Abstract
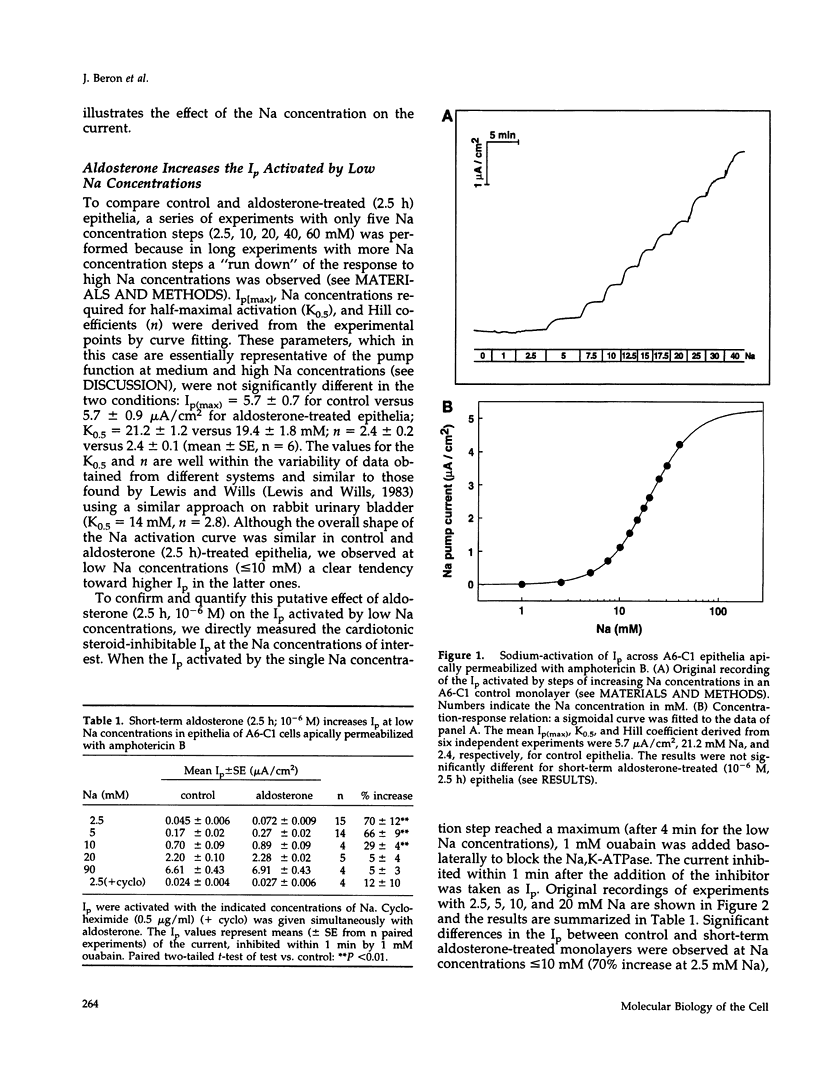
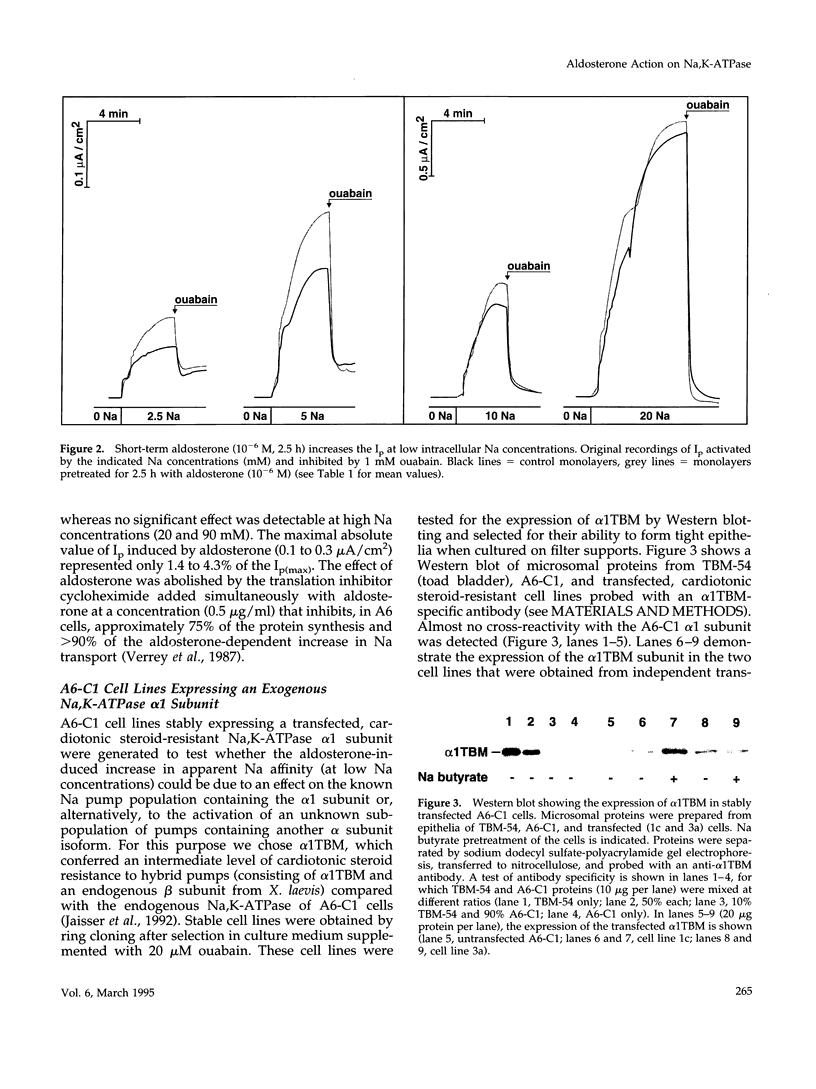
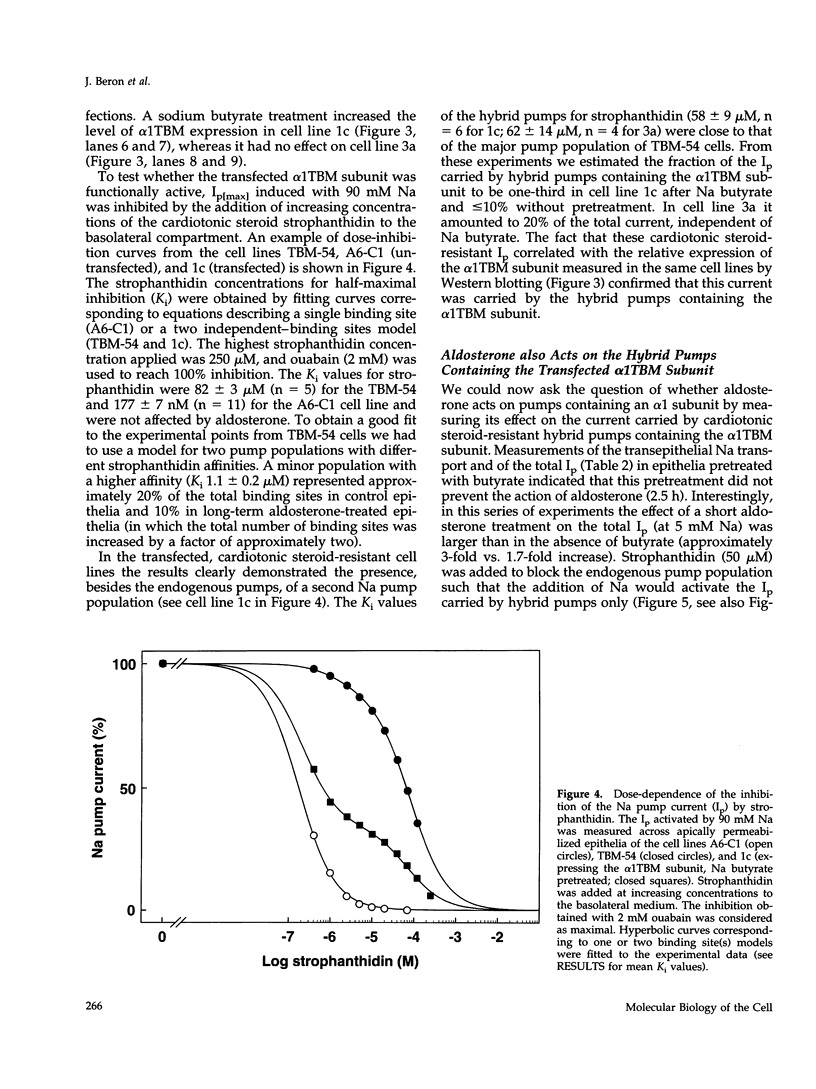
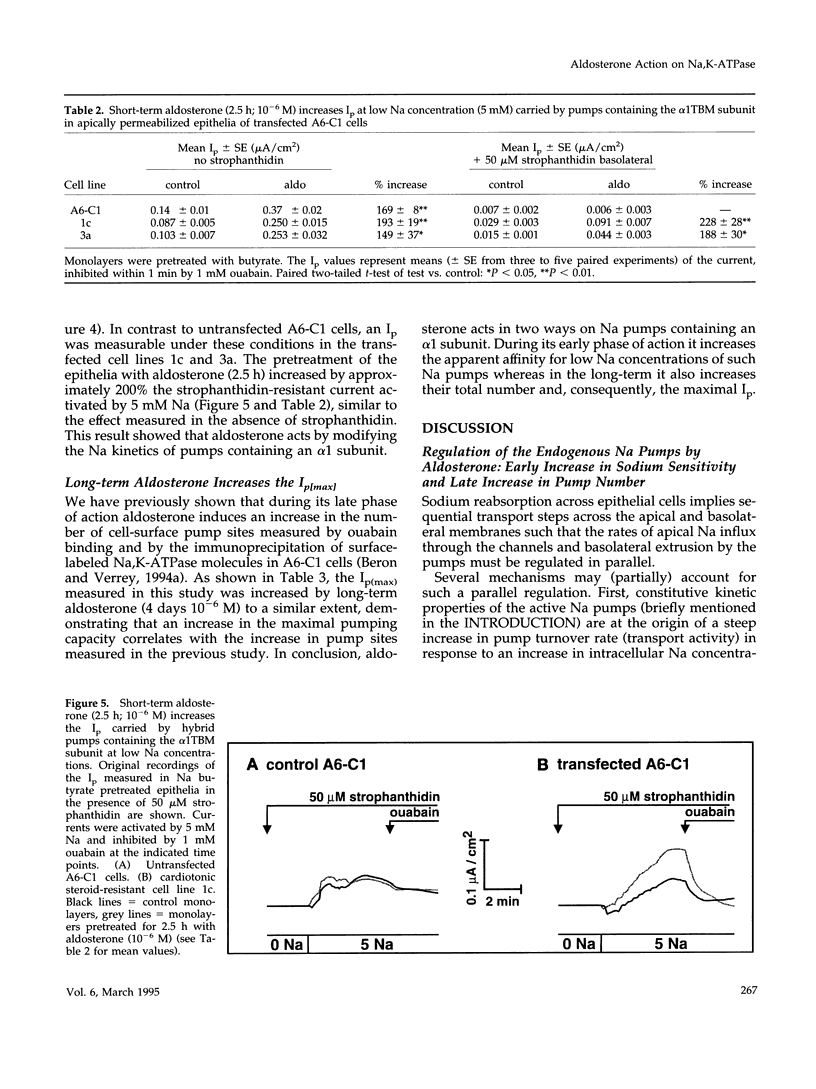
Short-term aldosterone (10(-6) M, 2.5 h) induces in A6-C1 cell epithelia an increase in Na transport, which is due to the in situ activation of the apical Na channel and, presumably, the basolateral Na pump (Na,K-ATPase). We have now directly measured the effect of aldosterone on the transport activity of endogenous Na pumps and hybrid Na pumps containing an exogenous alpha 1 subunit by measuring the pump current (Ip) across epithelia apically permeabilized with amphotericin B. Aldosterone (2.5 h) had no significant early effect on the maximal Ip, nor on the Na concentration required for half-maximal activation. In contrast, it increased the Ip at physiological intracellular Na concentrations (1.7-fold at 5 mM Na). This effect was blocked by the protein synthesis inhibitor cycloheximide. Hybrid pumps containing the transfected cardiotonic steroid-resistant alpha 1 subunit of Bufo marinus were also stimulated by aldosterone (2.5 h). A long aldosterone treatment (4 days) increased the maximal Ip produced by the endogenous pumps 1.5 to 2.1-fold. In conclusion, aldosterone acts on Na pumps containing an alpha 1 subunit in two ways. During its early phase of action it stimulates their transport activity by increasing their apparent Na affinity at physiological intracellular Na concentrations. In the long term it produces an increase in the maximal transport capacity, which corresponds to the known increase in the number of Na pumps.
Full text
PDF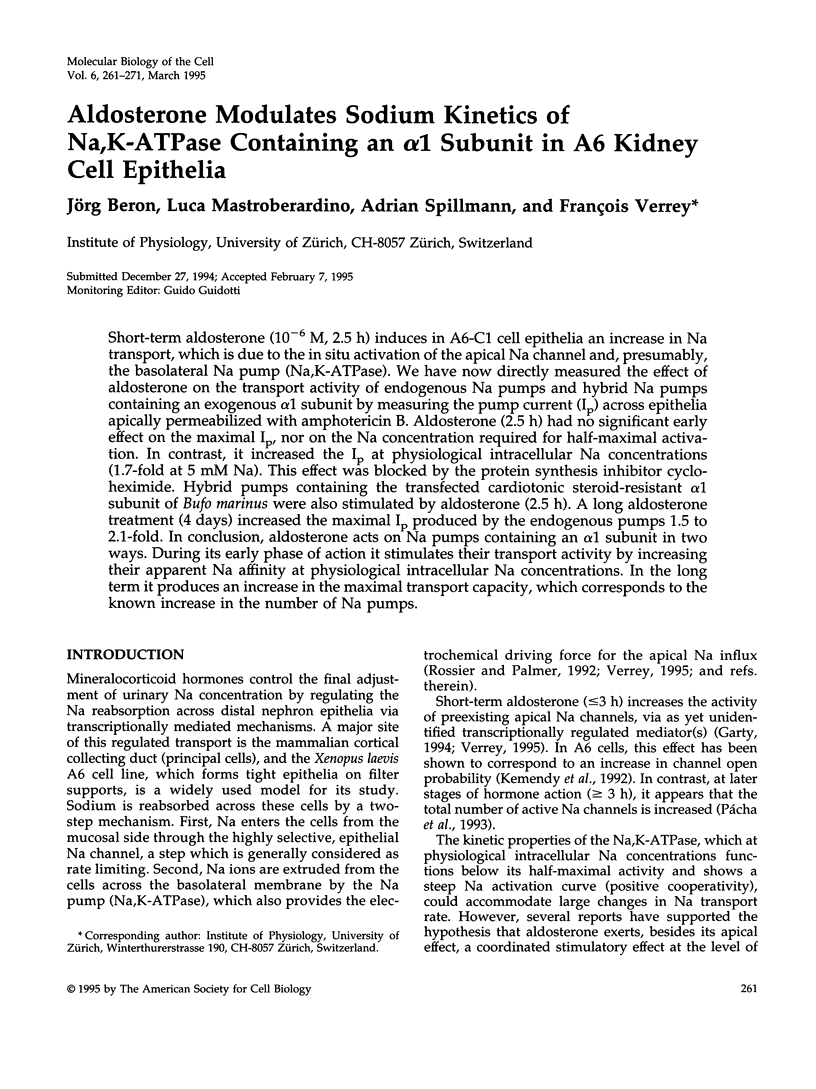






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann U., Geering K. Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 1990 Aug 20;269(1):105–108. doi: 10.1016/0014-5793(90)81130-g. [DOI] [PubMed] [Google Scholar]
- Aperia A., Ibarra F., Svensson L. B., Klee C., Greengard P. Calcineurin mediates alpha-adrenergic stimulation of Na+,K(+)-ATPase activity in renal tubule cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7394–7397. doi: 10.1073/pnas.89.16.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlet-Bas C., Khadouri C., Marsy S., Doucet A. Sodium-independent in vitro induction of Na+,K+-ATPase by aldosterone in renal target cells: permissive effect of triiodothyronine. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1707–1711. doi: 10.1073/pnas.85.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastl C. P., Hayslett J. P. The cellular action of aldosterone in target epithelia. Kidney Int. 1992 Aug;42(2):250–264. doi: 10.1038/ki.1992.284. [DOI] [PubMed] [Google Scholar]
- Beron J., Verrey F. Aldosterone induces early activation and late accumulation of Na-K-ATPase at surface of A6 cells. Am J Physiol. 1994 May;266(5 Pt 1):C1278–C1290. doi: 10.1152/ajpcell.1994.266.5.C1278. [DOI] [PubMed] [Google Scholar]
- Broillet M. C., Horisberger J. D. Basolateral membrane potassium conductance of A6 cells. J Membr Biol. 1991 Oct;124(1):1–12. doi: 10.1007/BF01871359. [DOI] [PubMed] [Google Scholar]
- Chuard F., Durand J. Coupling between the intracellular pH and the active transport of sodium in an epithelial cell line from Xenopus laevis. Comp Biochem Physiol Comp Physiol. 1992 May;102(1):7–14. doi: 10.1016/0300-9629(92)90003-9. [DOI] [PubMed] [Google Scholar]
- Frindt G., Silver R. B., Windhager E. E., Palmer L. G. Feedback regulation of Na channels in rat CCT. II. Effects of inhibition of Na entry. Am J Physiol. 1993 Mar;264(3 Pt 2):F565–F574. doi: 10.1152/ajprenal.1993.264.3.F565. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Takemoto F., Katz A. I. Early effects of aldosterone on Na-K pump in rat cortical collecting tubules. Am J Physiol. 1990 Jul;259(1 Pt 2):F40–F45. doi: 10.1152/ajprenal.1990.259.1.F40. [DOI] [PubMed] [Google Scholar]
- Féraille E., Carranza M. L., Rousselot M., Favre H. Insulin enhances sodium sensitivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol. 1994 Jul;267(1 Pt 2):F55–F62. doi: 10.1152/ajprenal.1994.267.1.F55. [DOI] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994 May;8(8):522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Granitzer M., Leal T., Nagel W., Crabbe J. Apical and basolateral conductance in cultured A6 cells. Pflugers Arch. 1991 Jan;417(5):463–468. doi: 10.1007/BF00370940. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Steele R. E., Sahib M. K., Wade J. B., Preston A. S., Lawson N. L., Johnson J. P. Toad urinary bladder epithelial cells in culture: maintenance of epithelial structure, sodium transport, and response to hormones. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4151–4155. doi: 10.1073/pnas.76.8.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. J., Thomas S. R., Ehrenfeld J. Intracellular pH controls cell membrane Na+ and K+ conductances and transport in frog skin epithelium. J Gen Physiol. 1988 Dec;92(6):767–791. doi: 10.1085/jgp.92.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisser F., Canessa C. M., Horisberger J. D., Rossier B. C. Primary sequence and functional expression of a novel ouabain-resistant Na,K-ATPase. The beta subunit modulates potassium activation of the Na,K-pump. J Biol Chem. 1992 Aug 25;267(24):16895–16903. [PubMed] [Google Scholar]
- Kemendy A. E., Kleyman T. R., Eaton D. C. Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol. 1992 Oct;263(4 Pt 1):C825–C837. doi: 10.1152/ajpcell.1992.263.4.C825. [DOI] [PubMed] [Google Scholar]
- Kent R. B., Emanuel J. R., Ben Neriah Y., Levenson R., Housman D. E. Ouabain resistance conferred by expression of the cDNA for a murine Na+, K+-ATPase alpha subunit. Science. 1987 Aug 21;237(4817):901–903. doi: 10.1126/science.3039660. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Halm D. R., Dawson D. C. Active sodium transport by turtle colon via an electrogenic Na-K exchange pump. Nature. 1980 Sep 18;287(5779):237–239. doi: 10.1038/287237a0. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr, Kraehenbuhl J. P. The cellular pool of Na+ channels in the amphibian cell line A6 is not altered by mineralocorticoids. Analysis using a new photoactive amiloride analog in combination with anti-amiloride antibodies. J Biol Chem. 1989 Jul 15;264(20):11995–12000. [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K. Apical membrane permeability and kinetic properties of the sodium pump in rabbit urinary bladder. J Physiol. 1983 Aug;341:169–184. doi: 10.1113/jphysiol.1983.sp014799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccolat M. P., Geering K., Gaeggeler H. P., Rossier B. C. Aldosterone regulation of Na+ transport and Na+-K+-ATPase in A6 cells: role of growth conditions. Am J Physiol. 1987 May;252(5 Pt 1):C468–C476. doi: 10.1152/ajpcell.1987.252.5.C468. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Speez N. Stimulation of apical Na permeability and basolateral Na pump of toad urinary bladder by aldosterone. Am J Physiol. 1986 Feb;250(2 Pt 2):F273–F281. doi: 10.1152/ajprenal.1986.250.2.F273. [DOI] [PubMed] [Google Scholar]
- Pellanda A. M., Gaeggeler H. P., Horisberger J. D., Rossier B. C. Sodium-independent effect of aldosterone on initial rate of ouabain binding in A6 cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C899–C906. doi: 10.1152/ajpcell.1992.262.4.C899. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Tam J. P. Multiple antigenic peptide method for producing antipeptide site-specific antibodies. Methods Enzymol. 1989;178:739–746. doi: 10.1016/0076-6879(89)78048-4. [DOI] [PubMed] [Google Scholar]
- Price E. M., Lingrel J. B. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988 Nov 1;27(22):8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Pácha J., Frindt G., Antonian L., Silver R. B., Palmer L. G. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993 Jul;102(1):25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Frindt G., Windhager E. E., Palmer L. G. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am J Physiol. 1993 Mar;264(3 Pt 2):F557–F564. doi: 10.1152/ajprenal.1993.264.3.F557. [DOI] [PubMed] [Google Scholar]
- Truscello A., Geering K., Gäggeler H. P., Rossier B. C. Effects of butyrate on histone deacetylation and aldosterone-dependent Na+ transport in the toad bladder. J Biol Chem. 1983 Mar 10;258(5):3388–3395. [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. J Membr Biol. 1994 Feb;138(1):65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Verrey F., Kraehenbuhl J. P., Rossier B. C. Aldosterone induces a rapid increase in the rate of Na,K-ATPase gene transcription in cultured kidney cells. Mol Endocrinol. 1989 Sep;3(9):1369–1376. doi: 10.1210/mend-3-9-1369. [DOI] [PubMed] [Google Scholar]
- Verrey F. Regulation of gene expression by aldosterone in tight epithelia. Semin Nephrol. 1990 Jul;10(4):410–420. [PubMed] [Google Scholar]
- Verrey F., Schaerer E., Zoerkler P., Paccolat M. P., Geering K., Kraehenbuhl J. P., Rossier B. C. Regulation by aldosterone of Na+,K+-ATPase mRNAs, protein synthesis, and sodium transport in cultured kidney cells. J Cell Biol. 1987 May;104(5):1231–1237. doi: 10.1083/jcb.104.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling P. A., Caplan M., Sutters M., Giebisch G. Aldosterone-mediated Na/K-ATPase expression is alpha 1 isoform specific in the renal cortical collecting duct. J Biol Chem. 1993 Nov 5;268(31):23469–23476. [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]