. E65, o1978. [DOI] [PMC free article] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
- Zhu, D.-X., Sun, W., Yang, G.-F. & Ng, S. W. (2007). Acta Cryst. E63, o4830.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810047914/si2310sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810047914/si2310Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report