Abstract
In this review we integrate ideas about regional and systemic circulatory capacities and the balance between skeletal muscle blood flow and cardiac output during heavy exercise in humans. In the first part of the review we discuss issues related to the pumping capacity of the heart and the vasodilator capacity of skeletal muscle. The issue is that skeletal muscle has a vast capacity to vasodilate during exercise [~300 mL (100 g)−1 min−1], but the pumping capacity of the human heart is limited to 20–25 L min−1 in untrained subjects and ~35 L min−1 in elite endurance athletes. This means that when more than 7–10 kg of muscle is active during heavy exercise, perfusion of the contracting muscles must be limited or mean arterial pressure will fall. In the second part of the review we emphasize that there is an interplay between sympathetic vasoconstriction and metabolic vasodilation that limits blood flow to contracting muscles to maintain mean arterial pressure. Vasoconstriction in larger vessels continues while constriction in smaller vessels is blunted permitting total muscle blood flow to be limited but distributed more optimally. This interplay between sympathetic constriction and metabolic dilation during heavy whole-body exercise is likely responsible for the very high levels of oxygen extraction seen in contracting skeletal muscle. It also explains why infusing vasodilators in the contracting muscles does not increase oxygen uptake in the muscle. Finally, when ~80% of cardiac output is directed towards contracting skeletal muscle modest vasoconstriction in the active muscles can evoke marked changes in arterial pressure.
Keywords: cardiac output, exercise hyperaemia, sympatholysis, VO2max
Overview of exercise, energy demand, muscle blood flow and cardiac output
During exercise the overall energy demand increases to sustain the contractile activity of the active skeletal muscle, the respiratory muscles and the myocardium. When exercise is performed for durations of longer than a minute or two and primarily ‘aerobic’ in nature, increases in skeletal muscle blood flow are critical to ensure the appropriate supply of O2 to the active skeletal muscles and to remove the metabolic byproducts.
During heavy exercise, i.e. an exercise intensity eliciting maximal oxygen uptake (VO2max), which can be sustained at a constant intensity for 5–10 min, whole-body exercise capacity is, in general, limited by the maximal rate of oxidative ATP resynthesis, which depends on oxygen delivery. However, under certain circumstances, for example in hypoxia and hyperthermia, fatigue may be elicited by mechanisms originating in the central nervous system (CNS) or triggered by afferent feedback (Fitts 1994, Amann et al. 2009).
The hyperaemic response to exercise is initially elicited by local factors of mechanical and metabolic origin and likely adjusted depending of the degree of deoxygenation of the capillary blood, and modulated by neural mechanisms. Exercise-induced skeletal muscle hyperaemia is essentially due to the selective vasodilation of vascular units perfusing the active muscle fibres. For vasodilation to result in a significant elevation of skeletal muscle blood flow cardiac output (Q) has to increase at least to the same extent as skeletal muscle blood flow, otherwise blood pressure would fall, reducing the effectiveness of skeletal muscle vasodilation. Despite the effort made by several generations of physiologists over the last 150 years, many questions remain on the regulatory mechanisms that elicit and maintain skeletal muscle hyperaemia and how cardiac output and muscle hyperaemia are coupled during heavy exercise in humans.
Systemic and muscular oxygen delivery
Maximal systemic O2 delivery is the product of maximal cardiac output (Qmax) and the arterial O2 content (CaO2) at peak exercise. Maximal cardiac output is determined by the stroke volume (SV) and heart rate (HR) at maximal or near-maximal exercise (Q = SV × HR), with SV playing a predominant role, inasmuch as maximal HR changes little and cannot be increased to compensate for a reduction in SV (Saltin et al. 1968). SV depends on the size and compliance of the heart, the preload, the afterload, myocardial contractility and the displacement of the atrioventricular (AV) plane from the base of the ventricles towards the apex in systole.
Athletic mammals have a greater heart mass when compared with non-athletic animals of similar body mass (Carew & Covell 1978, Gunn 1989). Athletic quadrupeds have almost two to threefold as much heart mass/body mass than humans (Carew & Covell 1978, Lorenz et al. 1999). Elite endurance human athletes have large hearts, with similarly enhanced left (35–38%) and right ventricular (RV; 37–48%) masses adjusted per body surface area and, hence, the LV/RV mass ratio (~3) is similar to that of controls (Scharhag et al. 2002, Perseghin et al. 2007). The increased heart size of the endurance athlete is due to the combination of genetic factors and the effect of training (Levine et al. 1991, Busjahn et al. 2009). That the size of the heart plays a critical role in determining maximal exercise capacity in humans is also supported by the fact that at near-maximal exercise levelling off of VO2 with increasing exercise intensity is preceded by a levelling off of cardiac output (Mitchell et al. 1958, Calbet et al. 2007). Moreover, a number of studies using different imaging techniques have reported a correlation (r = 0.80–0.89) between VO2max and left ventricular (LV) mass (Milliken et al. 1988, Scharhag et al. 2002).
To achieve and maintain a high SV when HR is close to maximal the heart must have a very high compliance and also must be able to contract and relax very fast (Levine et al. 1991). Both characteristics are enhanced in endurance elite athletes and in mammals with outstanding endurance capacities (Carew & Covell 1978, Levine et al. 1991, Gledhill et al. 1994, Perseghin et al. 2007), and endurance training in previously untrained humans improves LV diastolic function (Shapiro & Smith 1983, Levy et al. 1993). In contrast, systolic function is similar in highly trained and untrained humans, and does seem to contribute to the enlarged SV of the former (Blomqvist & Saltin 1983). Therefore, the main factors explaining the enlarged SV of trained endurance athletes are heart size and compliance and the mechanisms that help to fill first the right and left atrium very fast (Lundback 1986) and then their corresponding ventricular cavities, and to maintain preload at peak exercise, i.e. venous return and central blood volume (Blomqvist & Saltin 1983, Dawson et al. 2007) when HR is high.
In 1932, Hamilton & Rompf (1932) described a relative constancy of the total heart volume during the cardiac cycle. They concluded that through the displacement of the AV plane, the heart was able to pump blood but still maintain the same volume. This concept has been confirmed with modern techniques (Lundback 1986, Carlsson et al. 2004). Thus, the heart is working as a normal single water pump. The valve plane, relative to the ventricles, is moved back and forth by the papillary muscles in the blood stream, pushing the blood forwards. At a high HR and cardiac output, the momentum of the blood created by the descent of the AV plane facilitates a more rapid filling of the heart decreasing the requirement of outer volume change in the surrounding tissue, reducing the energy requirements of the pumping heart (Carlsson et al. 2004). The motion of the AV plane represents 60% of the total SV at rest (Carlsson et al. 2007). During exercise at 85–90% of the maximal HR the displacement of the AV plane increased from 15.6 to 17.6 in 12 females following 8 weeks of interval training (Slordahl et al. 2004). It remains to be determined to what extent can the displacement of the AV plane be a limiting factor for peak SV in humans.
In most circumstances VO2max is determined by systemic O2 transport (Saltin & Calbet 2006, Wagner 2006, Levine 2008). In 1924, Hill suggested that VO2max is limited by Qmax (Hill et al. 1924) and the supportive experimental evidence was obtained during the last three decades (Andersen & Saltin 1985, Calbet et al. 2004). This concept has been challenged by Noakes, who postulated that VO2max is limited by the ‘Central Governor’, presumably the CNS (Noakes 1997). In his theory Noakes defends that the CNS limits the recruitment of motor units at very high exercise intensities to prevent a ‘disturbance of homeostasis’ and avoid catastrophic damage to heart by a ‘reflex mechanism’ triggered by the heart itself (Noakes 1997, Noakes & St Clair Gibson 2004, Noakes et al. 2004). However, this theory cannot explain why some humans with ischaemic hearts are able to perform exercise until chest pain develops or why in some circumstances exercise can be performed until collapse. Although we do not know yet what limits Qmax, recent experiments have shown that Qmax does not seem to be limited by the maximal working capacity of the heart (Brink-Elfegoun et al. 2007, Lundby et al. 2008b).
Systemic O2 delivery also depends on CaO2. At peak exercise at sea level CaO2 is a little lower than at rest, due to the rightward shift in the oxygen dissociation curve of the haemoglobin caused by acidosis and hyperthermia (Nielsen et al. 2002), combined with some impairment in pulmonary gas exchange due to ventilation/perfusion (VA/Q) mismatch and diffusion limitation for O2 at high Qmax (Dempsey & Wagner 1999). Increasing blood haemoglobin concentration ([Hb]) by either autotransfusion (Ekblom et al. 1976) or treatment with erythropoietin (Ekblom & Berglund 1991, Lundby et al. 2008b) increases exercise capacity and VO2max, without modification of Qmax, peak exercise SV or HRmax. Conversely, isovolemic haemodilution reduces VO2max during whole-body exercise (Ekblom et al. 1976), and also VO2 peak during exercise with a small muscle (Koskolou et al. 1997) without altering peak exercise Q, SV or HR. Additionally, every 10% increase in [Hb] translates into an ~7% increase in VO2max (Calbet et al. 2006a).
During exercise in acute hypoxia systemic O2 delivery is reduced and so is VO2max. Up to an inspired O2 (FIO2) of 12% the main mechanism explaining the reduction in systemic O2 delivery with acute hypoxia is the reduction in CaO2 whilst Q, SV and HR are not affected, although peak HR may be slightly reduced in moderate hypoxia (Lundby et al. 2001). At higher levels of hypoxia (FIO2 < 0.12) the reduction in systemic O2 delivery is explained by the combination of a blunted Qmax (with a similar contribution of SV and HR) and the reduction in CaO2 (Calbet et al. 2003a). For example, with an FIO2 = 0.105 (equivalent to an altitude of 5300 m above sea level) Qmax is reduced by 15% compared to sea level (Calbet et al. 2003a). Intriguingly, increasing arterial [Hb] enhances VO2max at sea level and at simulated altitudes below 4000 m, but not above 4000 m (Robach et al. 2008). This phenomenon is due in part to an alteration in the distribution of blood flow (Robach et al. 2008), but has been also attributed to a limitation in pulmonary O2 diffusing capacity with more severe hypoxia (Calbet & Boushel 2010). In chronic hypoxia, [Hb] increases and pulmonary gas exchange improves resulting in an elevation of CaO2 to levels similar or higher than those observed at sea level prior to the altitude exposure (Calbet et al. 2003b). Nevertheless, maximal exercise systemic O2 transport remains below the sea level values due to a reduction in Qmax (Pugh 1964, Calbet et al. 2003b). Despite the fact that with altitude acclimatization systemic O2 transport is markedly improved, VO2max only increases marginally compared with the value registered in acute hypoxia. This is explained by an alteration in the distribution of blood flow, such that a lower fraction of the cardiac output is directed to the exercising muscles in chronic hypoxia (Calbet et al. 2003b).
At maximal whole-body exercise the active skeletal muscle mass for a 175 cm tall man can be estimated to lie in between 16 and 20 kg. A peak exercise 5–6 L min−1 of blood flow is required to perfuse the CNS, the coronary vessels, the respiratory muscles and other tissues apart from the active muscles, leaving 14–15 L min−1 for the active muscles. An elite endurance athlete with a Qmax of 35 L min−1 will be able to perfuse his active muscles with 29–30 L min−1 of blood flow, i.e. about twice as much as the sedentary subject. Supposing a similar active muscle mass in both cases the relative perfusion of the skeletal muscle at peak exercise should lie in between 88 and 188 mL kg−1 of muscle mass. However, much higher levels of relative skeletal muscle perfusion have been reported during small muscle exercise in humans (Andersen & Saltin 1985).
It should also be pointed out that there are far fewer invasive measurements in both trained and untrained women on the maximal capacities of various elements of the oxygen transport system during exercise. What can clearly be said is that VO2max expressed per kg of body weight is typically about 10% lower in females than in males with comparable training histories. The likely explanations for this include the higher levels of body fat in women and slightly lower haemoglobin and haematocrit levels. In highly trained subjects VO2max expressed per kg of fat free mass is similar in both sexes, suggesting that when scaled for body size and adjusted for differences in body composition there are not fundamental differences in either cardiac performance of skeletal muscle vasodilator capacity in men and women (Joyner 1993). Cardiac structural and functional adaptations to training are similar in men and women (Petersen et al. 2006). However, it does appear that the tendency for at least some highly trained subjects to demonstrate arterial desaturation during heavy exercise is more common in women as a result of structural differences in the their lungs (Harms et al. 1998, McClaran et al. 1998).
What are the determinants of ‘local’ exercise capacity in humans?
Local or peripheral fatigue, defined either as a task failure or a reduction in force or power generation capacity, may be elicited by a number of factors, which act by reducing the rate of ATP re-synthesis and/or by interfering with the biochemical processes involved in muscle contraction and relaxation (Fitts 1994). During sprint-like or high-intensity all-out exercise muscle fatigue appears to be independent of O2 delivery (Weyand et al. 1999, Calbet et al. 2003c), as well as during isometric contractions (Fulco et al. 1994). However, the maximal power attained during incremental exercise to exhaustion (Wmax) either with a large or a small muscle mass is reduced when CaO2 is reduced either by hypoxia (Fulco et al. 1996, Calbet et al. 2009), carbon monoxide breathing (Ekblom et al. 1975, Gonzalez-Alonso et al. 2001) or isovolemic haemodilution (Ekblom et al. 1976, Koskolou et al. 1997). A tentative conclusion that can be derived from these studies is that it seems that regardless of the size of the active muscle mass, Wmax is reduced when CaO2 is reduced irrespective of FIO2.
Before the 1980s it was believed that Qmax could sufficiently supply the active skeletal muscles with blood flow and maintain blood pressure even during maximal whole-body exercise (Mellander & Johansson 1968). This concept was based on the observation that peak muscle blood flow was about 50–60 mL (100 g)−1 min−1 (Kjellmer 1964, Grimby et al. 1967), implying that with a cardiac output of 20 L min−1 there was enough blood flow to supply ~25 kg of active muscle mass, leaving 5 L min−1 to supply the rest of the body. However, these pioneer measurements were carried out with xenon or venous occlusion plethysmography and both techniques underestimate muscle blood flow (Saltin 2007). In 1985, Andersen and Saltin reported values of 250 mL (100 g tissue)−1 min−1 for the quadriceps muscle during one leg knee extension exercise using the continuous infusion thermodilution technique to measure femoral vein outflow (Andersen & Saltin 1985) and Doppler to measure the arterial inflow (Rådegran et al. 1999). Peak muscle blood flow is even higher in elite cyclists with values of ~400 mL (100 g tissue)−1 min−1 reported (Richardson et al. 1993). The combination of these studies indicates that humans have a peak hyperaemic response which is essentially similar to that previously reported in other mammals including athletic species with very large hearts (Parks & Manohar 1983, Armstrong & Laughlin 1985, Manohar 1986, Musch et al. 1987).
If this magnitude of peak quadriceps muscle perfusion could be reached by other skeletal muscles then the activation of just 10 kg of muscle mass, i.e. less than during maximal exercise on the cycle ergometer, would overwhelm the pumping capacity of even the human with the highest Qmax causing hypotension. Regional differences in the peak hyperaemic response to exercise may exist as muscles differ in their daily use and endurance-trained muscle may have 40–60% higher hyperaemic responses than untrained muscles (Andersen & Saltin 1985, Snell et al. 1987, Richardson et al. 1993). Nevertheless, peak arm blood flow responses to exercise are lower than observed for knee extension exercise, even in arm-trained subjects (Ahlborg & Jensen-Urstad 1991, Volianitis et al. 2003). However, by studying elite cross-country skiers who have highly trained arm and leg muscles it was definitively shown that as initially proposed by Saltin (1985) the combined vasodilatory capacity of the arm and leg muscles exceeds the pumping capacity of the heart (Calbet et al. 2004) (Fig. 1). The latter finding is consistent with the idea that muscle vasodilation has to be restrained to avoid hypotension during whole-body exercise in humans.
Figure 1.
The combined vasodilatory capacity of the arm and leg muscles exceeds the pumping capacity of the heart (Calbet et al. 2004). Cross-country skiers were studied during sub-maximal (76% VO2max) skiing while using arm and legs (diagonal technique), only arm (double poling technique) and leg skiing (like skating). They were also studied during maximal exercise with the diagonal technique. Trunk and head perfusion at maximal diagonal was calculated by subtracting peak leg and arm blood flows from peak cardiac output. The maximal theoretical cardiac output was calculated by adding the maximal values observed for leg blood flow (during maximal diagonal), the peak arm blood flow (observed during double poling) and the 5 L min−1 of blood flow necessary to perfuse the head and trunk. The latter gave 4 L min−1 more cardiac output than actually measured, implying that in humans with well trained arm and leg muscles the combined peak perfusion of the head trunk and arm muscles exceeds the pumping capacity of the heart. This also implies that during maximal upright arm and leg combined exercise muscle vasodilation must be restrained to avoid hypotension (Calbet et al. 2004).
In general, during submaximal dynamic exercise, with either a small or large muscle mass, reductions in CaO2 are compensated for by increases in blood flow to maintain O2 delivery constant. Despite the fact that during maximal exercise with a small muscle mass only a fraction of Qmax is recruited, implying that there is substantial functional reserve, peak blood flow is not enhanced to account for the reduction in CaO2 elicited by either lowering FIO2 or [Hb] (Roach et al. 1999). This could indicate that during small muscle mass exercise skeletal muscle blood flow is already maximal likely due to maximal vasodilation in the active regions, implying that muscle perfusion cannot be increased unless perfusion pressure is increased.
To determine if there is still some vasodilatory reserve at peak exercise in the active skeletal muscle, a vasodilator was infused intra-arterially when exercise-induced hyperaemia was maximal (Calbet et al. 2006b, Lundby et al. 2008a). During whole-body exercise (cycle ergometer exercise) at sea level, the infusion of maximal doses of ATP, i.e. the dose that elicits maximal vasodilation under resting conditions, increased leg vascular conductance by 17% (Calbet et al. 2006b). Mean arterial pressure (MAP) was not affected by the infusion of ATP into the femoral artery, due to a small (6%) but significant elevation of Qmax. About half of the increase in Qmax was directed to the ATP-infused leg which showed a trend (P = 0.08) for 0.8 L min−1 higher leg blood flow (LBF) (8% more than under control conditions). However, at the same time fractional O2 extraction was reduced, indicating that the distribution of blood flow was altered by the vasodilator, and some flow went to less active muscle fibres or other vascular beds of the leg apart from the active muscle fibres. The same subjects were also studied after 8–12 days of residence at 4559 m above sea level, when their resting sympathetic nerve active should have been remarkably increased (Hansen & Sander 2003), and their resting MAP was elevated by ~20 mmHg. As expected, peak exercise Q was reduced by 19% and peak leg blood flow was 18% lower than at sea level. Thus, at maximal exercise in chronic hypoxia there was a functional reserve (about 5 L min−1) to increase Q and LBF in response to the ATP infusion. Maximal exercise leg vascular conductance was increased, but not LBF because MAP was reduced with ATP. Moreover, as observed at sea level, ATP reduced O2 extraction across the leg also indicating that ATP causes VO2/Q mismatch. These two experiments clearly show that exercise-induced skeletal muscle hyperaemia cannot be increased without a concomitant elevation of cardiac output. This conclusion is further supported by experiments performed with a small muscle mass (one or two-legged knee extension) exercise, in which neither isovolemic anaemia (Koskolou et al. 1997), isovolemic anaemia combined with hypoxia (Roach et al. 1999) nor severe acute hypoxia (Calbet et al. 2009) elicited elevations in normoxic peak LBF.
In the only study where adenosine was infused at maximal doses intra-arterially during maximal knee extension exercise, adenosine was combined with hyperoxia (FIO2 = 1) and in the four subjects from whom maximal data were obtained peak exercise vascular conductance across the whole leg was not increased by adenosine (Barden et al. 2007). Although this study is in support with the concept of limited vasodilatory reserve at maximal exercise in humans, there is a limitation that precludes any definite conclusion from this study, as only the quadriceps muscle was recruited during the exercise but adenosine was infused into the common femoral artery, implying that some vasodilation may have occurred outside the working muscles.
Peak LBF and vascular conductance is increased with endurance (Roca et al. 1992, Mourtzakis et al. 2004) and high-intensity intermittent exercise training (Juel et al. 2004). Roca et al. (1992) reported a 26% higher peak LBF during semi-recumbent cycle ergometer exercise after 9 weeks of endurance training, but vascular conductance was not assessed. In another study, 5 weeks of knee extension exercise (1 h day−1 × 5 days week−1) increased peak LBF and vascular conductance by 17% (Mourtzakis et al. 2004). A similar enhancement was reported by Juel et al. (2004) after 7–8 weeks of high-intensity intermittent training (fifteen 1 min bouts at approx. 150% of thigh maximal O2 uptake per day, 3–5 times week−1). The specific (mL kg−1 muscle mass) perfusion achieved after training in these studies (Juel et al. 2004, Mourtzakis et al. 2004) remains far below the high levels of skeletal muscle blood flow and vascular conductance reported in elite endurance athletes (Richardson et al. 1993).
Matching O2 demand and supply during whole-body exercise
Saltin (1985) showed that the human skeletal muscle has a vasodilatory capacity that, if similar in all muscles, would require a Qmax two to threefold higher than actually reached to maintain MAP, it was postulated that some vasoconstriction must restrain vasodilation during whole-body exercise in humans. This concept also implies that during maximal whole-body exercise a competition is established between active skeletal muscles, respiratory muscles, myocardium and the CNS for a limited and ultimately insufficient amount of Qmax. Flow goes where the resistance is lower, but the critical questions are: how is the overall haemodynamic response to exercise integrated? How is the distribution of blood flow governed? How are perfusion priorities established? Consistent with these ideas were observations made in early 1960s that blood pressure falls during supine or head down tilt exercise in subjects who had undergone surgical sympathectomy for severe hypertension (Marshall et al. 1961a). Under these circumstances unchecked vasodilation in the active muscles likely contributed to the fall in blood pressure.
Another important clue was provided by Secher et al. (1977) who reported that superimposing intense arm exercise (oxygen uptake in the arms representing more than 40% of whole-body oxygen uptake) on leg exercise reduced leg blood flow and VO2 without changes in MAP, indicating that leg blood flow was restrained by a neurogenic vasoconstricting mechanism. A similar restraint of LBF was also observed during whole-body exercise when the work of the respiratory muscles was increased (Harms et al. 1997, Dempsey et al. 2006). The same type of response, but less marked, was observed when leg cycling at 60% of VO2max was superimposed on arm cranking at 80% of VO2max (Volianitis & Secher 2002), causing a small reduction in arm blood flow. However, when the muscle mass recruited during the exercise was smaller, static and static-ischaemic arm exercise, causing a two to fourfold increase in muscle sympathetic nerve activity and a 15–32% increase in MAP, reduced leg vascular conductance without a net effect on LBF (Strange 1999), indicating a local autoregulatory effect in the contracting muscles when cardiac output is capable of meeting muscle blood flow demands.
More definitive evidence was obtained by studying a group of cross-country skiers in which Qmax, leg blood flow and arm blood flow were measured simultaneously, while skiing with different techniques (Calbet et al. 2004). Arm hyperaemia reached maximal levels when the skiers used the ‘double poling’ technique, in which both arms press on the poles simultaneously to propel the body. However, during maximal skiing with the diagonal technique, where arm and leg contribute to propulsion, the arm blood flow response was blunted, suggesting that during combined arm and leg exercise in the upright position the perfusing priority was given to the legs. Moreover, it was estimated that without the arm flow restriction MAP would have fallen to ~ 75 mmHg from the observed ~95 mmHg.
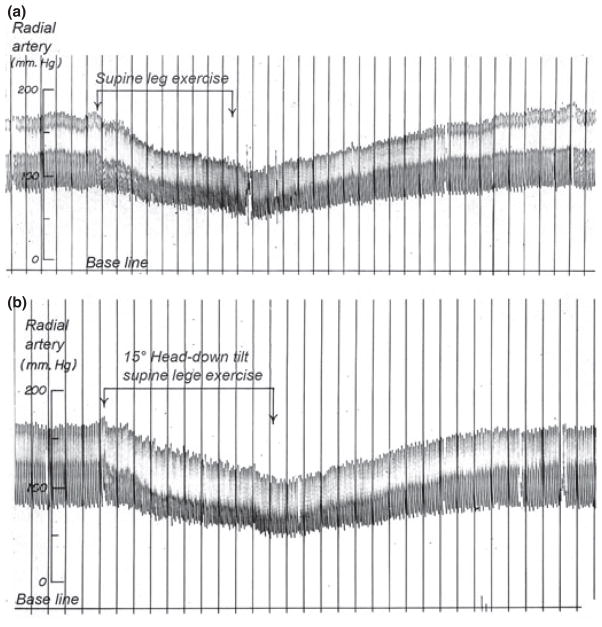
Several studies with patients provide evidence for the sympathetic nervous system as the main mechanism restraining muscle blood flow in the abdominal viscera and non-active skeletal muscles, and also in active skeletal muscle during heavy whole-body exercise (Lang et al. 1997, Dela et al. 2003). For example, MAP drops to values between 60 and 70 mmHg a minute or two after the onset of exercise in sympathectomized men performing exercise in both the supine position and with 15° head down tilt (Marshall et al. 1961b) (Fig. 2). Similarly, MAP is reduced during electrostimulation-induced exercise on the cycle ergometer in paraplegics who lack a functional sympathetic system (Dela et al. 2003). In patients with chronic heart failure sympathetic inhibition with clonidine results in greater vascular conductance and peak exercise blood flow in the exercising legs (Lang et al. 1997).
Figure 2.
Original record of arterial pressure during supine and head down tilt leg exercise in a 44-year-old male who had undergone thoracolumbar sympathectomy for the treatment of severe hypertension. The patient had normal autonomic innervation of the heart and could increase his heart rate and cardiac output. Thus, the fall in blood pressure during exercise seen in this figure is some of the first evidence that the sympathetic nerves must restrain blood flow to active muscles to regulate arterial pressure during exercise. Note that even the head down position used to maximize cardiac filling does not prevent the fall in arterial pressure during exercise. Figure from Marshall et al. (1961b).
A similar response is observed in patients with idiopathic hyperhidrosis, which has been attributed to overactivity of the sympathetic fibres, in which sympathectomy increases peak forearm vascular conductance and exercise capacity during handgrip exercise (Kardos et al. 2000). Nevertheless, studies with patients should be interpreted with caution as adaptations other than that anticipated may have developed.
Sympathetic vasoconstriction can be also blocked with ATP (Rosenmeier et al. 2004). However, ATP infusion into one femoral artery during maximal whole-body exercise in chronic hypoxia caused vasodilation without increasing leg blood flow, due to a reduction in perfusing pressure of ~20 mmHg to values that were similar to those observed at maximal exercise at sea level (Lundby et al. 2008a). Interestingly, in this experiment O2 extraction was marginally increased in the contralateral leg (not ATP-infused leg) that is compatible with a reduction in leg blood flow in the contra-lateral exercising leg. Thus, it seems that there was a response to the reduction in MAP but this response was likely blunted in the ATP-infused leg while the other vascular beds were already subject to high levels of vasoconstriction.
This result together with the observation of a relatively low MAP during upright maximal cross-country skiing (Calbet et al. 2004) and during running uphill on the treadmill (Hermansen et al. 1970) indicates that a small reduction in MAP during whole-body exercise is tolerable.
As illustrated in Figure 3, LBF, leg vascular conductance and femoral venous oxygen saturation were all reduced in old compared with young subjects, during submaximal cycle ergometer exercise. These findings are consistent with the idea that there is greater sympathetic restraint of blood flow in the active muscles of the older subjects due to their more limited cardiac output and higher levels of sympathetic outflow. In agreement with this view Magnusson et al. (1997) showed that patients with moderate chronic heart failure can reach a peak skeletal muscle perfusion and a leg oxygen uptake comparable to that of healthy individuals when a sufficiently small muscle mass is activated. However, if the exercise involves a larger muscle mass, peak LBF, leg vascular conductance and VO2 are markedly reduced, and these effects are accompanied by increased noradrenaline spillover (an indirect measure of sympathetic activation). Thus, in conditions with limited Qmax, sympathetic overactivity is even more necessary to limit exercise-induced skeletal muscle vasodilation to preserve minimal perfusion levels for the brain, heart and respiratory muscles.
Figure 3.
Estimates of two leg blood flow made using thermodilution in trained younger (open bars) and older (filled bars) subjects during cycle exercise. Leg blood flow, vascular conductance and femoral venous oxygen saturation were all reduced in the older subjects. These findings are consistent with the idea that there is greater sympathetic restraint of blood flow in the active muscles of the older subjects due to their more limited cardiac output and higher levels of sympathetic outflow. The reduced venous oxygen saturation in the older subjects indicates that oxygen consumption is maintained by greater extraction. These data are also consistent with emerging ideas about sympathetic regulation of arterial blood pressure during exercise and physiological ‘strategies’ that facilitate high levels of oxygen uptake even when total blood flow is limited. Figure from Proctor et al. (2003). *P < 0.05 compared to young subjects.
Sympathetic activation is needed to maintain blood pressure while sympatholysis permits VO2/Q matching
In the previous sections of this review, we have stressed that during whole-body exercise, VO2max under most circumstances is largely dependent on factors associated with maximum oxygen delivery. In this context, a high cardiac output generally driven by a large SV is perhaps the ‘dominant’ determinant of VO2max. We have also pointed out that during large muscle mass whole-body exercise the capacity of the contracting skeletal muscles to ‘demand’ blood flow via their impressive ability to vasodilate could potentially threaten blood pressure regulation in the context of a ‘limited’ cardiac output. In other words, if a sufficient mass of skeletal muscle were active, the vasodilator capacity of the contracting muscles could outstrip maximum cardiac output and blood pressure would fall. While this clearly happens in patients with autonomic failure, it does not happen in normal subjects (Marshall et al. 1961b, Schrage et al. 2004).
In this context, the reason it does not happen is that there is sympathetic ‘restraint’ of blood flow to contracting muscles. This restraint appears to occur in vivo even though there is evidence from isolated contracting skeletal muscles that sympathetic control of blood flow to contracting skeletal muscle can be abolished (functional sympatholysis).
In the early 1960s Remensnyder et al. (1962) used an isolated perfused hind limb preparation and showed that the rise in perfusion pressure, seen at a series of fixed flow rates, associated with sympathetic activation caused by carotid occlusion was lost during exercise. They reasoned that this could only have happened if the sympathetic nerves were unable to cause constriction in the hind limb. In later studies (Thomas et al. 1997, Thomas & Victor 1998) in isolated rat skeletal muscle, it was clearly demonstrated that nitric oxide (NO) released in association with contractions limited the ability of the sympathetic nerves to evoke vasoconstriction in active skeletal muscle. However, the role of NO in limiting the sympathetically mediated vasoconstriction in contracting human muscles is less clear, and some studies have suggested that it is and is not obligatory (Chavoshan et al. 2002, Dinenno & Joyner 2003).
What can be said about the sympathetic control of blood flow to contracting human muscles is that ‘sympatholysis’ is not absolute and that there is evidence from a variety of models showing at least some sympathetic control of blood flow to contracting skeletal muscles (Marshall et al. 1961b, Tschakovsky et al. 2002, Calbet et al. 2004). There is also evidence of ongoing sympathetic control of blood flow in contracting muscles in conscious dogs performing voluntary treadmill exercise (Ruble et al. 2002). In both humans and dogs, the substance or substances that limit the ability of the sympathetic nerves to cause vasoconstriction is unclear. Recently ATP release (perhaps from deoxygenated red cells) has emerged as a possible candidate (Rosenmeier et al. 2004, 2008, Kirby et al. 2008). This substance is attractive for a number of reasons but infusions of ATP or related compounds in either the contracting forearm or contracting leg can essentially eliminate sympathetic control in vasodilated limbs in a more dramatic way than is seen during exercise.
The retention of at least some sympathetic control of blood flow to contracting muscles has important implications for whole-body exercise and the regulation of blood pressure in whole-body exercise. Under these circumstances, 70–80% (or perhaps more) of cardiac output is directed towards the active muscles, and even small changes in blood flow to the contracting muscles will have a marked impact on MAP if the circulatory conditions remain unchanged in the other vascular beds. For example, if 80% of cardiac output is directed towards contracting skeletal muscles, a 20% reduction in flow to those tissues will cause a 16% increase in arterial pressure. Fortunately, this can be accomplished in most cases without limiting oxygen consumption by the contracting skeletal muscles. Along these lines, the very high muscle blood flow values observed during one-leg kicking in humans (and other forms of small muscle mass exercise) are associated with limited oxygen extraction by the active muscles (Andersen & Saltin 1985). In other words, deep venous saturation is relatively high (30–40%) in most of these models and this is in contrast to the very low deep venous oxygen saturations seen in the femoral vein during activities like heavy two-leg cycling (Proctor et al. 1998). This means that reductions in flow during small muscle mass exercise can be accommodated and oxygen consumption protected by increased oxygen extraction in the active muscles.
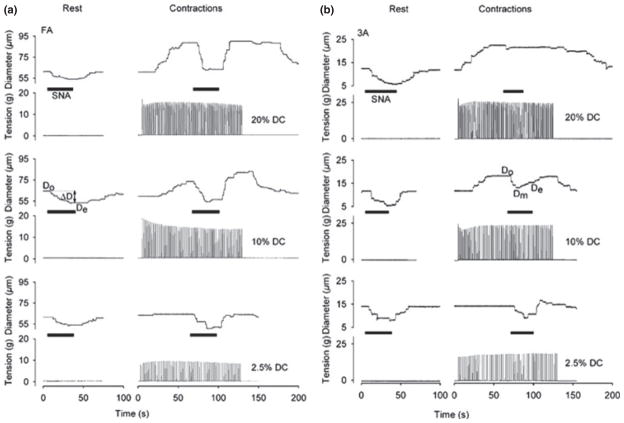
How might this increased oxygen extraction occur in a way that matches somewhat limited blood flow with the demand for oxygen? One clue comes from a study conducted by Van Teeffelen & Segal (2003) in the microcirculation of hamster skeletal muscles. As illustrated in Figure 4, these investigators showed that sympathetic control of blood flow to the smaller elements of the microcirculation was lost in contracting skeletal muscle, but retained in the larger microvessels. This finding is consistent with differing distributions of postjunctional alpha-1 and alpha-2 adrenergic receptors on the microvasculature and their differing sensitivities to metabolites and pH (there are more postjunctional alpha-2 receptors in the smallest microvessels, and their ability to constrict is limited by a number of metabolites and a fall in pH). When the findings of Van Teeffelen and Segal are extrapolated to the control of blood flow in the whole muscle it means that an increase in sympathetic outflow would constrict blood vessels that distribute flow to various regions of the contracting muscle but that loss of sympathetic control in the smallest vessels would tend to direct flow to the most metabolically stressed elements of the active muscle (Joyner & Thomas 2003).
Figure 4.
Individual records of the responses of a feed artery (FA panel a, left) and a 3A arteriole (panel b, right) to sympathetic stimulation at rest and during contractions caused by three levels of direct current stimulation in hamster retractor muscle. In the feed artery sympathetic stimulation (SNA) caused a reduction in vessel diameter at rest and during all levels of contraction showing that contraction did not inhibit sympathetic vasoconstriction. In the 3A arteriole the ability of sympathetic stimulation to reduce vessel diameter was lost during the highest level of contraction. When concepts arising from this paper are applied to whole-body exercise in humans, they suggest that continued sympathetic control of larger arteries during exercise might regulate or restrain total muscle blood flow. However, loss of sympathetic control in the smaller vessels would optimize the distribution of flow within the active muscles. Such a mechanism would also direct the limited flow to the most metabolically active areas in the contracting muscles and likely enhance oxygen extraction. Figure from Van Teeffelen & Segal (2003).
This concept has important implications for whole-body exercise because it permits the minimal level of flow to support a given level of contraction to be achieved under many circumstances. In other words, the retained ability to cause vasoconstriction in the larger blood vessels would limit total flow to a muscle, and the loss of sympathetic control in the smallest vessels would direct the limited flow to the areas of the muscle where it is most needed. Together this physiological compromise would permit oxygen extraction to be maximized in a given muscle, the largest fraction of total muscle mass to receive adequate or nearly adequate blood flow, and arterial blood pressure would be regulated at the same time. Along these lines, when vasodilators are infused into contracting leg muscles oxygen consumption in the exercising leg is not augmented in a way that suggests the vasodilation is limited either to non-active or less active areas of the muscle (Calbet et al. 2006b). These findings are also consistent with the idea that sympathetic control is lost in the smallest vessels and that they are in fact maximally dilated.
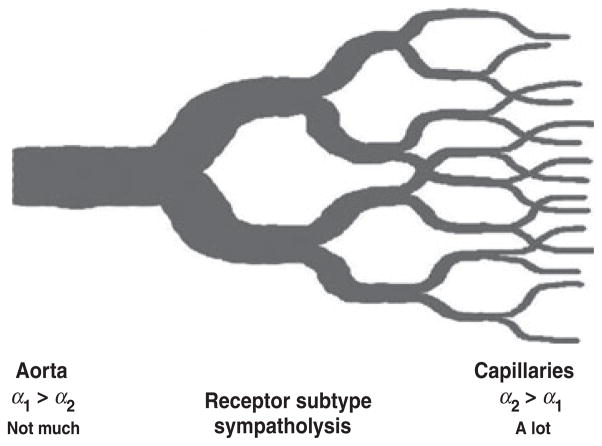
In summary, sympathetic control of blood flow to contracting muscles is critical for the regulation of arterial pressure in humans. This restraint of flow is especially evident during whole-body exercise like cross-country skiing or in older humans who have age-related reductions in maximum cardiac output (Proctor et al. 1998, Calbet et al. 2004). The mechanisms responsible for generating and targeting the sympathetic outflow required to ‘manage’ this competition between systemic cardiac output and local vasodilation are unclear and while there is some evidence that baroreflexes and baroreflex resetting play an important role in this phenomenon, there are still a number of questions about this topic (Ogoh et al. 2003, Joyner 2006). Additionally, the substances released by the active muscles, which limit the ability of the sympathetic nerves to cause vasoconstriction in the active muscles, are also incompletely understood with ATP recently emerging as an important candidate substance that could explain most of this phenomenon. More importantly, observations in the microcirculation are now consistent with observations in the macrocirculation and provide a unifying framework (Fig. 5) for both systemic blood pressure regulation and blood flow ‘management’ to skeletal muscle during heavy exercise in humans.
Figure 5.
Ideas about how blood flow and metabolism are matched. There is a progressive increase in sympatholysis from conducting blood vessels to the capillaries in skeletal muscle. This tends to eliminate sympathetic restraint of blood flow to the most active areas within a contracting muscle but permits total flow to that muscle to be regulated upstream. Such a scheme would explain the almost total extraction of O2 across active skeletal muscle vascular beds during heavy large muscle mass exercise in humans. It would also permit relatively more total muscle to be perfused in the context of the ‘limited’ cardiac output seen in humans. Data from animal models suggest that differences in alpha-adrenergic receptor subtype distribution from large to small blood vessels might contribute to this phenomenon, but this idea is unproved in humans.
Acknowledgments
J.A.L. Calbet’s work on this topic was supported by a Grant from Ministerio de Educación y Ciencia (DEP2009-11638). M.J. Joyner’s work on this topic was supported by NIH HL-46493, RR-024150 and the Caywood Professorship via the Mayo Foundation. Both authors would like to thank the many subjects who have participated in their experiments over the years and also their collaborators along with the dedication of their technical support staffs.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Ahlborg G, Jensen-Urstad M. Arm blood flow at rest and during arm exercise. J Appl Physiol. 1991;70:928–933. doi: 10.1152/jappl.1991.70.2.928. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and VO2max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2491–H2497. doi: 10.1152/ajpheart.01396.2006. [DOI] [PubMed] [Google Scholar]
- Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- Brink-Elfegoun T, Kaijser L, Gustafsson T, Ekblom B. Maximal oxygen uptake is not limited by a central nervous system governor. J Appl Physiol. 2007;102:781–786. doi: 10.1152/japplphysiol.00566.2006. [DOI] [PubMed] [Google Scholar]
- Busjahn CA, Schulz-Menger J, Abdel-Aty H, Rudolph A, Jordan J, Luft FC, Busjahn A. Heritability of left ventricular and papillary muscle heart size: a twin study with cardiac magnetic resonance imaging. Eur Heart J. 2009;30:1643–1647. doi: 10.1093/eurheartj/ehp142. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Boushel B. Integrative conductance of oxygen during exercise at altitude. Adv Exp Med Biol. 2010 doi: 10.1007/978-1-4899-7678-9_26. (in press) [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003a;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003b;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, De Paz JA, Garatachea N, Cabeza De Vaca S, Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol. 2003c;94:668–676. doi: 10.1152/japplphysiol.00128.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, Van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Koskolou M, Boushel R. Importance of hemoglobin concentration to exercise: acute manipulations. Respir Physiol Neurobiol. 2006a;151:132–140. doi: 10.1016/j.resp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006b;291:R447–R453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol. 2009;587:477–490. doi: 10.1113/jphysiol.2008.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TE, Covell JW. Left ventricular function in exercise-induced hypertrophy in dogs. Am J Cardiol. 1978;42:82–88. doi: 10.1016/0002-9149(78)90989-x. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Cain P, Holmqvist C, Stahlberg F, Lundback S, Arheden H. Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol. 2004;287:H243–H250. doi: 10.1152/ajpheart.01125.2003. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;292:H1452–H1459. doi: 10.1152/ajpheart.01148.2006. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson EA, Shave R, Whyte G, Ball D, Selmer C, Jans O, Secher NH, George KP. Preload maintenance and the left ventricular response to prolonged exercise in men. Exp Physiol. 2007;92:383–390. doi: 10.1113/expphysiol.2006.035089. [DOI] [PubMed] [Google Scholar]
- Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sorensen F, Kjaer M. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation. 2003;107:2127–2133. doi: 10.1161/01.CIR.0000065225.18093.E4. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom B, Berglund B. Effect of erythropoietin administration on maximal aerobic power. Scand J Med Sci Sports. 1991;1:88–93. [Google Scholar]
- Ekblom B, Huot R, Stein EM, Thorstensson AT. Effect of changes in arterial oxygen content on circulation and physical performance. J Appl Physiol. 1975;39:71–75. doi: 10.1152/jappl.1975.39.1.71. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Wilson G, Astrand PO. Central circulation during exercise after venesection and reinfusion of red blood cells. J Appl Physiol. 1976;40:379–383. doi: 10.1152/jappl.1976.40.3.379. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fulco CS, Cymerman A, Muza SR, Rock PB, Pandolf KB, Lewis SF. Adductor pollicis muscle fatigue during acute and chronic altitude exposure and return to sea level. J Appl Physiol. 1994;77:179–183. doi: 10.1152/jappl.1994.77.1.179. [DOI] [PubMed] [Google Scholar]
- Fulco CS, Lewis SF, Frykman PN, Boushel R, Smith S, Harman EA, Cymerman A, Pandolf KB. Muscle fatigue and exhaustion during dynamic leg exercise in normoxia and hypobaric hypoxia. J Appl Physiol. 1996;81:1891–1900. doi: 10.1152/jappl.1996.81.5.1891. [DOI] [PubMed] [Google Scholar]
- Gledhill N, Cox D, Jamnik R. Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc. 1994;26:1116–1121. [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G, Haggendal E, Saltin B. Local xenon 133 clearance from the quadriceps muscle during exercise in man. J Appl Physiol. 1967;22:305–310. doi: 10.1152/jappl.1967.22.2.305. [DOI] [PubMed] [Google Scholar]
- Gunn HM. Heart weight and running ability. J Anat. 1989;167:225–233. [PMC free article] [PubMed] [Google Scholar]
- Hamilton WF, Rompf JH. Movement of the base of the ventricle and the relative constancy of the cardiac volume. Am J Physiol. 1932;102:559–565. [Google Scholar]
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507(Pt 2):619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen L, Ekblom B, Saltin B. Cardiac output during submaximal and maximal treadmill and bicycle exercise. J Appl Physiol. 1970;29:82–86. doi: 10.1152/jappl.1970.29.1.82. [DOI] [PubMed] [Google Scholar]
- Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Part V. The recovery process after exercise in men. Proc R Soc B. 1924;97:96–138. [Google Scholar]
- Joyner MJ. Physiological limiting factors and distance running: influence of gender and age on record performances. Exerc Sport Sci Rev. 1993;21:103–133. [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. doi: 10.1113/jphysiol.2003.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–E251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Kardos A, Taylor DJ, Thompson C, Styles P, Hands L, Collin J, Casadei B. Sympathetic denervation of the upper limb improves forearm exercise performance and skeletal muscle bioenergetics. Circulation. 2000;101:2716–2720. doi: 10.1161/01.cir.101.23.2716. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer I. Studies on exercise hyperemia. Acta Physiol Scand Suppl. 1964;244:1–27. [PubMed] [Google Scholar]
- Koskolou MD, Roach RC, Calbet JA, Radegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol. 1997;273:H1787–H1793. doi: 10.1152/ajpheart.1997.273.4.H1787. [DOI] [PubMed] [Google Scholar]
- Lang CC, Rayos GH, Chomsky DB, Wood AJ, Wilson JR. Effect of sympathoinhibition on exercise performance in patients with heart failure. Circulation. 1997;96:238–245. doi: 10.1161/01.cir.96.1.238. [DOI] [PubMed] [Google Scholar]
- Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure–volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116–126. doi: 10.1161/01.cir.88.1.116. [DOI] [PubMed] [Google Scholar]
- Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- Lundback S. Cardiac pumping and function of the ventricular septum. Acta Physiol Scand Suppl. 1986;550:1–101. [PubMed] [Google Scholar]
- Lundby C, Araoz M, van Hall G. Peak heart rate decreases with increasing severity of acute hypoxia. High Alt Med Biol. 2001;2:369–376. doi: 10.1089/15270290152608543. [DOI] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008a;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JA. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008b;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylven C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Allwood MJ, Keck EW, Shepherd JT. Measurement of cardiac output and “central” blood volume by various systems. J Appl Physiol. 1961a;16:541–544. doi: 10.1152/jappl.1961.16.3.541. [DOI] [PubMed] [Google Scholar]
- Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961b;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol. 1998;84:1872–1881. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- Mellander S, Johansson B. Control of resistance, exchange, and capacitance functions in the peripheral circulation. Pharmacol Rev. 1968;20:117–196. [PubMed] [Google Scholar]
- Milliken MC, Stray-Gundersen J, Peshock RM, Katz J, Mitchell JH. Left ventricular mass as determined by magnetic resonance imaging in male endurance athletes. Am J Cardiol. 1988;62:301–305. doi: 10.1016/0002-9149(88)90228-7. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest. 1958;37:538–547. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourtzakis M, Gonzalez-Alonso J, Graham TE, Saltin B. Hemodynamics and O2 uptake during maximal knee extensor exercise in untrained and trained human quadriceps muscle: effects of hyperoxia. J Appl Physiol. 2004;97:1796–1802. doi: 10.1152/japplphysiol.00169.2004. [DOI] [PubMed] [Google Scholar]
- Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63:2269–2277. doi: 10.1152/jappl.1987.63.6.2269. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Bredmose PP, Stromstad M, Volianitis S, Quistorff B, Secher NH. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J Appl Physiol. 2002;93:724–731. doi: 10.1152/japplphysiol.00398.2000. [DOI] [PubMed] [Google Scholar]
- Noakes TD. 1996 J.B. Wolffe Memorial Lecture. Challenging beliefs: ex Africa semper aliquid novi. Med Sci Sports Exerc. 1997;29:571–590. doi: 10.1097/00005768-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Noakes TD, St Clair Gibson A. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br J Sports Med. 2004;38:648–649. doi: 10.1136/bjsm.2003.009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes TD, St Clair Gibson A, Lambert EV. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans. Br J Sports Med. 2004;38:511–514. doi: 10.1136/bjsm.2003.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CM, Manohar M. Distribution of blood flow during moderate and strenuous exercise in ponies (Equus caballus) Am J Vet Res. 1983;44:1861–1866. [PubMed] [Google Scholar]
- Perseghin G, De Cobelli F, Esposito A, Lattuada G, Terruzzi I, La Torre A, Belloni E, Canu T, Scifo P, Del Maschio A, Luzi L, Alberti G. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: a magnetic resonance imaging and phosphorus 31 spectroscopy study. Am Heart J. 2007;154:937–942. doi: 10.1016/j.ahj.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Hudsmith LE, Robson MD, Doll HA, Francis JM, Wiesmann F, Jung BA, Hennig J, Watkins H, Neubauer S. Sex-specific characteristics of cardiac function, geometry, and mass in young adult elite athletes. J Magn Reson Imaging. 2006;24:297–303. doi: 10.1002/jmri.20633. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Pugh LGCE. Cardiac output in muscular exercise at 5800 m (19000 ft) J Appl Physiol. 1964;19:441–447. [Google Scholar]
- Rådegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Robach P, Calbet JA, Thomsen JJ, Boushel R, Mollard P, Rasmussen P, Lundby C. The ergogenic effect of recombinant human erythropoietin on VO2max depends on the severity of arterial hypoxemia. PLoS ONE. 2008;3:e2996. doi: 10.1371/journal.pone.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol. 1992;73:1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Yegutkin GG, Gonzalez-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol. 2008;586:4993–5002. doi: 10.1113/jphysiol.2008.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42D–47D. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;583:819–823. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Calbet JA. Point: in health and in a normoxic environment, VO2max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:744–745. doi: 10.1152/japplphysiol.01395.2005. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–VII78. [PubMed] [Google Scholar]
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Dinenno FA, Roberts SK, Johnson CP, Sandroni P, Low PA, Joyner MJ. Effects of midodrine on exercise-induced hypotension and blood pressure recovery in autonomic failure. J Appl Physiol. 2004;97:1978–1984. doi: 10.1152/japplphysiol.00547.2004. [DOI] [PubMed] [Google Scholar]
- Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand. 1977;100:288–297. doi: 10.1111/j.1748-1716.1977.tb05952.x. [DOI] [PubMed] [Google Scholar]
- Shapiro LM, Smith RG. Effect of training on left ventricular structure and function. An echocardiographic study. Br Heart J. 1983;50:534–539. doi: 10.1136/hrt.50.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slordahl SA, Madslien VO, Stoylen A, Kjos A, Helgerud J, Wisloff U. Atrioventricular plane displacement in untrained and trained females. Med Sci Sports Exerc. 2004;36:1871–1875. doi: 10.1249/01.mss.0000145444.01292.3d. [DOI] [PubMed] [Google Scholar]
- Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol. 1987;62:606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol. 1999;514(Pt 1):283–291. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506(Pt 3):817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Teeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Krustrup P, Dawson E, Secher NH. Arm blood flow and oxygenation on the transition from arm to combined arm and leg exercise in humans. J Physiol. 2003;547:641–648. doi: 10.1113/jphysiol.2002.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. Counterpoint: in health and in normoxic environment VO2max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:745–747. doi: 10.1152/japplphysiol.01395a.2005. discussion 747–748. [DOI] [PubMed] [Google Scholar]
- Weyand PG, Lee CS, Martinez-Ruiz R, Bundle MW, Bellizzi MJ, Wright S. High-speed running performance is largely unaffected by hypoxic reductions in aerobic power. J Appl Physiol. 1999;86:2059–2064. doi: 10.1152/jappl.1999.86.6.2059. [DOI] [PubMed] [Google Scholar]