1. Introduction
The title of our previous talk “Acoustic targeted drug delivery in neurological tissue” was changed to “Acoustic enhanced Evans blue dye perfusion in neurological tissues” along with minor details of the corresponding abstract to this paper. This was done to provide a more direct title and abstract of our research study for indexing and searching by the scientific community.
The success of treating brain cancer such as neuroblastomas and neurofibromatosis has not been very effective, and is in fact the leading cause of cancer-related death in patients younger than age 35. Despite the development of drugs that combat these malignancies, the prognosis for patients remains poor. One reason for the poor outcome is that migrating cells escape removal during tumor resection, and in many cases avoid radiation and chemotherapy after resection. This allows the tumor to regrow, usually aggressively, and often at a location close to the site of the original tumor (Giese et al., 2003 and Lefranc et al., 2005). In the last 10 years recent developments in drug delivery methods have allowed doctors to implant/inject time-release drugs into the tumor cavity that allow for continuous release of chemotherapy; however results from these studies have not been as successful as anticipated. Gliadel wafers impregnated with BCNU (also called carmustine) are implanted into the resection cavity to deliver high concentrations of drug to the surrounding tissue. However, the diffusivity of BCNU in tissue limits its penetration to a few millimeters from the wafer before it is eliminated by a variety of mechanisms (Westphal et al., 2003).
Convection-enhanced drug delivery has been used to bypasses the blood-brain barrier by infusing drugs directly into the brain through a needle or microcatheter. The infusion establishes a pressure gradient that induces a flow in the brain away from the needle. Small molecules can be delivered over relatively large distances in the brain, but larger molecules, including certain proteins with proven efficacy against malignant cells, are hindered in their transport (Bobo et al, 1994 and Vogelbaum, 2007). As a result, they cannot travel sufficiently far to reach migratory malignant cells. Drugs can be packaged inside nanoparticles such as polymeric spheres or liposomes, which protects the drug from elimination, but transport of these nanoparticles in the interstitium is even more hindered than that of proteins (Panyam et al. 2003 and Müller et a. 2000).
The transport of proteins and nanoparticles can be improved by increasing the porosity or the effective “pore size” of the brain interstitium. For example, transport is enhanced when the proteins or nanoparticles are infused in a hyperosmolar solution that induces tissue swelling locally by drawing fluid into the interstitium from surrounding blood vessels and cells. Infusing an enzyme that temporarily degrades the extracellular matrix, which increases the permeability of the interstitium to proteins and nanoparticles, also enhances transport (Neeves et al. 2007). Although these methods work to some extent, additional transport enhancement is required to realize the potential of convection enhanced drug delivery.
In this study we utilize 1.58 MHz focused ultrasound to increase the perfusion of locally delivered Evans blue dye that is widely used in tissue diffusion experiments (Woitzik et al., 2007, Aoki et al., 2002 and Chan et al., 1983), into an agar gel that is used routinely as a mimic of brain tissue for drug delivery studies (Chen et al., 2004), equine brain and avian muscle tissue. The ultimate goal is to increase the rate of transport of pharmaceutical agents relative to their elimination rate, and thereby extend the distance that drugs penetrate and maintain therapeutically useful concentrations.
2. Methods
Specimen setup and EBD application
Neurological tissue mimicking phantoms were prepared by filling off-the-shelf 12 oz Solo Cups (Tops Super Market Brand, Ithaca, NY) with a solution of 0.6 wt% agar powder (Product #19461580, MP Biomedicals, Solon, OH). The powder was dissolved in 100 degree Celsius distilled water for 5 minutes and pored into the cups to a height of 2.5cm. The cups were then covered, set aside, and allowed to cool and gel (approximately 20 minutes). Equine horse brain was harvested from the Cornell Vet School two minutes post-mortem and prepared into 3×3×3 cm slices (the cortex surface was used for the ultrasound enhanced EBD perfusion). The equine brain experiments were conducted within 30 minutes of brain harvesting. Avian muscle tissue was purchased fresh from a local supermarket and cut into 3×3×3 cm squares for the study. Well characterized Evans blue dye (Product #203163, MP Biomedicals, Solon, OH) diluted in distilled water to 0.25 wt% was used to mimic water soluble drug and apply contrast to determine the extent of perfusion. During ultrasound application, the fresh tissues were placed in 12 oz cups, and all samples were covered by 200 ml of EBD and secured in position.
Ultrasound setup, dosing and analysis
Ultrasound energy in the samples was generated by a lead zirconate titanate (PZT-4), 1.58 MHz, 25.4mm diameter piezoelectric ceramic with a radius of curvature corresponding to 40mm. The ceramic, air-backed and housed in a PVC plastic assembly, was driven at 1.58 MHz, by a +/- 200 volt amplifying circuit (TC6320 high speed ultrasound driver, Supertex Inc., San Jose, CA.). The acoustic source power was modeled mathematically using Mason’s model (Redwood, 1961, Morris and Hutchens, 1986, and Warren, 1985), and measured directly with a calibrated hydrophone (Model #S158, Transducer Engineering Inc., Andover, MA). All samples were sonicated 100% duty cycle at the average intensity of 3.00 watts/cm2 (peak-focus-intensity 15 watts/cm2) in the samples geometric center for durations of 1-4 minutes. The location of the focus of the transducer was positioned so that the focus at 40mm from the front surface of the US was placed on the sample/dye interface as shown in figure 1. The US was oscillated at 0.25 Hz over a 10mm translation perpendicular to the samples surface to increase the sonicated volume for the duration of all experiments.
Fig 1.
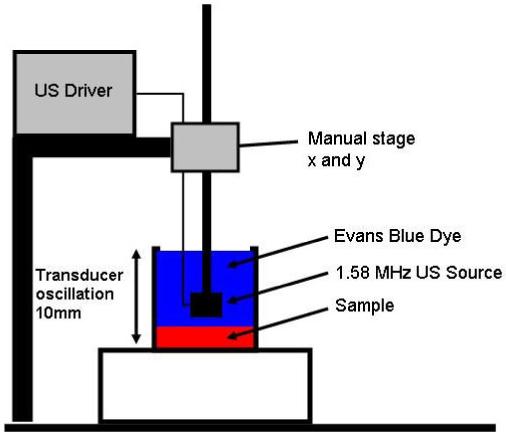
The experimental setup for the sample study is shown to the left. The sonicated phantom is covered in 0.25 wt% diluted Evans blue dye and sonicated at 100% duty while simultaneously the transducer is oscillated up and down at 0.25Hz 10mm amplitude. The beginning position of the transducer was 40mm above the sample/dye interface.
For analysis purposes the location of the sample/dye interface was taken to be zero. Sectional readings of EBD perfusion were taken every minute for the control and sonicated samples (four at each time point totaling 12 for each material). The section of the sample was preformed by taking a 2mm geometric center slice from the sample and imaging the location of the US focused energy with a CCD camera/microscope system (Nikon Cool Pix 995, Nikon Inc. USA and Olympus BX51, Olympus Inc. USA). The digitally captured images were imported into Mat Lab 6.5 (Math Works Inc. USA) to determine the extent of the Evans blue dye perfusion into the samples with and without applied US. By using Red-Green-Blue (RGB) color mapping and converting digital pixels into distance (mm) measurements, intensity vs. distance concentration curves of EBD perfusion into the samples were produced. The four spectral intensity curves corresponding to each sectional measurement for each time point were averaged, and the area under the concentration curves for the sonicated and control were evaluated to quantify the density of EBD uptake.
3. Results
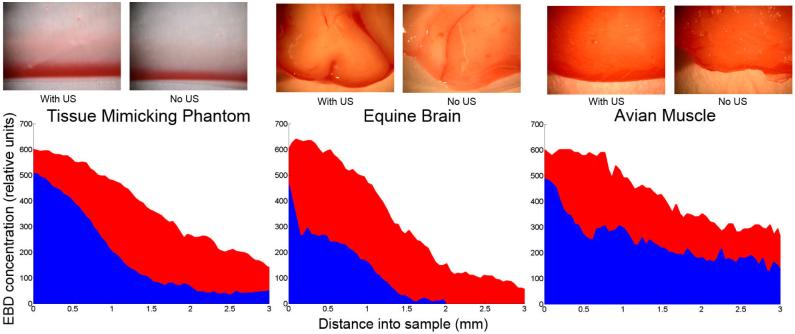
Analysis of the experimental data shows the sample tissues have a substantial increase in EBD perfusion when compared to the control. Shown in figure 2 are the sectional images of the EBD concentration gradient for three samples studied at two minutes (1, 3 and 4 minutes not shown). The red curves (peak uptake aprox. 600 for all) represents the EBD perfusion into the samples with US applied. The blue curves are the controls representing simple diffusion into the sample. Application of US enhanced EBD density of 61.5% (phantom), 93.2% (brain) and 52.8% (muscle) compared to the control.
Fig 2.
The above Figure shows the sectional images and the results of enhanced EBD delivery into a tissue mimicking phantom, equine horse brain and avian muscle tissues with the application of Therapeutic Ultrasound for two minutes in red, compared with the control in blue. The figure shows concentration curves of the EBD uptake into the samples and that local delivery of EBD in conjunction with the application of Therapeutic Ultrasound may significantly enhanced the amount of EBD delivery into tissue. Application of US enhanced EBD density of 61.5% (phantom), 93.2% (brain) and 52.8% (muscle) compared to the control.
During the four time points studied in this experiment and visible in figure 2, the EBD concentration readings of the control and sonicated samples are not equivalent at 0mm into the sample. The edge effect of concentration difference between experimental and control groups at the sample/dye interface was noticed in our prior study (Lewis and Olbricht, 2007). We now believe that this edge effect and mismatch of concentration in the control and experimental groups is a surface mass transport resistance, and the limited diffusion of the dye into the samples for the short time points resulting in poor pixel intensity image mapping. Experimentally with the phantom, we found that if we removed the phantoms gelling surface with a razor, the mass-transport resistance was not apparent and EBD more readily perfused the phantom. The focused ultrasound had a sonoporation type effect as in transdermal drug delivery, and has an impact on breaking down the surface resistance of all samples allowing for quicker perfusion of dye and overall dye density uptake in the sonicated samples.
4. Conclusion
In this study we looked at the effect of 1.58 MHz ultrasound on the perfusion of Evans blue dye into neurological tissue mimicking phantoms, equine brain and avian muscle tissue. We found a clear increase in EBD penetration and overall dye density in the sonicated verses control samples for all times. Experimentally, this was noticeable in figure 2 at 2 minutes with enhanced dye uptake of 61.5% (phantom), 93.2% (brain) and 52.8% (muscle) compared to the controls. The application of 1.58MHz focused ultrasound to brain mimicking phantom, equine brain and avian muscle, in conjunction with topical delivery of EBD was found to increase the surface mass-transfer of EBD into the three samples. We hypothesize that the acoustic pressures from the peak focal intensity of 15.2 watts/cm2 and oscillation of the transducer field figure 1, imparted radiation pressures of EBD into the sample, while also warming and agitating the porous matrix. These combined effects enhanced the perfusion of dye with sonication.
From the results presented we find that 1.58 MHz ultrasound is a promising new method to increase the penetration of hydrophilic drugs into brain tissue. The goal of this research is to use ultrasound to drive locally delivered chemotherapy agents past current diffusion limitations to reach migratory cancer cells. Continued research to study how changes in ultrasound parameters will affect the rate and level of dye perfusion in animal brain tissues will be our continuing focus. Different frequencies, powers, and pulse sequences appropriately below the brain tissue damage threshold will be analyzed to create a therapeutically useful regime.
Contributor Information
George K. Lewis, Jr., Department of Biomedical Engineering, Cornell University, 108 Olin Hall, Ithaca, NY 14850, george@cornellbme.com
Dr. William L. Olbricht, Department of Chemical Engineering, Cornell University, 108 Olin Hall, Ithaca, NY 14850, wlo1@cornell.edu
Dr. George K. Lewis, Sr., Transducer Engineering Inc., P.O. Box 4034, Andover, MA 01810, thearrayman@transducerengineering.com
References
- Giese A, Bjerkvig R, Berens M, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. American Cancer Society Journal of Clinical Oncology. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating cells to apoptosis. Journal of Clinical Oncology. 2005;23(10):2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hilt D, Bortey E, Delavault P, Olivares R, Warnke P, Whittle I, Jääskeläinen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo R, Laske D, Akbasak A, Morrison P, Dedrick R, Oldfield E. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelbaum M. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. Journal of Neuro-Oncology. 2007;83:97–109. doi: 10.1007/s11060-006-9308-9. [DOI] [PubMed] [Google Scholar]
- Müller R, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Neeves K, Sawyer A, Foley C, Saltzman W, Olbricht W. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer. Brain Research. 2007;1180:121–132. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitzik J, Schilling L. A new method for superselective middle cerebral artery infusion in the rat. J Neurosurg. 2007;106(5):872–880. doi: 10.3171/jns.2007.106.5.872. [DOI] [PubMed] [Google Scholar]
- Aoki T, Sumii T, Mori T, Wang X, Lo E. Blood-Brain Barrier Disruption and Matrix Metalloproteinase-9 Expression During Reperfusion Injury. Journal of Stroke. 2002;33:2711. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- Chan P, Fishman R, Caronna J, Schmidley J, Prioleau G, Lee J. Induction of brain edema following intracerebral injection of arachidonic acid. Anneals of Neurology. 1983;13(6):625–632. doi: 10.1002/ana.410130608. J. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gillies G, Broaddus W, Prabhu S. A realist realistic tissue phantom for intraparenchymal infusion studies. Journal of Neurosurgery. 2004;101:314–322. doi: 10.3171/jns.2004.101.2.0314. [DOI] [PubMed] [Google Scholar]
- Redwood M. Transient Performance of a Piezoelectric Transducer. JASA. 1961;33(4):527–536. [Google Scholar]
- Morris S, Hutchens C. Implementation of Mason’s Model on Circuit Analysis Programs. IEEE Trans. Ultrasonics, Ferroelectrics and Frequency Control. 1986;33(3):295–298. doi: 10.1109/t-uffc.1986.26832. [DOI] [PubMed] [Google Scholar]
- Warren M. Physical Acoustics and the Properties of Solids. D. Van Nostrand Company; Toronto, Canada: 1958. Library of Congress Number 58-11114. [Google Scholar]
- Lewis G, Olbricht W. A phantom feasibility study of acoustic enhanced perfusion. Proceedings of IEEE, LISA. 2007:67–70. 2007. Article 39. [Google Scholar]