Abstract
Background
Clonidine, a centrally acting antihypertensive agent, has been used successfully in pregnancy. We sought to describe the pharmacodynamic effects of clonidine in pregnancy and the associated impact on fetal growth.
Methods
A retrospective cohort study was performed. Maternal hemodynamics were measured before and after treatment. Responses to clonidine were categorized by the predominant hemodynamic effect: decreased vascular resistance, decreased cardiac output, or mixed.
Results
Sixty-six pregnant women were studied. Treatment was associated with a reduction of mean arterial pressure, (-9.2 mmHg, P<0.001), a reduction in total peripheral resistance, (-194 dyne•cm•sec-5, P<0.001), and an increase in cardiac output, (+0.5 L/min, P<0.001). The hemodynamic response was characterized by decreased resistance in 34 women; decreased cardiac output in 22; and mixed effect in 10. Women with a higher dose of clonidine (>0.15mg/day) and those with a lower creatinine clearance were more likely to experience a primary reduction in cardiac output. Mean birth weight percentile was lower in the group that experienced a reduction in cardiac output compared to the group with a reduction in vascular resistance, (26.1 vs 43.6, P=0.02). The rate of birth weight <10th percentile was also higher in the group experiencing decreased cardiac output, (41% vs 8.8%, P=0.008)
Conclusion
The hemodynamic effect of clonidine in pregnancy is heterogeneous. The category of effect, reduction in vascular resistance vs. reduction in cardiac output, significantly impacts fetal growth. A reduction in heart rate after therapy identifies pregnancies at risk for reduced fetal growth.
Keywords: pregnancy, hypertension, clonidine, pharmacodynamics, hemodynamics Clonidine in Pregnancy
Introduction
Clonidine is an antihypertensive agent that achieves its hypotensive effect by stimulating α2 adrenergic receptors in the brainstem thereby decreasing central adrenergic output. The mechanism of action is similar to that of alpha-methyldopa, but the onset of action of clonidine is more rapid. The incidence of serious side effects is less with clonidine. Horvath et al. have reported successful and safe use of clonidine as an antihypertensive in pregnancy but the hemodynamics effects of clonidine, when used in pregnancy, have not been previously reported.(1)
Antihypertensive therapy of all pregnant women at the University of Washington is individualized based on noninvasive measurement of cardiac output with the intent of not only lowering blood pressure but also normalizing cardiac output and vascular resistance. We have considerable experience with the use of clonidine in pregnancy. In general, we have used clonidine in directed therapy to achieve a reduction in vascular resistance. We have observed that while clonidine effectively lowers blood pressure, the hemodynamic response is inconsistent, lowering cardiac output in some patients and vascular resistance in others. We have reported that an excessive reduction in cardiac output or permitting a rise in vascular resistance when treating blood pressure in pregnancy can be associated with a reduction in fetal growth.(2,3)
The purpose for this investigation was to first describe the pharmacodynamics of clonidine in pregnancy with particular attention to differences in individual hemodynamic responses. Second, we wanted to determine if differences in individual hemodynamic responses had an impact on fetal growth and birth weight. Finally, we wanted to determine if the pattern of hemodynamic response could be predicted by maternal characteristics or by baseline hemodynamic parameters that could be ascertained without noninvasive measurement of cardiac output.
Methods
A retrospective cohort study was performed in patients cared for at the University of Washington Obstetric Hypertension Clinic. All pregnant patients treated non-emergently with antihypertensive agents have an assessment of maternal hemodynamics before treatment and follow-up measurements after treatment. The study was approved by the University of Washington Human Subjects Review Committee.
Study Population
Charts were reviewed from 1997-2007 to identify subjects started on clonidine mono-therapy after 16 weeks gestational age. Pregnancy is associated with significant changes in maternal hemodynamics in the first and early second trimesters. Women were included when clonidine therapy was initiated after 16 weeks’ to avoid these confounding effects. In general, patients were started on clonidine if their vascular resistance was elevated. Subjects were excluded when post-treatment hemodynamic data were not available, for treatment with other antihypertensive medications or for an acute hypertensive illness such as preeclampsia or hypertensive crisis during the study interval. “Hypertensive crisis” was defined by rapidly increasing blood pressure in the face of antihypertensive therapy requiring admission to the hospital, treatment with magnesium sulfate, administration of IV fluids and aggressive adjustment of medications. These clinical interventions would confound interpretation of the hemodynamic effect of clonidine. Subjects were not excluded for the development of preeclampsia subsequent to the second measurement.
Hemodynamic measurements were made immediately before the initiation of treatment with clonidine. The post-treatment measurement was the next hemodynamic assessment after the initiation of therapy. Blood pressure (BP) was measured at rest in the left lateral recumbent position using an automated cuff (Accutorr; Datascope, Paramus, NJ). Stroke volume (SV) and cardiac output (CO) were measured using a previously validated Doppler technique (4,5) (UltraCOM Cardiac Output Monitor; Lawrence Medical, Redmond, WA). Mean arterial pressure (MAP) and total peripheral resistance (TPR) were calculated: MAP=(2dBP+sBP)/3; TPR=80•MAP/CO.
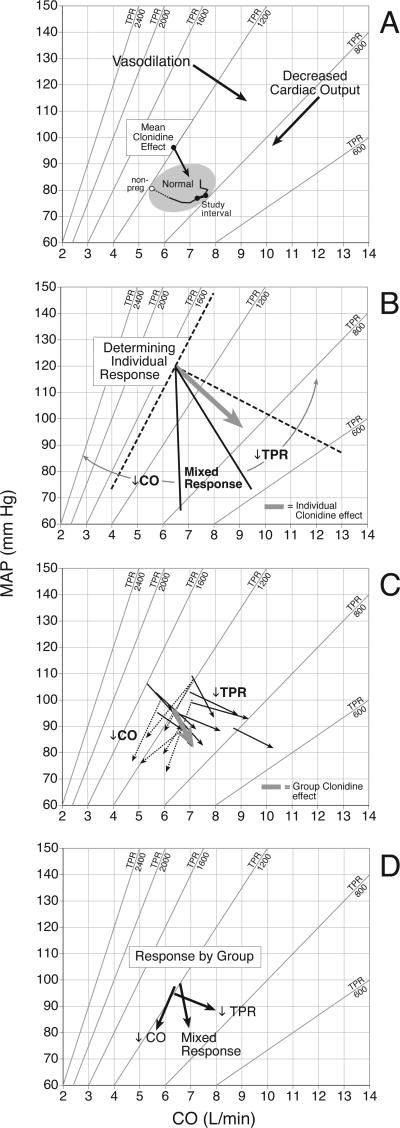
Hemodynamic responses of individual subjects and groups of subjects were plotted on a graph of MAP vs. CO where TPR is represented by isometric lines of vascular resistance, (Figure 1-A). Individual hemodynamic responses were categorized into one of three groups: (A)-↓TPR, (B)-↓CO, and (C)-Mixed Response as described in Figure 1-B.
Figure 1.
A) Describes the hemodynamic relationships between cardiac output (CO) on the X-axis, mean arterial pressure (MAP), on the Y-axis and total vascular resistance (TPR) as defined by Ohm's Law where MAP=CO•TPR/80. Diagonal lines represent the locus of points with the same vascular resistance. Hemodynamic actions are described by vectors of change. Vectors that run perpendicular to isometric lines of resistance represent describe vasodilation. Vectors that run parallel describe a reduction in cardiac output. The mean hemodynamic effect of clonidine is plotted. Hemodynamic changes in normal pregnancy are plotted in the normal region. The open data point identifies hemodynamic values obtained more than 6 weeks’ postpartum. Filled data points identify hemodynamic changes in normal pregnancy over the study interval.
B) The response groups were determined by plotting individual vectors of response for each subject, (eg BOLD VECTOR). An isometric line of resistance was drawn through the point determined by the individual's pretreatment hemodynamics, (ie the tail of the individual's vector). (DIAGNONAL DOTTED LINE). A second dotted line was drawn perpendicular to the isometric line of resistance. Within the right angle determined by the dotted lines, three sectors of clonidine action were then defined by 2 lines drawn at 30 degree intervals: ↓TPR –vasodilation; ↓CO -decreased cardiac output; Mixed Response. In this example, the individual has a ↓TPR response, (BOLD VECTOR).
C) The bold, grey vector describes the mean hemodynamic effect of clonidine for the entire cohort. Vectors from a selection of individual subjects are plotted demonstrating the heterogeneity of hemodynamic response. Solid vectors indicate a vasodilatory response. Dotted vectors indicate responses characterized by decreased cardiac output.
D) Describes the mean hemodynamic response of each category.
Timed urine collections for assessment of creatinine clearance and proteinuria were collected at home less than one week prior to the first hemodynamic measurement and initiation of clonidine therapy.
Birth weight percentiles were calculated using normative data from the State of Washington.(6) Paired T-test was used to compare pre- and post-treatment hemodynamic data. T-test, chi-squared and Fischer's exact tests were used to compare maternal characteristics and pregnancy outcomes between groups. Significant comparisons were adjusted with a Bonferroni correction. A multinomial logistic regression model was used to determine whether certain maternal characteristics were associated with response group to treatment with clonidine. The maternal characteristics age, creatinine clearance, clonidine dose and pre-existing diabetes were entered as independent variables (covariates) in the model. The hemodynamic response group (↓TPR, ↓CO or Mixed Response) was the dependent variable. The likelihood ratio of each maternal characteristic was calculated, and those that were significantly associated with the hemodynamic response group were tested for inclusion in the model.
Multinomial logistic regression was also used to determine whether there was an association between the hemodynamic response group and neonatal birth weight. The independent variables in this model included gestational age of delivery, BMI, chronic hypertension, severe preeclampsia, maternal age and race. These covariates were decided upon a priori. Neonatal birth weight was assessed in increments of 100 gm.
Results
Seventy-two women were identified who had been treated with clonidine monotherapy. One was excluded for acute preeclampsia. Five subjects did not have any appreciable change in blood pressure or other hemodynamic measurements such that a vector of change could not be generated. They were excluded from data analysis due to concerns for noncompliance. Their exclusion did not affect hemodynamic results (data not shown). Patients were treated with an oral dose of 0.05 mg three times daily (TID) or 0.1 mg TID. One patient was treated with a clonidine patch for a total of 0.4 mg per 24 hours, (8 weeks between measurements). The interval from initiation of clonidine to post-treatment hemodynamic measurement ranged from 1 day to 12 weeks with, on average, a 4-week interval between measurements.
Table 1 describes the demographics of the cohort. Treatment started in the late second trimester. Asian women represented 25% of the cohort that is more than would be expected from the ethnic distribution of our obstetrical population, (12%). Demographic variables did not predict response group. Women in the ↓CO Group were on a higher dose of clonidine, (> 0.15 mg/day).
Table 1. Patient Demographics expressed in means, medians, and percentages.
± standard deviation, n (%), [interquartile range]
| All | ↓TPR | ↓CO | P* | Mixed | P† | |
|---|---|---|---|---|---|---|
| N | 66 | 34 | 22 | 10 | ||
| Age (yrs) | 30.7 ± 6.0 | 30.3 ± 5.8 | 29.2 ± 6.0 | 0.52 | 34.8 ± 5.7 | 0.15 |
| Initial GA (wks) | 23.0 ± 5.2 | 22.5 ± 4.7 | 24.2 ± 5.8 | 0.72 | 22.3 ± 4.7 | 0.92 |
| Interval (wks) § | 4.7 [2.4 – 6.4] | 4.8 [2.7-6.0] | 4.5 [2.0-7.0] | 0.92 | 4.5 [2.0-6.3] | 0.74 |
| Race | 1.00 | 0.32 | ||||
| White | 32 (58) | 15 (56) | 9 (50) | 8 (80) | ||
| Asian | 14 (25) | 7 (26) | 5 (28) | 2 (20) | ||
| Other | 9 (16) | 5 (19) | 4 (22) | 0 | ||
| BMI (kg/m2) | 31.3 ± 7.2 | 32.9 ± 7.9 | 29.2 ± 6.3 | 0.21 | 30.4 ± 6.3 | 0.90 |
| Nulliparity | 34 (52) | 17 (50) | 12 (55) | 0.78 | 5 (50) | 0.49 |
| Diabetes | 6 (9) | 6 (18) | 0 | 0.07 | 0 | 0.31 |
| cHTN | 40 (61) | 22 (65) | 13 (59) | 0.78 | 5 (50) | 0.47 |
| > 0.15 mg/24hr | 14 (21) | 2 (6) | 9 (41) | <0.01 | 3 (30) | 0.21 |
| CrCl (mL/24hr) | 153 ± 44 | 167 ± 39 | 142 ± 47 | 0.14 | 124 ± 36 | <0.05 |
| Proteinuria (mg/24hr) § | 129 [56-198] | 142 [91-198] | 138 [76-163] | 0.64 | 56 [44-136] | 0.27 |
value comparing ↓TPR group to ↓CO group.
value comparing ↓TPR group to Mixed group.
median [interquartile range]
Interval - time interval between measurement of hemodynamics prior to treatment and second measurement
BMI - body mass index
cHTN - chronic hypertension
Cr Cl - creatinine clearance
Maternal age, creatinine clearance, clonidine dose and pre-existing diabetes were associated with response group and tested in the regression model. A multivariate analysis was performed using the ↓TPR group as the reference group. Covariates were added to the model in a stepwise fashion starting with the variable with the largest univariate effect. Covariates were kept in the model if the association changed by at least 10%. The final model included clonidine dose and maternal creatinine clearance and did not show a statistically significant association between these maternal characteristics and hemodynamic response (Table 2). Six women with diabetes were included in the study. All six women had a vasodilatory response to clonidine. The multivariate analysis was repeated excluding women diabetes. The results did not change (data not shown).
Table 2.
Multinomial regression model to identify maternal characteristics associated with type of hemodynamic response.
| Response | β coefficient | RRR | 95% CI | P |
|---|---|---|---|---|
| ↓CO Group | ||||
| Clonidine dose | 1.73 | 5.65 | 0.97-32.92 | 0.054 |
| CrCl | -0.21 | 0.81 | 0.59-1.11 | 0.18 |
| Mixed Group | ||||
| Clonidine dose | 1.42 | 4.12 | 0.52-32.88 | 0.18 |
| CrCl | -0.45 | 0.64 | 0.42-0.97 | 0.04 |
CrCl – creatinine clearance
RRR – relative risk ratio
Table 3 describes the hemodynamic response to clonidine. Normative data from 89 nulliparous women who developed neither gestational hypertension or preeclampsia are included for comparison.(7) With all data combined, a decrease in MAP was associated with a decrease in TPR and a modest rise in CO. The mean hemodynamic response is described in Figure 1-C by the bold vector. However, the mean vector does not accurately characterize the response of individual subjects. The heterogeneity of response is described by a selection of individual responses in Figure 1-C. The solid vectors represent a largely vasodilatory effect, (↓TPR), which was seen in approximately half of the study cohort. The dotted vectors represent an effect similar to ß-blockade, (↓CO), which was present in approximately a third of the cohort. Each group experienced a reduction in MAP, although the response in the ↓TPR Group was more modest. The ↓CO Group experienced a substantial reduction in HR with an associated reduction in CO and a very modest change in TPR. Mean group responses are described in Figure 1-D. Pre-treatment hemodynamic profile, (e.g. MAP, CO, HR, SV, TPR), did not predict response as demonstrated by the similar starting point of each response vector. Table 4 describes pregnancy outcomes. As might be expected, many women delivered prior to term, and the cohort experienced a high rate of preeclampsia. Mean birth weight percentile was lower in the ↓CO Group, (26.1), compared to the ↓TPR Group, (43.6), P=0.02.
Table 3.
Maternal hemodynamics before and with clonidine treatment
| MAP (mmHg) | CO (L/min) | HR (b/min) | SV (mL) | TPR (dyne•sec•cm-4) | |
|---|---|---|---|---|---|
| Total (66) | |||||
| Pre | 96.1 ± 7.1 | 6.4 ± 1.0 | 81.5 ± 12.3 | 79.3 ± 17.7 | 1231 ± 223 |
| Post | 86.9 ± 6.8 | 6.9 ± 1.4 | 78.1 ± 12.1 | 89.7 ± 16.0 | 1037 ± 191 |
| Δ | -9.2 | +0.5 | -3.4 | +10.4 | -194 |
| P | <0.001 | <0.001 | <0.05 | <0.001 | <0.001 |
| ↓TPR (34) | |||||
| Pre | 94.6 ± 6.8 | 6.4 ± 1.0 | 83.4 ± 12.2 | 76.2 ± 19.7 | 1215 ± 177 |
| Post | 89.7 ± 6.2 | 7.7 ± 1.4 | 84.6 ± 11.3 | 92.2 ± 16.3 | 951 ± 134 |
| Δ | -4.9 | +1.3 | +1.2 | +16 | -264 |
| P | <0.001 | <0.001 | 0.43 | <0.001 | <0.001 |
| ↓CO (22) | |||||
| Pre | 97.4 ± 6.8 | 6.4 ± 1.1 | 81.7 ± 12.2 | 76.5 ± 15.1 | 1261 ± 293 |
| Post | 83.8 ± 6.8 | 5.8 ± 0.9 | 72.0 ± 8.5 | 81.3 ± 13.7 | 1183 ± 198 |
| Δ | -13.6 | -0.6 | -9.7 | +4.8 | -78 |
| P | <0.001 | <0.001 | <0.001 | 0.34 | 0.09 |
| Mixed (10) | |||||
| Pre | 98.6 ± 7.7 | 6.6 ± 0.9 | 74.8 ± 11.7 | 89.2 ± 13.0 | 1223 ± 204 |
| Post | 84.7 ± 4.8 | 6.9 ± 0.8 | 69.5 ± 9.6 | 99.5 ± 11.9 | 1005 ± 153 |
| Δ | -13.9 | +0.3 | -5.3 | +10.3 | -218 |
| <0.001 | <0.05 | 0.12 | <0.05 | <0.001 | |
| Normative Data (89) | |||||
| 23 weeks | 76.5 ± 6.8 | 7.3 ± 1.2 | 78.0 ± 11.5 | 94.7 ± 2.0 | 865 ± 160 |
| 28 weeks | 78.0 ± 7.2 | 7.6 ± 1.6 | 80.5 ± 12.8 | 95.8 ± 1.9 | 855 ± 185 |
| Δ | +1.5 | +0.3 | +1.5 | +1.1 | -10.0 |
Table 4. Pregnancy Outcomes.
± standard deviation or n (%).
| Total | ↓TPR | P* | ↓CO | Mixed Response | |
|---|---|---|---|---|---|
| N | 65 | 34 | 22 | 10 | |
| Gestational Age (wks) | 36.4 ± 2.6 | 36.4 ± 2.8 | 0.88 | 36.5 ± 2.5 | 35.9 ± 1.9 |
| Birth weight (g) | 2767 ± 754 | 2938 ± 784 | 0.08 | 2553 ± 726 | 2638 ± 616 |
| Birth weight percentile | 36.1 ± 27 | 43.6 ± 28 | 0.02 | 26.1 ± 24 | 31.2 ± 21 |
| N | 58 | 31 | 18 | 9 | |
| Preeclampsia | 16 (28%) | 7 (23%) | 0.73 | 5 (28%) | 4 (44%) |
value comparing ↓TPR group to ↓CO group.
Missing data Neonatal Outcome – 1
Preeclampsia - 8
The hemodynamic response group was associated with birth weight controlling for gestational age of delivery, chronic hypertension, maternal age, race and BMI, (Table 5). The ↓CO Group was less likely to be associated with a higher birth weight compared to the ↓TPR Group (RRR 0.81; 95% CI 0.66-0.98) for an increase in birth weight by 100g. The Mixed Group exhibited a trend towards a lower birth weight baby relative to the ↓TPR Group (RRR 0.83; 95% CI 0.64-1.07).
Table 5.
Multinomial regression model to identify maternal characteristics associated with birth weight.
| Response | β coefficient | RRR | 95% CI | P |
|---|---|---|---|---|
| ↓CO Group | ||||
| Birth weight (100g increments) | -0.21 | 0.81 | 0.66-0.98 | 0.03 |
| Gestational age (delivery) | 0.53 | 1.71 | 1.00-2.92 | 0.05 |
| Maternal age | -0.05 | 0.95 | 0.81-1.11 | 0.51 |
| Maternal race | 0.27 | 1.31 | 0.70-2.42 | 0.40 |
| Body Mass Index | -0.07 | 0.93 | 0.83-1.04 | 0.22 |
| Chronic hypertension | 0.05 | 1.05 | 0.19-5.91 | 0.95 |
| Severe preeclampsia | 0.44 | 1.55 | 0.19-12.74 | 0.69 |
| Mixed Group | ||||
| Birth weight (100g increments) | -0.19 | 0.83 | 0.64-1.07 | 0.15 |
| Gestational age (delivery) | 0.28 | 1.32 | 0.70-2.51 | 0.39 |
| Maternal age | 0.39 | 1.48 | 0.97-2.26 | 0.07 |
| Maternal race | -1.87 | 0.15 | 0.02-1.00 | 0.15 |
| Body Mass Index | -0.20 | 0.82 | 0.60-1.12 | 0.21 |
| Chronic hypertension | -3.22 | 0.04 | 0.00-1.35 | 0.07 |
| Severe preeclampsia | 3.49 | 32.71 | 0.38-2805 | 0.13 |
RRR – relative risk ratio
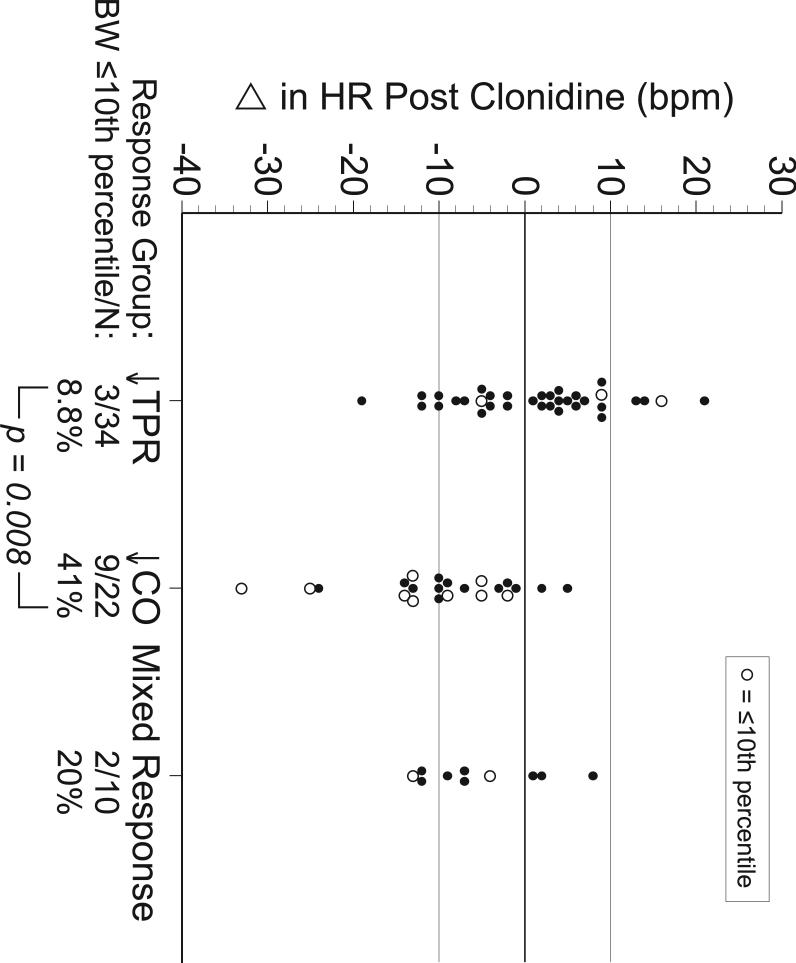
Figure 2 describes the change in heart rate, (ΔHR), for each subject by category of response. Subjects delivering babies at <10th percentile are identified by an open circle. In the ↓CO Group, 9/22 (41%) of birth weights were <10th percentile compared to 3/34 (8.8%) in the ↓TPR Group, (P = .008). Women in the ↓CO Group were more prone to have a reduction in heart rate. However, ΔHR alone does not discriminate between groups. While 20/22 (91%) of women in the ↓CO Group had a reduction in heart rate, only 20/41 (49%) of women with a reduction in HR were in the ↓CO Group.
Figure 2.
Change in heart rate, (ΔHR), with treatment is plotted for each subject by category of response. Open circles indicate cases where infants were born at a birth weight ≤10th percentile.
Discussion
Clonidine was found to have heterogeneous hemodynamic effects when used to treat hypertension in pregnancy. Fifty-two percent of patients experienced a primary reduction in vascular resistance. Twenty-two percent experienced a response characterized by a reduction in cardiac output similar to that seen with ß-blockers. Women whose response was characterized by a reduction in cardiac output delivered infants with a lower birth rate percentile and an increased incidence of birth weight < 10th percentile. While a reduction in heart rate associated with treatment identified 91% of women with a reduction in cardiac output, this finding was not specific.
Clonidine is a centrally acting adrenergic agonist with a mechanism of action similar to alpha-methyldopa. Horvath et al. performed a small, randomized trial that suggested a similar efficacy of blood pressure control between the two agents.(1) Although apparent oral clearance of clonidine is increased in pregnancy by 80%, (440±168 ml/min vs. 245±72 ml/min), renal clearance is not increased.(8) Only 36% of clonidine is excreted unchanged in the urine in pregnancy compared to 59% in nonpregnant subjects.(8) The mechanism of increased clonidine metabolism in pregnancy has yet to be determined.
Clonidine reduced the blood pressure of our pregnant subjects by approximately 9 mmHg. When examined in aggregate, the reduction in blood pressure seems to be due to a reduction in TPR associated with a modest compensatory increase in CO. The average hemodynamic effect did not accurately describe the effect in individual subjects. Thirty-four women (53%) had a clear vasodilatory response. Twenty-two (34%) had a response comparable to a ß-blocker, eg. atenolol.(9) The pharmacokinetic response is heterogeneous and more complex than other hemodynamic agents we have studied.(9-12)
We have previously reported that baseline hemodynamic conditions associated with hypertension in pregnancy are associated with different pregnancy outcomes.(13) Women with hypertension characterized by high vascular resistance. (≥ 1150 dyne•cm•sec-5) are at risk for babies delivered earlier, (31.7 vs. 35.7 weeks, P<0.001) and lower birth weight percentile, (18.7 vs. 38.3, P<0.003). Given the high baseline TPR in this cohort, the pregnancies were at risk for reduced fetal growth.
In a small, randomized trial, Butters el al. reported a reduction in birth weight in women assigned to atenolol, (up to 200 mg/day), compared to placebo.(14) In a meta-analysis of blood pressure trials in pregnancy, VonDadleszen et al. reported a modest reduction in birth weight (145 g/10 mmHg blood pressure reduction) associated with treatment of blood pressure in pregnancy independent of the agent or class of agents used.(15) In a randomized trial of early treatment of high cardiac output to prevent preeclampsia, we reported a modest reduction in birth weight without an increase in fetuses born at <10th percentile.(3) Notably, the smallest babies in our study were associated with a reduction in cardiac output to less than the mean for gestational age of normal pregnancies. In a report of pregnant women with high cardiac output and risk factors for preeclampsia treated with atenolol, we found that fetal growth <10th percentile was associated with prior pregnancies complicated by fetal growth restriction, cardiac output that fell below the mean for gestational age in normal pregnancy, and vascular resistance that rose above 1150 dyne•cm•sec-5.(13) In summary, baseline hemodynamic status and the hemodynamic response to antihypertensive therapy seem to impact fetal growth.
In this report, women were treated with clonidine experienced a heterogeneous response. Women in the ↓CO Group delivered babies with a lower birth weight percentile (26.1 vs. 43.6, P=0.02) compared to the ↓TPR Group. Forty-one percent of babies born to the ↓CO Group were at <10th percentile compared to 8.8% in the ↓TPR Group, (P = 0.008). The differential effect on fetal growth may be attributable to the differential hemodynamic effects of the drug. Alternatively, unknown factors intrinsically associated with the different responses could be responsible for the observed effect on growth.
Most maternal characteristics that we assessed in our cohort were not associated with the hemodynamic response group. A higher dose of clonidine occurred more commonly in the ↓CO Group. Since both situations would be associated with a higher maternal clonidine concentration, plasma clonidine concentration could be a determinant of the type of response group. We do not have serum clonidine levels in this cohort to evaluate this hypothesis.
Clonidine decreases renin secretion, probably through a reduction in central adrenergic output. Pregnancy is a condition of high renin activity. Renin activity in hypertensive pregnancies is more heterogeneous. Individual variation in renin activity during pregnancies complicated by hypertension may be in part responsible for the heterogeneous hemodynamic response to clonidine that we have observed. We do not have data on renin activity to test this hypothesis.
The heterogeneous response to treatment with clonidine seems to be associated with an impact on fetal growth. The ↓CO Group had smaller babies, consistent with observations we have in the context of treatment with atenolol when CO is reduced below the mean for gestational age of women with normal pregnancies. Similar fetal growth findings with clonidine that has a different mechanism of action than atenolol, suggest that the effect is due to the common effect on maternal hemodynamics. At this point these observations regarding mechanism are speculative. Our suggestion that fetal growth can be positively impacted by adjusting therapy after treatment with clonidine based on hemodynamic response group requires confirmation.
As might be expected, a reduction in heart rate characterized the ↓CO Group with 20/22 women (91%) having a ΔHR < 0. All babies in this group with birth weights <10th percentile were delivered to women with a reduction in heart rate. In the entire cohort, a ΔHR < 0 identified 12/14 babies born at <10th percentile. However, 41/64 women (64%) of the entire cohort had a ΔHR < 0. While a decrease in heart rate was sensitive in predicting response group and small babies, it was not very specific.
In a system such as ours where cardiac output can be and is routinely measured non-invasively, treatment can be adjusted based on an observed response. The dose of clonidine can be reduced if >0.15 mg/24 hours and a direct vasodilator such as hydralazine added to lower vascular resistance and increase cardiac output. When treating without the benefit of maternal hemodynamics, achieving optimal therapy is more difficult, but some direction can be offered from our findings. First, by limiting the dose of clonidine to 0.15 mg/day and adding a second agent such as hydralazine when an additional reduction in blood pressure is needed, the chance of a ↓CO effect will be reduced. Second, if a decrease in heart rate is observed with clonidine, addition of a vasodilator such as hydralazine should again be considered. While this strategy would again decrease the chance of a ↓CO response, 33% of women whose treatment was altered might not have needed an additional agent to avoid a ↓CO response. If blood pressure was not excessively reduced, this strategy would not be likely to have adverse effects.
Our study is limited by being a retrospective evaluation of hemodynamic data in women treated with clonidine. The interval between measurements was not standardized but was on average 4 weeks apart. Although the hemodynamic changes in pregnancy are modest over the studied interval, (Figure 1-A), some of the effect attributed to clonidine could have been due to changes due to pregnancy. On the other hand, the long interval between evaluations permitted equilibration of compensatory changes in hemodynamics associated with steady state or chronic therapy. We had no direct measure of compliance with therapy. Five subjects were excluded for suspected noncompliance. Alternatively, a small number of pregnant women may have a negligible response to clonidine. Our study may not be sufficiently powered to exclude demographic variables that might be associated with response.
We believe that clonidine is a valuable tool in the management of hypertension in pregnancy. Clonidine's unique mechanism of action may be important in controlling blood pressure in women with severe and resistant chronic hypertension. While individualized hemodynamic effect can be achieved in a straightforward manner using noninvasive measurements of cardiac output, these are not yet routinely available. However, by limiting the total dose of clonidine as a single agent and by monitoring heart rate response, a regimen may be achieved that works to optimize the potential for fetal growth without hemodynamic measurements.
Acknowledgments
The project described was supported by Award Number U10HD047892 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.)
References
- 1.Horvath JS, Phippard A, Korda A, Henderson-Smart DJ, Child A, Tiller DJ. Clonidine hydrochloride-a safe and effective antihypertensive agent in pregnancy. Obstet Gynecol. 1985;66:634–8. [PubMed] [Google Scholar]
- 2.Easterling TR, Carr DB, Brateng D, Diederichs C, Schmucker B. Treatment of Hypertension in Pregnancy: The Impact on Maternal Disease, Preterm Delivery, and Fetal Growth. Obstet Gynecol. 2001;98:427–33. doi: 10.1016/s0029-7844(01)01477-6. [DOI] [PubMed] [Google Scholar]
- 3.Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of Preeclampsia: A randomized trial of atenolol in hyperdynamic patients prior to the onset of hypertension. Obstet Gynecol. 1999;93:725–33. doi: 10.1016/s0029-7844(98)00522-5. [DOI] [PubMed] [Google Scholar]
- 4.Easterling TR, Watts DH, Schmucker BC, Benedetti TJ. Measurement of cardiac output during pregnancy: Validation of Doppler technique and clinical observations in preeclampsia. Obstet Gynecol. 1987;69:845–50. [PubMed] [Google Scholar]
- 5.Easterling TR, Carlson KL, Schmucker BC, Brateng DA, Benedetti TJ. Measurement of cardiac output in pregnancy by Doppler technique. Amer J Perinat. 1990;7:220–1. doi: 10.1055/s-2007-999486. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky S, Easterling TR, Holt VL, Critchlow CW. Detecting Small-for-Gestational Age: The Development of a Population-based Reference for Washington State. American Journal of Perinatology. 2005;22:405–12. doi: 10.1055/s-2005-872595. [DOI] [PubMed] [Google Scholar]
- 7.Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: A longitudinal study. Obstet Gynecol. 1990;76:1061–9. [PubMed] [Google Scholar]
- 8.Buchanan ML, Easterling TR, Carr DB, Shen DD, Risler LJ, Nelson WL, Mattison DR, Hebert MF. Clonidine Pharmacokinetics in Pregnancy. Drug Metab Dispos. 2008 Dec 30; doi: 10.1124/dmd.108.024984. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easterling TR, Benedetti TJ, Schmucker BC, Carlson KL. Antihypertensive therapy in pregnancy directed by noninvasive hemodynamic monitoring. Amer J Perinat. 1989;6:86–9. doi: 10.1055/s-2007-999553. [DOI] [PubMed] [Google Scholar]; Obstet Gynecol. 1987;69:845–50. [PubMed] [Google Scholar]
- 10.Easterling TR, Carr DB, Davis C, Diedrichs C, Brateng DA, Schmucker B. Low dose, short acting angiotensin converting enzyme inhibitors: Use in pregnancy. Obstet Gynecol. 2000;96:956–61. doi: 10.1016/s0029-7844(00)01037-1. [DOI] [PubMed] [Google Scholar]
- 11.Carr DB, Gavrila D, Brateng D, Easterling TR. Maternal hemodynamic changes associated with furosemide treatment. Hypertens Pregnancy. 2007;26:173–8. doi: 10.1080/10641950701204489. [DOI] [PubMed] [Google Scholar]
- 12.Easterling TR. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005 Jan;45(1):25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 13.Easterling TR, Benedetti TJ, Carlson KL, Brateng DA, Wilson, Schmucker BC. The effect of maternal hemodynamics on fetal growth in hypertensive pregnancies. Amer J Obstet Gynecol. 1991;165:902–6. doi: 10.1016/0002-9378(91)90436-u. [DOI] [PubMed] [Google Scholar]
- 14.Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. BMJ. 1990;301:587–9. doi: 10.1136/bmj.301.6752.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. 2000;355:87–92. doi: 10.1016/s0140-6736(98)08049-0. [DOI] [PubMed] [Google Scholar]