Abstract
The study of human evolution depends upon a fair assessment of the ability of hominin individuals to gain access to necessary resources. We expect that the morphology of extant and extinct populations represents a successful locomotory system that allowed individuals to move across the environment gaining access to food, water and mates while still maintaining excess energy to allocate to reproduction. Our assessment of locomotor morphology must then incorporate tests of fitness within realistic environments—environments that themselves vary in terrain and whose negotiation requires a variety of gait and speeds. This study assesses muscular activity (measured as the integrated signal from surface electromyography) of seven thigh and hip muscle groups during walking and running across a wide range of speeds and inclines, in order to systematically assess the role that morphology can play in minimizing muscular activity and thus energy expenditure. Our data suggest that humans are better adapted to walking than running at any slope, as evidenced by small confidence intervals and even trends across speed and incline. We find that while increasing task intensity unsurprisingly increases muscular activity in the lower limb, individuals with longer limbs show significantly reduced activity during both walking and running, especially in the hip adductors, gluteus maximus and hamstring muscles. People with a broader pelvis show significantly reduced activity while walking in the hip adductor and hamstring muscles.
Keywords: human locomotion, EMG, incline, gluteus maximus
In the study of human locomotion, it has been generally suggested that muscle activity, as measured using electromyography (EMG), will offer important clues to selective pressures shaping the locomotor elements of human form (Basmajian, 1972; Zihlman, 1978; Tuttle et al., 1979; Stern and Susman, 1981; Marzke et al., 1988; Lieberman et al., 2006). Despite that humans perform a relatively small array of gaits [e.g. walking and running, though for skipping see (Srinivasan, 2006)], they perform them over a wide variety of terrain and with varying speed combinations. Consequently, effectively filtering through possible selection pressures on locomotor morphology requires a systematic comparison of morphology (lower limb length and pelvis breadth being a reasonable place to begin), in addition to the systematic comparison of the activity of human locomotor musculature across gait, speed and incline [for lower limb muscle activity during other activities—throwing, digging, gathering, etc.—see (Marzke et al., 1988)]. Building ecological models of mobility [e.g. (Foley, 1992; Foley and Elton, 1998; Kramer, 2004)] depends upon accurate assessments of the benefits of bipedalism, and the benefits of bipedalism cannot be understood without better modelling of the influence of variable terrain and non-level surfaces. To this end integrating the interactions between speed and incline is vital for understanding selection on hominin locomotor morphology, such as lower limb length and pelvis shape (width).
In addition, previous studies have made much of the fact that certain muscle groups in people are larger than in non-human primates (e.g. gluteus maximus) (Stern and Susman, 1981; Lovejoy, 1988; Marzke et al., 1988; Lieberman et al., 2006) or smaller than in non-human primates (e.g. hip adductors, biceps femoris, medial hamstrings) [for chimpanzees: (Thorpe et al., 1999)]. This has prompted a series of studies suggesting the expansion of the gluteus maximus in particular in response to upright posture (Stern and Susman, 1981; Lovejoy, 1988), throwing (Marzke et al., 1988), or to running (Lieberman et al., 2006). For example, Lieberman et al. (2006) suggest that the larger size of the gluteus maximus is attributable to increased activity during running as compared to during walking. The logic being that the larger muscles have more actin-myosin cross bridges in parallel and thus habitually produce more force, specifically during the activities that the muscle has evolved to perform. However, it seems logical that any lower limb locomotor task of high intensity (here we will test increases in both speed and incline) will cause an increase in gluteus maximus activity, as part of a systematically more intense engagement of the lower limb musculature. We thus expect the activity (integrated EMG) of several lower limb and pelvic muscle groups to increase during intense human locomotion, such as that experienced while moving up sloped terrain and/or at the higher speeds within each gait.
In better understanding the relationship between muscle form (size) and function (activity levels), it is valuable to not simply look at which locomotor conditions (gait, speed, incline) produce muscle activity increases, but also at the range of locomotor conditions that can be produced with relatively consistent muscle activity levels within and between individuals. In those situations where we do not see substantial increases in muscular activity level or variability, we might expect those activities to cost less metabolic energy and thus be more efficient [c.f. (Zange et al., 2008; Hepple et al., 2010)]. In general, individuals able to negotiate variations in slope and speed with minimal increases in muscular activity will require less locomotor-related metabolic energy (Reilly et al., 2007), leading to an increase in their reproductive fitness (Gibson and Mace, 2006).
It is reasonable to suppose that changing the morphology of the locomotor apparatus could lead to a minimizing of muscular activity during habitually practiced forms of locomotion. Previous work has suggested a close relationship between metabolic cost, muscular force and morphology [body or limb mass: (Taylor et al., 1982; Myers and Steudel, 1985); lower limb length: (Kramer, 1999; Steudel-Numbers and Tilkens, 2004); pelvis width: (Rak, 1991; Wall-Scheffler et al., 2007)]. Because we see variation in the postcranial morphology of hominins, in particular in lower limb length and pelvic breadth, we need to continue to work towards understanding the functional significance of the variation. Some models purport direct energetic benefits of long limbs (Steudel-Numbers and Tilkens, 2004; Steudel-Numbers et al., 2007) or a broad pelvis (Wall-Scheffler et al., 2007) but typically these models are only assessing single speed, single task, or single postcranial variable. What we seek to assess in this research is how postcranial morphology (specifically lower limb length and pelvis breadth) interacts with muscle activity and thus impacts locomotor costs across multiple gaits, inclines and speeds.
We specifically address the following questions: 1. Does gait affect the pattern of muscle recruitment? 2. Does incline affect the pattern of muscle recruitment? 3. If yes to 1 & 2, is the pattern associated with increased speed (via changes in gait) different from that of increasing incline? 4. Are the effects of incline, speed and gait additive or interactive? 5. How does morphology affect muscle recruitment pattern(s)?
Which muscles to choose?
In the study of human locomotion, thigh and hamstring muscles have been reasonably well-studied. During running the hamstrings are activated prior to footstrike (Gazendam and Hof, 2007) and at push-off (Fields et al., 2005); the quadriceps are active during early swing phase (Swanson and Caldwell, 2000) and seem to be controlling the position of the body’s center of mass after landing (McClay et al., 1990; Fields et al., 2005). The hip adductors are continuously active throughout the running gait and function in stabilizing the pelvis (with respect to the thigh) during stance and vice versa during swing (McClay et al., 1990). The gluteus maximus and medius become active in late swing and are generally considered to be decelerating the thigh and assisting in stabilizing the thigh and pelvis throughout early stance (McClay et al., 1990; Fields et al., 2005).
During walking, the gluteus maximus, medius, and vastus lateralis provide the majority of support during early stance (Gazendam and Hof, 2007) and prevent the dropping of the body’s opposite side (Knutson and Soderberg, 1995). Gluteus medius activity is extended at higher speeds and shows involvement during late swing phase to increase stability and well as late stance phase to help clear the foot from the ground (Knutson and Soderberg, 1995). While the hamstrings and rectus femoris do not seem to offer much support during stance on level surfaces, the rectus femoris in particular seems to offer some support of the knee at upward elevation of the center of mass (Tokuhiro et al., 1985) during incline walking. Other quadriceps muscles are likely also involved in the stabilizing of the knee as the torso is carried forward (Basmajian, 1967). Additionally, the quadriceps and hamstring muscles (acting antagonistically) are significant during phase transitions (from swing to stance, and from stance to swing as speed increases) (Knutson and Soderberg, 1995). The hamstrings are active during the latter half of swing for decelerating the shank (Knutson and Soderberg, 1995; Gazendam and Hof, 2007), and both hamstrings and quadriceps are vital at the onset of stance to stabilize the knee joint (Tokuhiro et al., 1985; Knutson and Soderberg, 1995).
While a consensus seems to exist in the literature that, within a gait, moving from a level to inclined surface increases muscular activity (Swanson and Caldwell, 2000; Lay et al., 2007), recent research suggests in running this may relate to the speed at which an incline is taken, with faster speeds eliciting more activity at high inclines (Yokozawa et al., 2007). Walking studies have rarely reported EMG results of incline walking at more than a single self-selected speed [(Tokuhiro et al., 1985; Kawamura et al., 1991; McIntosh et al., 2006; Lay et al., 2007) though see (Arendt-Nielsen et al., 1991)], so much needs to be done in this area. Examining the possible overlap between gait, speed and incline in their influence on muscle activity seems a key next step in our understanding of selection pressures related to locomotion.
METHODS
Detailed methods have been laid out in Chumanov et al.(2008), however a brief summary follows. We collected data on 34 human subjects (17 males and 17 females), between the ages of 18–37 (mean=22.9); each signed a written informed consent form approved by the UW-Madison IRB. The protocol consisted of walking and running on a treadmill at a series of randomly ordered speeds and inclines (www.random.org). The speeds consisted of 1.2, 1.5, 1.8, 2.7, and 3.6 ms−1. Participants walked at 1.2, 1.5 and 1.8 ms−1 and ran at 1.8, 2.7, and 3.6 ms−1. Inclines included 0% (0°), 10% (5.7°), 15% (8.6°) and 20% (11.5°). Each incline was performed at each speed/gait for a total of 24 conditions. While we purposely chose individuals from a wide range of morphologies, we did ask each individual to do the same wide range of tasks. Since we are assessing the relationships between morphology, muscle activity, and locomotor conditions, looking at the effect of increasing absolute speed and incline for individuals differing in size and morphology was of particular interest. In addition, as individuals can spend part of their locomotory time moving with other individuals of different sizes (Kramer, 1998; Pontzer and Wrangham, 2006; Costa, 2010), assessing the impact of the range of activity has implications for selection on the mobility strategies of the group.
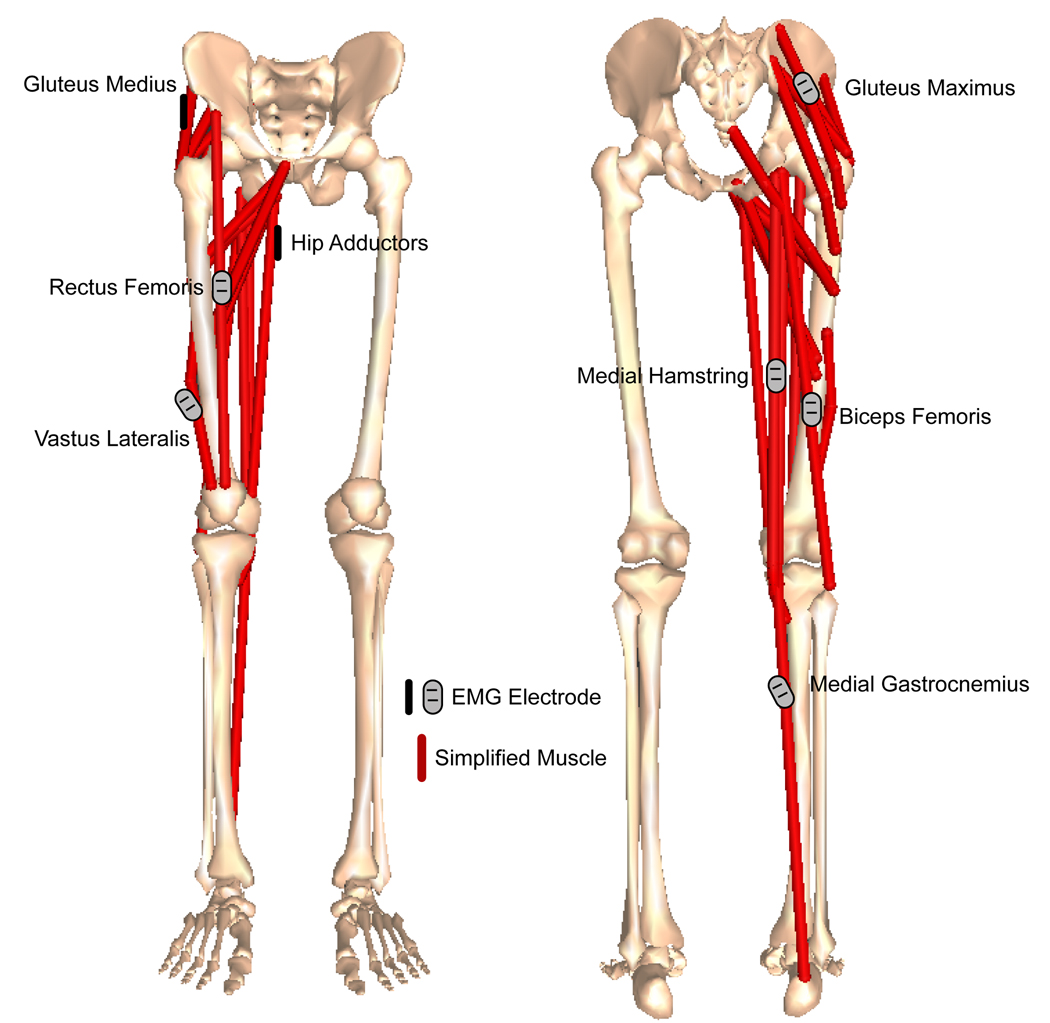
Each subject had electromyography (EMG) surface electrodes placed on seven thigh and hip muscle groups following Basmajian’s protocol (Basmajian and Blumenstein, 1989) which ensured each EMG reading is coming from the muscle of interest (Clancy et al., 2002; Rainoldi et al., 2004) (Figure 1). Proper electrode placement is further important to maximize electrical activity recording of the desired muscle while minimizing the crosstalk that can occur from adjacent muscles. As such, placement of surface electrodes is typically near a motor endplate of the muscle while maintaining adequate distance from neighboring muscles (Kamen and Caldwell, 1996). Once placed, these electrodes were secured with athletic tape and not moved for the duration of the trial (all 24 conditions) ensuring consistent and comparable readings for each individual. The muscle groups included the hamstring muscles (biceps femoris and medial hamstrings (which include semitendinosus and semimembranosus)), 2 quadriceps muscles (vastus lateralis and rectus femoris), hip adductors, hip abductors (gluteus medius) and the gluteus maximus. We decided to consider two groups of hamstrings and two of quadriceps to assess differences in activation between two muscles that have somewhat similar anatomical locations.
Figure 1.
All EMG signals were first full-wave rectified and low pass filtered using a 6th order Butterworth filter with a cutoff frequency of 50Hz. For each participant, the mean activity for each muscle was found during the slowest walking speed (1.2ms−1) on a level surface; this value was then used as the normalization factor. EMG signals vary from day to day, person to person (Kadaba et al., 1989; Knutson and Soderberg, 1995) based on placement (i.e. how close you get to the motor neuron will change the signal intensity) so normalization is necessary to compare between subjects (essentially brings all participants to the same base level). The actual factor used for normalization is not as important as the consistency between participants (Burden et al., 2003). Integrated muscle activities were found using the trapezoidal method, a numerical method for estimating integrals which uses the idea that point to point changes can each be represented as a trapezoid. The area for each trapezoid can then be found and summed to get an overall representation for the integral (Kaw and Egwu, 2009). Each muscle integrated activity was further divided by the respective normalization factor. Essentially integrated muscle activity is a measure of how active the muscle is over time. Mathematically it is the area under the curve after the EMG has been rectified and low pass filtered. Peak muscle activity and onset of muscle activity are less desirable measures because they will vary depending on electrode placement, sub-cutaneous fat, and participant hygiene (Kleissen et al., 1997). The order of speed-incline combinations was randomized for each participant, with approximately 10s of data (a minimum of 5 strides) recorded for each condition. The choice to use 5 gait cycles was based on Kadaba et al. (1989) who demonstrated repeatable kinematic, kinetic and EMG data during locomotion from as few as 3 gait cycles (Kadaba et al., 1989). Our kinematic data varied less than 2.5 degrees within each condition for all measured angles leading us to conclude that 5 strides for each condition was sufficient to accurately characterize the locomotion pattern.
Anthropometric measurements (mass, stature, bi-trochanteric breadth, biiliac breadth, thigh length and shank length (medial and lateral)) were taken on each participant (Chumanov et al., 2008) (Table 1). Thigh length was obtained by measuring the distance between the proximal portion of the greater trochanter to the lateral midpoint of the knee (equal distance between the femoral epicondyles and the tibial plateau). Lateral measures of the shank included the distance between the lateral midpoint of the knee to the most lateral portion of the lateral malleolus. Together, thigh length and lateral shank length constituted the total length of the lower limb. Medial length of the shank was also taken, following Porter (1996) in order that a correlation could be made between the external measures and skeletal measures, and thus crural index could be calculated (Porter, 1996). All lower limb measurements were collected using an anthropometer.
Table 1.
Participant anthropometric measures [mean (standard deviation)].
| Measure | Male (N=17) | Females (N=17) |
|---|---|---|
| Stature (cm) | 182.3 (7.97) | 166.5 (9.1) |
| Mass (kg) | 79.8 (13.0) | 61.0 (6.9) |
| Lower Limb Length (cm) | 88.5 (5.1) | 80.0 (4.6) |
| Bitrochanteric Breadth (cm) | 31.0 (2.1) | 30.3 (2.0) |
| Biiliac Breadth (cm) | 27.0 (2.0) | 25.5 (1.6) |
| Crural Index | 0.86 (0.04) | 0.83 (0.06) |
Statistical analysis was accomplished using SPSS 17.0 for Windows (now called PASW Statistics). Repeated measures ANOVA’s, t-tests, and predictive regressions were used to test the relationships between muscle activity, anthropometrics and task.
RESULTS
When speed is held constant
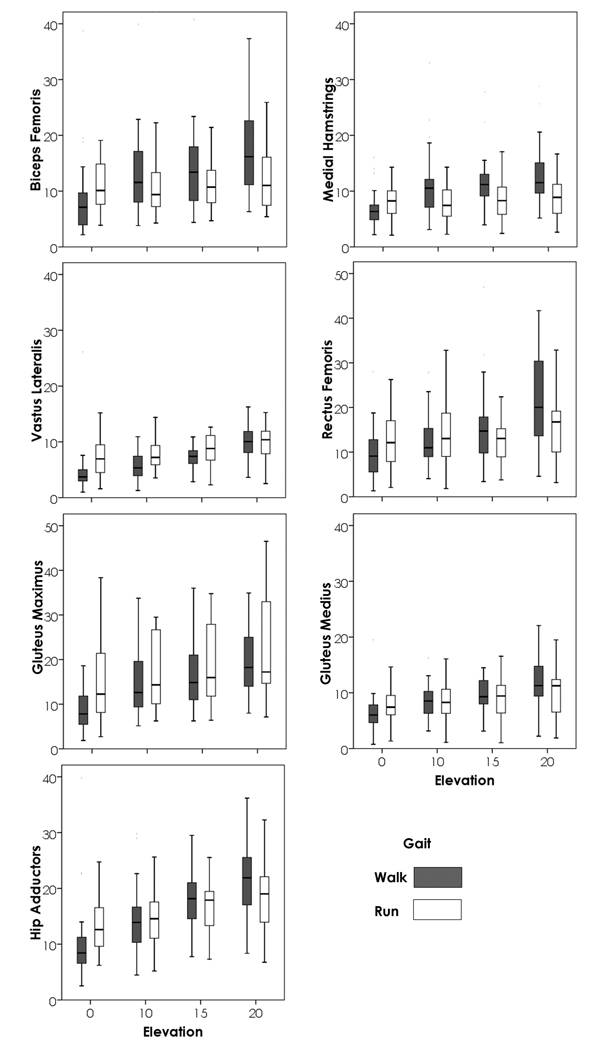
A repeated measures ANOVA was used to determine the importance of gait and incline in determining activity in each of the seven muscle groups when speed was held constant at 1.8 ms−1 (the highest walking and the lowest running speed). The repeated measures ANOVA was run both including 0% grade locomotion, and not including 0% grade locomotion. The difference between these two models is essentially the difference between being on a level or not (all 4 inclines) and whether once you are on an incline, the steepness of the slope continues to increase activity. Figure 2 illustrates these relationships. Particularly interesting to note when viewing Fig. 2 is that the relationship between walking and running changes based on incline. Whether walking requires more activity in a muscle than does running often changes between the lowest inclines and the highest (rectus femoris, hip adductors and gluteus maximus being good examples) when speed is held constant. For all muscle groups, irrespective of whether 0% grade was included, incline was of highly significant importance (p<0.001). When 0% grade was included, the interaction between gait and incline was also significant for all muscle groups (p<0.03); however, when inclines above 0% grade were considered, only the rectus femoris, biceps femoris and hip adductors (p<0.04) had significant interactions between gait and incline. When all inclines were included, gait had a significant impact on the model (p<0.01) for all muscle groups except rectus femoris, gluteus medius, and hip adductors. When 0% grade was not included, gait was not significant for rectus femoris and gluteus medius.
Figure 2.
When each gait is assessed separately
For walking
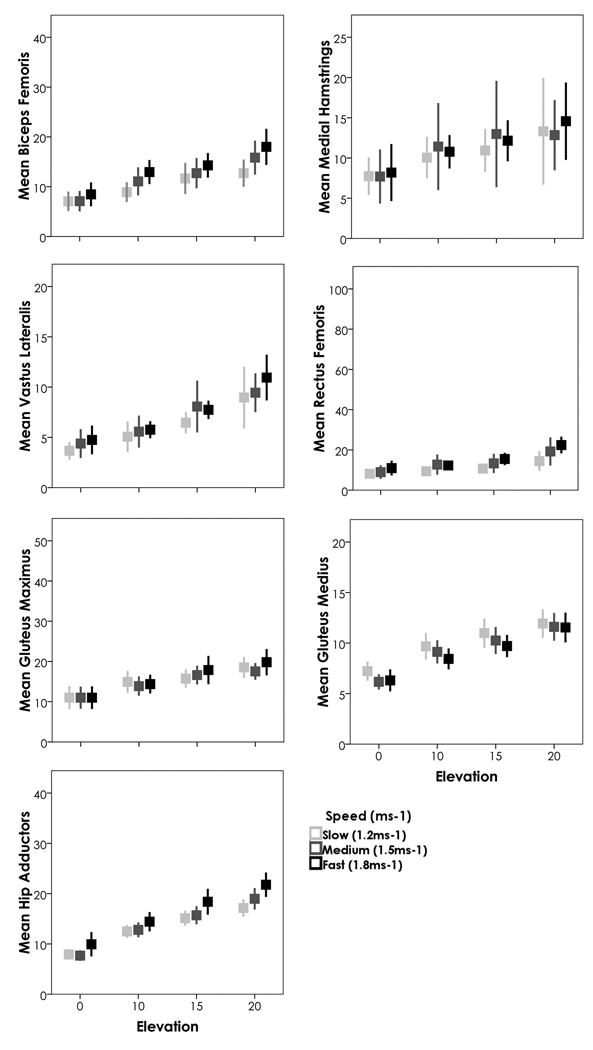
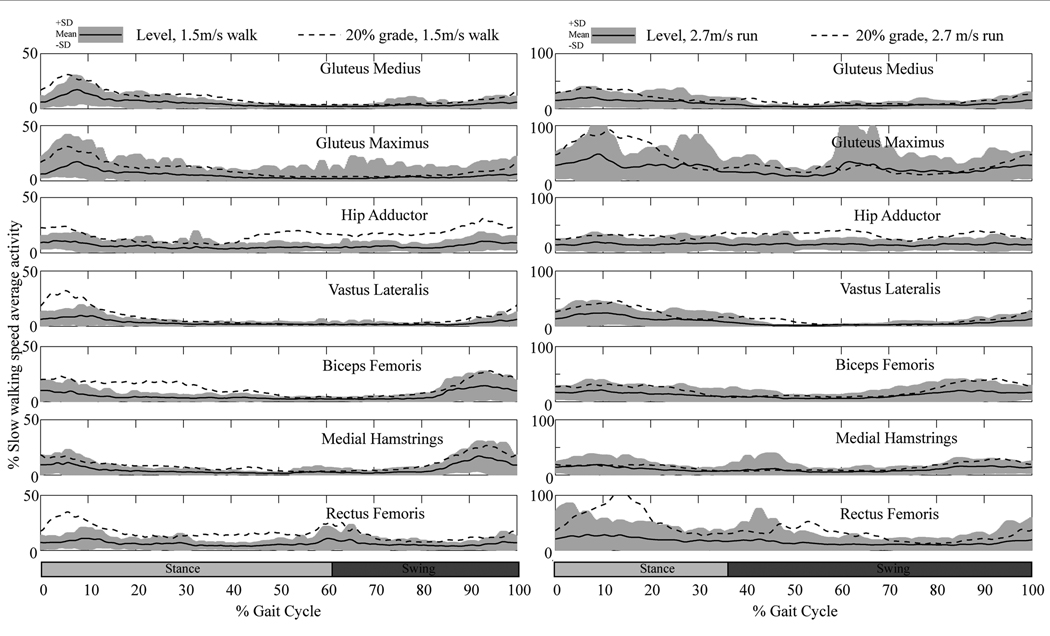
A repeated measures ANOVA was run for each muscle group assessing the roles of incline and speed (1.2, 1.5, and 1.8 ms−1) on each muscle group’s activity. Sex was also included in the model as a between-subject’s factor [previously published research showed significant differences between the sexes (Chumanov et al., 2008)]. All muscle groups had a highly significant relationship with incline (p<0.001) (Figure 3). All muscle groups except for gluteus maximus had a significant relationship with speed (p<0.006 for all other groups; p=0.3 for gluteus maximus). The interaction between speed and incline was more variable and depended both upon the muscle group and whether 0% incline was included in the model (that is, whether 3 inclines (10%, 15%, 20%) were included or 4 (0%, 10%, 15%, 20%). When all 4 inclines were included, hip adductors, rectus femoris, vastus lateralis, medial hamstrings and biceps femoris all showed significant interactions between speed and incline (p<0.008); gluteus medius and gluteus maximus did not (p>0.357). When level treadmill walking was not included, biceps femoris and medial hamstrings did not show a significant interaction between speed and incline (p>0.160). Thus, for the hip adductors (which remain active throughout the stride) and the quadriceps (activity occurs early in stance phase) speed exacerbates the effects of increasing incline itself (from 10% to 15% for example as seen in Figure 3) and significantly increases the activity of these muscle groups (See Figure 4 for activity patterns across the gait cycle). For the hamstrings (activity late in swing phase), once a person begins on an incline, speed has less of an exacerbating effect on activity. Sex showed significant interactions with the activity of the gluteus medius and speed (p<0.05).
Figure 3.
Figure 4.
For running
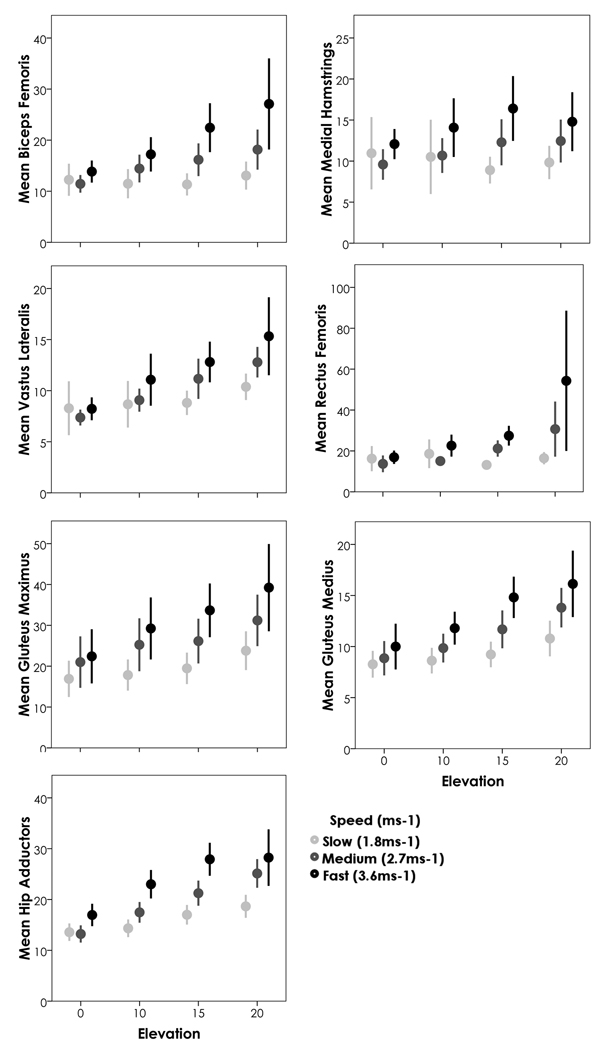
As with walking, a repeated measures ANOVA was run for each muscle group assessing the roles of incline and speed (1.8, 2.7, and 3.6 ms−1) on each muscle’s activity. Sex was again included in the model as a between-subject’s factor, though did not show significant interactions. Because only a subset of our sample performed the 20% incline at 3.6 ms−1 this analysis includes a smaller sample (N=18). When all four levels were included, incline significantly impacted every muscle group’s activity (p<0.03) (Figure 5). When only 3 inclines were included, neither rectus femoris (p=0.098) nor the medial hamstrings (p=0.790) reached significance, thus only the lateral side of the limb (biceps femoris and vastus lateralis) continue to increase activity while running up a slope. Both of these muscle groups show similar activity patterns, with increased activity during stance phase (Figure 4). The biceps femoris does pick up a bit of additional activity during the latter parts of swing on an inclined surface. Conversely the medial hamstrings do not particularly increase activity throughout the gait cycle, whereas the rectus femoris shows dramatic increases between level and inclined surfaces throughout the beginning of stance, and again at the beginning of swing; however, this increase appears to occur immediately upon reaching the incline and once the person is on the incline, then there is not such a dramatic interaction for the rectus femoris. Speed was significant for all muscle groups, irrespective of what levels were included (p<0.01). When running, the interaction between speed and incline was only consistently significant for hip adductors (p<0.01) (as with walking, hip adductors are active throughout the stride and activity increases across the gait cycle). Gluteus medius showed some interaction at inclines above 0% (p=0.041) and showed much higher activity particularly throughout stance phase, with activity remaining consistent throughout swing phase; the medial hamstrings showed some interaction when all inclines were included (p=0.015) but the increase in activity seems more prevalent at the end of swing phase. Now, if we drop the 20% grade from the analysis and re-run the above models including our full sample of 34 participants, the only difference is the significance of the interactions between speed and incline (p<0.05) for all muscle groups (for the gluteus maximus, the interaction is only significant when the 0% grade is included and primarily consists of increased activity at the beginning of stance phase). This suggests that while running, the interaction between speed and incline may be more subtle than the effect of each separately (thus the larger sample is necessary), though does not need particularly high levels of the variables (e.g. 20% incline at 3.6 ms1) to be detected.
Figure 5.
The influence of pelvis shape and lower limb length on muscle activity
Anthropometrics show the most regular relationships with the activity of biceps femoris, hip adductors, gluteus medius and gluteus maximus. The following results are from linear regressions in which the anthropometric measurements (mass, lower limb length, bitrochanteric breadth, biiliac breadth, and crural index) were put in each muscle activity model (sex, incline and speed included) in a stepwise fashion. These models thus show the significant impact of morphology after statistically controlling for all the differences identified in the previous sections (Table 2). For both walking and running, crural index showed a significant negative relationship with the activity of biceps femoris and gluteus medius (p<0.001)—the smaller the crural index (longer the femur in relation to the tibia), the greater the activity. Bitrochanteric breadth showed a significant positive relationship on each of the four muscle groups listed in Table 2 during walking (p<0.001) and with gluteus medius and biceps femoris for running (p<0.001). Lower limb length shows negative relationships for walking and running for the muscle activity of biceps femoris, hip adductors and gluteus maximus (p<0.04). Biiliac breadth is the only measure that showed a different relationship for walking and running (p<0.03): biiliac breadth has a negative relationship with hip adductor activity during walking, but a positive relationship with hip adductor activity during running, suggesting more narrow ilia have higher hip adductor activity during walking and lower activity during running. The ilia have a strong positive relationship for both walking and running for the gluteus maximus (p≤0.001) and negative for the biceps femoris (walking, p=0.05).
Table 2.
This table identifies the effect of each significant anthropometric measure on the muscle activity of the defined muscle group. Each box identifies the positive or negative effect in a model that included sex, incline, and speed. Anthropometric variables were entered into the model in a stepwise fashion; measurements not included in the table were not significant contributors to the model. See text for p-values (all p<0.05).
| Anthropometrics | Walking | Running | |
|---|---|---|---|
|
Gluteus medius |
Bitrochanteric Breadth | Positive | Positive |
| Biiliac Breadth | NS | NS | |
| Lower Limb Length | Positive | NS | |
| Crural Index | Negative | Negative | |
|
Gluteus maximus |
Bitrochanteric Breadth | Positive | NS |
| Biiliac Breadth | Positive | Positive | |
| Lower Limb Length | Negative | Negative | |
| Crural Index | NS | Positive | |
|
Hip Adductors |
Bitrochanteric Breadth | Positive | NS |
| Biiliac Breadth | Negative | Positive | |
| Lower Limb Length | Negative | Negative | |
| Crural Index | NS | Negative | |
|
Biceps femoris |
Bitrochanteric Breadth | Positive | Positive |
| Biiliac Breadth | Negative | NS | |
| Lower Limb Length | Negative | Negative | |
| Crural Index | Negative | Negative | |
DISCUSSION
The purpose of this study has been to emphasize the range of motor activities necessary for survival in a given ecological context and to provide evidence that these selection pressures could have acted on hominin locomotor morphology, in particular pelvis breadth and limb length. Our data illustrate that as locomotor intensity increases, either through rising speed, incline or their combination, lower limb muscle activity increases, but these increases are modulated by morphology. While patterns of increased muscle activity demonstrated in the present study are similar to results of other studies, generally speed or incline have only been considered within a gait, or gait has been varied but on a level treadmill [walking: (Murray et al., 1984; Tokuhiro et al., 1985), running: (Swanson and Caldwell, 2000; Yokozawa et al., 2007), level walking and running: (Gazendam and Hof, 2007)]; the present study systematically combines these variables. We have systematically shown how muscular activity is affected by incline, speed, their interaction with each other, and their interaction with morphology.
Gluteus maximus activity
Multiple authors (Lovejoy, 1988; Marzke et al., 1988) emphasize the role of the gluteus maximus in maintaining torso posture and this is not falsified in this study—the increase in gluteus maximus activity in response to increasing incline and speed is likely the result of maintenance of torso posture (Oddsson and Thorstensoon, 1990). Under a hypothesis that increased activity leads to increased selection, it is unlikely that running alone can account for the expansion of the gluteus maximus size since incline walking provided similar increases in activity. The usefulness of EMG in determining these sorts of selection pressures may be somewhat hindered by the finding that even ‘smaller’ (than great apes) muscle groups (hip adductors being a good example) also increase activity with locomotor intensity. In addition, as we expect evolution to shape morphological and neurological systems that result in some amount of energetic economy through minimized muscle activity (Tuttle et al., 1979; Reilly et al., 2007), dramatic increases in activity for a given muscle group should not suggest increased evolutionary fitness, but increased cost and potentially decreased reproductive fitness. This seems to be particularly true for chronic activity. Increased activity may suggest that muscle moment arms are not in fact optimally adapted to a particular use. While certain short-term activities that are high cost may be selected if they provide large benefits (cheetah sprinting being an obvious example), we generally expect that activities performed regularly (striding bipedality) will need to be economical. It is thus of particular interest from an evolutionary standpoint how the variation between individuals during walking are reduced compared with running (illustrated by the 95% confidence intervals in Figures 3 and 5, and dramatically with the standard deviations of Figure 4): during increases in intensity (incline and speed), the variation of increased activity is smaller while walking, suggesting this gait can be neurologically and kinematically tuned to produce economical locomotion across a wide range of morphologies. This systematic pattern of walking further suggests more active selection pressures on the walking gait, over and against running and does not appear to support arguments laid out by Bramble, Lieberman and colleagues (Bramble and Lieberman, 2004; Lieberman et al., 2005; Lieberman et al., 2006) that the large size of the gluteus maximus has been particularly selected for long distance running. It does, however, remain possible, that any selection for endurance running has occurred particularly late in hominin evolution (maybe simply our own species), so has not had enough time for extensive fine-tuning to occur.
Pelvic dimensions and lower limb length influence muscle activity patterns
We expect that morphology will consist of the sum of adaptations to selection pressures, and the two key morphology measures discussed in this paper (pelvis breadth and lower limb length) have generally been considered well-adapted for thermoregulatory pressures, with potentially costly side-effects for locomotion. For example, modern humans have a crural index around .81–.86 depending on the population (Trinkaus, 1983; Porter, 1999) and it has been suggested this is primarily due to the variability in length of the distal segment (Holliday, 1999). While shifting of the tibial length has been generally associated with climatic adaptations, this change also has the potential to impact the locomotory system by increasing the metabolic cost of walking (Weaver and Steudel-Numbers, 2005). The results in this paper suggest that cold pressures which lead to the selective advantage of shorter tibia, and smaller crural indices may lead to an increase in the activity of hamstrings and hip abductors and thus more costly stabilization of the knee joint (Tokuhiro et al., 1985). The general shortening of the lower limbs in cold conditions has a similar impact on gluteus maximus activity and the hip adductors. This increased activity may explain some of the increased relative cost of locomotion for shorter limbed people (Steudel-Numbers and Tilkens, 2004; Steudel-Numbers et al., 2007).
While thermoregulatory pressures on pelvic breadth are strongly supported among modern human populations (Ruff, 1994), the non-modern fossil evidence from low latitudes suggest a more complicated pattern of pelvic breadth strategy. The results here show the significant role biiliac breadth has on the muscle activity of the hip and pelvis; though increased breadth of the ilia does significantly increase muscle activity of the gluteus maximus (walking and running) and hip adductors (running), it significantly decreases activity during walking in both the hip adductors and hamstrings. This suggests that the broad pelvis characteristic of Australopithecines (Steudel, 1978; Lovejoy, 1988; Rak, 1991; Ward, 2002), H. erectus (Simpson, 2008), and mid-Pleistocene Homo (Pycraft, 1930; Rak and Arensburg, 1987; Arsuaga et al., 1999; Rosenberg et al., 2006) reduces hip adductor and hamstring activity during walking, even after speed and incline are considered, suggesting effective, efficient walking by these hominin species, especially in the stabilization of the knee joint.
Finally, this study further illustrates the importance of including inclines in any study trying to better understand human evolution. Studies, similar to those of Passmore and Durnin, with participants walking on variable terrain, should be reattempted with larger sample sizes (Passmore and Durnin, 1955). Surface diversity was likely a common environmental factor for hominin populations, and the results here suggest that while differences do exist between walking and running on level treadmills, such differences are significantly minimized when locomotor intensity (incline, speed and their interaction) increases. To better understand changes in morphology, we need to have a better understanding of the impact of variable terrain on morphology.
Acknowledgements
We are grateful to all of our participants for donating their time to this project. We further appreciate the helpful comments from C. Ruff, P. Kramer, M. Myers and 2 anonymous reviewers that have greatly improved this manuscript. B.H.’s work was funded by NIH (RR 25012). E.C.’s work was funded by a NSF Graduate Fellowship.
Contributor Information
Cara M. Wall-Scheffler, Department of Biology Seattle Pacific University Seattle, WA 98119-1997.
Elizabeth Chumanov, University of Wisconsin School of Medicine and Public Health Department of Orthopedics and Rehabilitation, Physical Therapy Program Madison, WI 53706-1532.
Karen Steudel-Numbers, Department of Zoology University of Wisconsin Madison, WI 53703.
Bryan Heiderscheit, University of Wisconsin School of Medicine and Public Health Department of Orthopedics and Rehabilitation, Physical Therapy Program Madison, WI 53706-1532.
Literature Cited
- Arendt-Nielsen L, Sinkjaer T, Nielsen J, Kallesoe K. Electromyographic patterns and knee-joint kinematics during walking at various speeds. J Electromyogr Kinesiol. 1991;1:89–95. doi: 10.1016/1050-6411(91)90002-M. [DOI] [PubMed] [Google Scholar]
- Arsuaga J-L, Lorenzo C, Carretero J-M, Gracia A, Martínez I, García N, Bermúdez de Castro J-M, Carbonell E. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399:255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Muscles alive: their functions revealed by electromyography. Baltimore: Williams & Wilkins Company; 1967. [Google Scholar]
- Basmajian JV. Biomechanics of human posture and locomotion: Perspectives from electromyography. In: Tuttle RH, editor. The functional and evolutionary biology of primates. Chicago: Aldine Atherton; 1972. pp. 292–304. [Google Scholar]
- Basmajian JV, Blumenstein R. Electrode placement in electromyographic biofeedback. Baltimore: Williams and Wilkins; 1989. [Google Scholar]
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Burden AM, Trew M, Baltzopoulos V. Normalisation of gait EMGs: a re-examination. J Electromyogr Kinesiol. 2003;13:519–532. doi: 10.1016/s1050-6411(03)00082-8. [DOI] [PubMed] [Google Scholar]
- Chumanov ES, Wall-Scheffler CM, Heiderscheit BC. Gender differences in walking and running on level and inclined surfaces. Clin Biomech. 2008;23:1260–1268. doi: 10.1016/j.clinbiomech.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Clancy EA, Morin EL, Merletti R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J Electromyogr Kinesiol. 2002;12:1–16. doi: 10.1016/s1050-6411(01)00033-5. [DOI] [PubMed] [Google Scholar]
- Costa M. Interpersonal distances in group walking. J Nonverbal Behav. 2010;34:15–26. [Google Scholar]
- Fields KB, Bloom OJ, Priebe D, Foreman B. Basic biomechanics of the lower extremity. Prim Care. 2005;32:245–251. doi: 10.1016/j.pop.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Foley RA. Evolutionary ecology of fossil hominids. In: Smith EA, Winterhalder B, editors. Evolutionary ecology and human behavior. New York: Aldine de Gruyter; 1992. pp. 131–164. [Google Scholar]
- Foley RA, Elton S. Time and energy: the ecological context for the evolution of bipedalism. In: Strasser E, Fleagle J, Rosenberger A, McHenry H, editors. Primate locomotion: recent advances. New York: Plenum Press; 1998. pp. 419–433. [Google Scholar]
- Gazendam MGJ, Hof AL. Averaged EMG profiles in jogging and running at different speeds. Gait Posture. 2007;25:604–614. doi: 10.1016/j.gaitpost.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gibson MA, Mace R. An energy-saving development initiative increases birth rate and childhood malnutrition in rural Ethiopia. PLoS Medicine. 2006;3:476–484. doi: 10.1371/journal.pmed.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Howlett RA, Kindig CA, Stary CM, Hogan MC. The O2 cost of the tension-time integral in isolated single myocytes during fatigue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R983–R988. doi: 10.1152/ajpregu.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday TW. Brachial and crural indices of European Late Upper Paleolithic and Mesolithic humans. J Hum Evol. 1999;36:549–566. doi: 10.1006/jhev.1998.0289. [DOI] [PubMed] [Google Scholar]
- Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GV. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res. 1989;7:849–860. doi: 10.1002/jor.1100070611. [DOI] [PubMed] [Google Scholar]
- Kamen G, Caldwell GE. Physiology and intepreptation of the electromyogram. J Clin Neurophysiol. 1996;13:366–384. doi: 10.1097/00004691-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Kaw A, Egwu EK. Numerical methods with applications. 2009 http://www.autarkaw.com.
- Kawamura K, Tokuhiro A, Takechi H. Gait analysis of slope walking: a study on step length, stride width, time factors and deviation in the center of pressure. Acta Med Okayama. 1991;45:179–184. doi: 10.18926/AMO/32212. [DOI] [PubMed] [Google Scholar]
- Kleissen RFM, Litjens MCA, Baten CTM, Harlaar J, Hof AL, Zilvold G. Consistency of surface EMG patterns obtained during gait from three laboratories using standardised measurement technique. Gait Posture. 1997;6:200–209. [Google Scholar]
- Knutson LM, Soderberg GL. EMG: Use and interpretation in gait. In: Craik RL, Oatis CA, editors. Gait analysis: theory and application. St. Louis: Mosby; 1995. pp. 307–325. [Google Scholar]
- Kramer PA. The costs of human locomotion: maternal investment in child transport. Am J Phys Anthropol. 1998;107:71–85. doi: 10.1002/(SICI)1096-8644(199809)107:1<71::AID-AJPA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kramer PA. Modeling the locomotor energetics of extinct hominids. J Exp Biol. 1999;202:2807–2818. doi: 10.1242/jeb.202.20.2807. [DOI] [PubMed] [Google Scholar]
- Kramer PA. The behavioral ecology of locomotion. In: Meldrum DJ, Hilton CE, editors. From biped to strider: the emergence of modern human walking, running and resource transport. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 101–115. [Google Scholar]
- Lay AN, Hass CJ, Nichols TR, Gregor RJ. The effects of sloped surfaces on locomotion: an electromyographic analysis. J Biomech. 2007;40:1276–1285. doi: 10.1016/j.jbiomech.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Pontzer H, Cutright-Smith E, Raichlen DA. Why is the human gluteus so maximus? Am J Phys Anthropol. 2005;126:138. [Google Scholar]
- Lieberman DE, Raichlen DA, Pontzer H, Bramble DM, Cutright-Smith E. The human gluteus maximus and its role in running. J Exp Biol. 2006;209:2143–2155. doi: 10.1242/jeb.02255. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. Evolution of human walking. Sci Am. 1988;256:118–125. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Longhill JM, Rasmussen SA. Gluteus maximus muscle function and the origin of hominid bipedality. Am J Phys Anthropol. 1988;77:519–528. doi: 10.1002/ajpa.1330770412. [DOI] [PubMed] [Google Scholar]
- McClay IS, Lake MJ, Cavanagh PR. Muscle activity in running. In: Cavanagh PR, editor. Biomechanics of distance running. Champaign: Human Kinetics Books; 1990. pp. 165–186. [Google Scholar]
- McIntosh AS, Beatty KT, Dwan LN, Vickers DR. Gait dynamics on an inclined walkway. J Biomech. 2006;39:2491–2502. doi: 10.1016/j.jbiomech.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Murray MP, Mollinger LA, Gardner GM, Sepic SB. Kinematic and EMG patterns during slow, free, and fast walking. J Orthop Res. 1984;2:272–280. doi: 10.1002/jor.1100020309. [DOI] [PubMed] [Google Scholar]
- Myers MJ, Steudel K. Effect of limb mass distribution on the energetic cost of running. J Exp Biol. 1985;116:363–373. doi: 10.1242/jeb.116.1.363. [DOI] [PubMed] [Google Scholar]
- Oddsson L, Thorstensoon A. Task specificity in the control of intrinsic trunk muscles in man. Acta Physiol Scand. 1990;129:123–131. doi: 10.1111/j.1748-1716.1990.tb08904.x. [DOI] [PubMed] [Google Scholar]
- Passmore R, Durnin JVGA. Human energy expenditure. Physiol Rev. 1955;35:801–840. doi: 10.1152/physrev.1955.35.4.801. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Wrangham RW. Ontogeny of ranging in wild chimpanzees. Int J Primatol. 2006;27:295–309. [Google Scholar]
- Porter AMW. Physique and the skeleton. PhD Dissertation. University of London; 1996. [Google Scholar]
- Porter AMW. Modern human, early modern human and Neanderthal limb proportions. Int J Osteoarchaeol. 1999;9:54–67. [Google Scholar]
- Pycraft WP. The pelvis of Rhodesian man. Man. 1930;30:117–121. [Google Scholar]
- Rainoldi A, Melchiorri G, Caruso I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods. 2004;134:37–43. doi: 10.1016/j.jneumeth.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Rak Y. Lucy's pelvic anatomy: its role in bipedal gait. J Hum Evol. 1991;20:283–290. [Google Scholar]
- Rak Y, Arensburg B. Kebara 2 Neanderthal pelvis: first look at a complete inlet. Am J Phys Anthropol. 1987;73:227–231. doi: 10.1002/ajpa.1330730209. [DOI] [PubMed] [Google Scholar]
- Reilly SM, McElroy EJ, Biknevicius AR. Posture, gait and the ecological relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology. 2007;110:271–289. doi: 10.1016/j.zool.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Rosenberg KR, Zuné L, Ruff CB. Body size, body proportions, and encephalization in a Middle Pleistocene archaic human from northern China. Proc Natl Acad Sci USA. 2006;103:3552–3556. doi: 10.1073/pnas.0508681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CB. Morphological adaptation to climate in modern and fossil hominids. Yrbk Phys Anthropol. 1994;37:65–107. [Google Scholar]
- Simpson SW. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322:1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. Why walk and run: energetic costs and energetic optimality in simple mechanics-based models of a bipedal animal. Ithaca: Cornell University; 2006. [Google Scholar]
- Stern JT, Susman RL. Electromyography of the gluteal muscles in Hylobates, Pongo, and Pan: implications for the evolution of hominid bipedality. Am J Phys Anthropol. 1981;55:153–166. [Google Scholar]
- Steudel-Numbers K, Tilkens M. The effect of lower limb length on the energetic cost of locomotion: implications for fossil hominins. J Hum Evol. 2004;47:95–109. doi: 10.1016/j.jhevol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Steudel-Numbers K, Weaver T, Wall-Scheffler CM. The evolution of human running: effects of changes in lower limb length on locomotor efficiency. J Hum Evol. 2007;53:191–196. doi: 10.1016/j.jhevol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Steudel K. A multivariate analysis of the pelvis of early hominids. J Hum Evol. 1978;7:583–595. [Google Scholar]
- Swanson SC, Caldwell GE. An integrated biomechanical analysis of high speed incline and level treadmill running. Med Sci Sports Exerc. 2000;32:1146–1155. doi: 10.1097/00005768-200006000-00018. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Heglund NC, Maloiy GM. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, Ker RF, Alexander RM. Dimensions and moment arms of the hind-and forelimb muscles of common chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tokuhiro A, Nagashima H, Takechi H. Electromyographic kinesiology of lower extremity muscles during slope walking. Arch Phys Med Rehabil. 1985;66:610–613. [PubMed] [Google Scholar]
- Trinkaus E. Neandertal postcrania and the adaptive shift to modern humans. In: Trinkaus E, editor. The Mousterian legacy: human biocultural change in the Upper Pleistocene. Oxford: BAR; 1983. pp. 165–200. [Google Scholar]
- Tuttle RH, Basmajian JV, Ishida H. Activities of pongid thigh muscles during bipedal behavior. Am J Phys Anthropol. 1979;50:123–136. doi: 10.1002/ajpa.1330500113. [DOI] [PubMed] [Google Scholar]
- Wall-Scheffler CM, Geiger K, Steudel-Numbers K. Infant carrying: the role of increased locomotory costs in early tool development. Am J Phys Anthropol. 2007;133:841–846. doi: 10.1002/ajpa.20603. [DOI] [PubMed] [Google Scholar]
- Ward CV. Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? Yrbk Phys Anthropol. 2002;45:185–215. doi: 10.1002/ajpa.10185. [DOI] [PubMed] [Google Scholar]
- Weaver T, Steudel-Numbers K. Does climate or mobility explain the differences in body proportions between Neandertals and their Upper Paleolithic successors? Evol Anthropol. 2005;14:218–223. [Google Scholar]
- Yokozawa T, Fujii N, Ae N. Muscle activities of the lower limb during level and uphill running. J Biomech. 2007;40:3467–3475. doi: 10.1016/j.jbiomech.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Zange J, Beisteiner M, Müller K, Shushakov V, Maassen N. Energy metabolism in intensively exercising calf muscle under a simulated orthostasis. Plfügers Arch - Eur J Physiol. 2008;455:1153–1163. doi: 10.1007/s00424-007-0361-9. [DOI] [PubMed] [Google Scholar]
- Zihlman AL. Interpretations of early hominid locomotion. In: Jolly C, editor. Early Hominids in Africa. London: Duckworth; 1978. pp. 361–377. [Google Scholar]