Abstract
Factors affecting larval growth and nutrition have consequences on adult fecundity. Since the mosquito larval midgut is the primary organ of digestion and nutrient absorption, factors that affect the growth and development of the midgut may have potential consequences on the reproductive potential of the adult. To gain a better understanding of mosquito midgut development the growth and metamorphic remodeling of the Aedes aegypti L. and Culex pipiens L. (Diptera: Culicidae) midguts were investigated. Cytological evidence was obtained suggesting that, in both the anterior and posterior Ae. aegypti larval midgut, diploid regenerative cells give rise to new endoreplicating cells that significantly contribute to the growth and metabolism of the midgut. This hypothesis was supported by BrdU incorporation studies showing that diploid cells, as well as large and small endoreplicating cells, synthesize DNA during the 2nd, 3rd and 4th instars. Cytological studies of the Cx. pipiens larval midgut suggest that anterior midgut growth in this species is primarily by cell enlargement. To study metamorphic remodeling of the midgut, DNA synthesis in Ae. aegypti 4th instar midguts was followed by using 5-bromo-2-deoxyuridine (BrdU) incorporation. During the 24 hr period after the last larval-larval molt both endoreplicating and diploid cells incorporate BrdU. After the critical weight is achieved, endoreplicating cell BrdU incorporation gradually ceases while diploid cells continue to replicate. The period of maximum diploid cell incorporation correlated with the period of maximum ecdysone titer.
Keywords: metamorphosis, Aedes aegypti, Culex pipiens, BrdU
Introduction
Factors controlling the growth and nutritional state of insect larvae affect the reproductive potential of the adult (Chambers and Klowden 1994; Soliman et al. 1995; Tu and Tatar 2003; Zhou et al. 2004). Small, poorly fed mosquito larvae produce adults of reduced reproductive potential (Briegel 1990; Renshaw et al. 1994; Briegel 2003; Noriega 2004; Telang and Wells 2004; Telang et al. 2006; Telang et al. 2007). Interfering with the normal development of the midgut might reduce the larva's ability to absorb, or store, nutrients and, as a consequence, reduce adult fecundity. Thus detailed knowledge of regulators of midgut development could identify targets that may be modulated to affect female fecundity and control mosquito populations.
The midgut is composed of an epithelial layer of large endoreplicating cells that have been identified as having polytene chromosomes (Trager 1937; Sutton 1942; Gillham 1957; Clements 1992). Interspersed among the endoreplicating cells are diploid regenerative cells (Trager 1937; Berger 1938; Richins 1945). The endoreplicating cells of the midgut perform functions such as ionic and osmotic regulation (Clements 1992), lipid and carbohydrate storage (Wigglesworth 1942; Clements 1992; Nishiura et al. 2007), control of the midgut lumen pH (Corena et al. 2002; Seron et al. 2004) and secretion of digestive enzymes and absorption of nutrients. In addition, the midgut has associated endocrine (Brown et al. 1986; Brown and Cao 2001; Moffett and Moffett 2005) and muscle cells (O'Brien 1965; Bernick et al. 2007). By measuring larval length, and the length of the anterior midgut epithelial cells, Trager (1937) concluded that Aedes aegypti L. (Diptera: Culicidae) midgut growth was, in large part, a result of cell enlargement and not cell proliferation. However little else is known concerning the development of the mosquito larval midgut and factors that control its growth.
Metamorphosis of the mosquito midgut is a remodeling process in which larval endoreplicating cells undergo programmed cell death while imaginai diploid cells replicate giving rise to the adult midgut epithelium. During the 4 (final) larval instar, midgut diploid cells increase in number, in both the anterior and posterior midgut regions. Most of the increase in diploid cell number occurs in the posterior midgut. Shortly before pupation, a decrease in larval size and weight is accompanied by a shortening of the midgut and endoreplicating larval cells start to detach from the basement membrane and slough off into the midgut lumen. The discarded endoreplicating cells eventually form the yellowish meconium of the pupal midgut (Richins 1938; Nishiura and Smouse 2000; Nishiura 2002).
The hormonal and transcriptional processes that control mosquito metamorphosis and midgut remodeling are beginning to be understood (Jenkins et al. 1992; Nishiura et al. 2003; Nishiura et al. 2005; Margam et al. 2006; Parthasarathy and Palli 2007). The critical weight for metamorphosis occurs by 24 hours after the last larval-larval molt, suggesting that by this time JH concentrations are low enough so that a subsequent increase in ecydysone concentration will initiate metamorphosis (Chambers and Klowden 1990; Nishiura et al. 2007; Telang et al. 2007). There is a biphasisc increase in ecdysone concentration after the critical weight is achieved that very likely initiates metamorphosis (Jenkins et al. 1992; Lan and Grier 2004; Margam et al. 2006; Telang et al. 2007).
Both diploid cell division and endoreplicating cell removal appear to be affected by JH concentrations since the application of juvenile hormone analogues to 4th instars affects both processes. High concentrations of JHA interfere with both diploid cell division and removal of endoreplicating cells. At lower concentrations, diploid cell division occurs but endoreplicating larval cells are not completely removed from the basement membrane (Nishiura et al. 2003; Nishiura et al. 2005; Wu et al. 2006; Parthasarathy and Palli 2007).
In this communication are reported cytological investigations into the growth and metamorphosis of the mosquito larval midgut of two species, Ae. aegypti and Culex pipiens. Addressed are two questions related to the process of cell division in the larval midgut. Does growth of the larval midgut occur only by cell enlargement? Secondly, when during midgut metamorphosis do diploid cells, that make-up most of the adult midgut, replicate? Results presented here suggest that during the period of larval growth newly formed endoreplicating cells arise in the Ae. aegypti anterior and posterior midgut. This process appears to be somewhat different in larval Cx. pipiens in that few newly formed endoreplicating cells are observed in the anterior midgut, but many are observed in the posterior midgut. In both species, newly formed endoreplicating cells appear to eventually make-up a substantial proportion of the midgut epithelial cells. To explain the origins of the newly formed endoreplicating cells, it is hypothesized that during larval growth, diploid cells divide and some differentiate into newly formed endoreplicating cells, and these cells substantially contribute to the growth of the larval midgut. If correct, growth of the mosquito larval midgut should be viewed as resulting from cell enlargement and cell division. This differs from a previous hypothesis suggesting that diploid cells give rise to endoreplicating cells only to replace those lost during the larval phase and midgut growth occurs by cell enlargement (Trager 1937). BrdU incorporation studies presented here indicate that approximately 48 hours after the last larval-larval molt, diploid cell division largely ceases in the Ae. aegypti anterior midgut but appears to accelerate in the posterior midgut. This cell division appears to be related to metamorphic midgut remodeling in that the dividing diploid cells persist, and are present in the pupal midgut, which eventually becomes the adult midgut. Overall, results presented here suggest that cell division may be important to both mosquito larval midgut growth and metamorphosis. An implication of this suggestion is that factors that affect cell division may have consequences on larval nutrition and adult fecundity.
Materials and Methods
Culture of mosquito larvae
Ae. aegypti eggs (Orlando strain) were obtained from Louisiana Biologicals and Cx. pipiens eggs were obtained from Carolina Biologicals. Larvae were grown at 24 °C and fed a mixture of TetraMin Cichlid food (8g), bakers yeast (4g) in 1.0 liter water. Under these conditions Ae. aegypti and Cx. pipiens 1st instars molted approximately 48 hours after egg hatching. Second instars were identified by the shed 1st instar cuticle and the clear head of a newly molted larva. The duration of the 2nd instar was approximately 24 hours. Likewise, 3rd instars were identified by the shed 2nd instar cuticle and clear head of a newly molted larva. The duration of the 3rd instar was approximately 24 hours. The collection of 4th instars, at various times after the last larval-larval molt, was carried out as described previously (Nishiura et al. 2005).
Larval midgut staining
Dissected midguts were fixed for 30 minutes at room temperature in 4% paraformaldehyde dissolved in phosphate buffered saline (PBS). They were then rinsed twice in distilled water and stained with a two-fold dilution of Mayer's hematoxylin solution (Sigma, www.sigmaaldrich.com). DAPI, 4′,6-diamidino-2-phenylindole, (Sigma), at a final concentration of 0.1 µg/ml, was added to the staining solution. After staining with hematoxylin and DAPI for 10 minutes the midguts were washed twice with water and mounted for microscopy.
The stained midguts were examined by bright field and fluorescence microscopy. Larval midguts had detectable autofluorescence when excited at 495nm and observed at 525nm using a Fluorescein isothiocyanate (FITC) filter set. The combination of hematoxylin stain and autofluorescence resulted in well delineated cell boundaries so cell and nuclear areas could be measured.
5-bromo-2-deoxyuridine (BrdU) labeling
First, 2nd and 3rd instars were exposed to 100 µg/ml BrdU (Sigma) for 24 hours at 24 °C. Midguts were then dissected and fixed overnight at 4 °C in 4% paraformaldehyde dissolved in PBS. The fixed midguts were serially equilibrated in solutions of increasing methanol concentrations (50%, 75% 100%), and then serially equilibrated in solutions of increasing ethanol concentrations (50% ethanol: 50% methanol; 75% ethanol: 25% methanol; 100% ethanol) and stored at -20 °C. Fourth instars, at various times after the last larval-larval molt, were exposed to 100 µg/ml BrdU for 8 to 12 hours and then midguts were dissected, fixed and stored as described above.
BrdU immunohistochemistry
Incorporated BrdU was detected by the DNase digestion method (Tkatchenki 2006). Midguts, fixed and stored as described above, were washed 5X in PBS containing 0.5% tween 20. They were then washed 3X in DNase buffer (50 mM Tris, pH 7.6; 10mM MgCl2). DNase (RNase-free, Ambion, www.ambion.com), at a final concentration of 0.1 unit/µl, was added and the midguts incubated at 37 °C for 2 hours. After incubation, the midguts were washed in TBS (50 mM Tris, pH 7.6; 100 mM NaCl) and heated at 70 °C for 10 minutes to inactivate the DNase. Midguts were then washed 5X in blocking buffer (TBS-5% BSA) composed of TBS plus 5% bovine serum albumin (BSA). They were then incubated with a 1:200 dilution of mouse anti-BrdU (Sigma) for 2 hrs at room temperature. After incubation, midguts were washed 5X with TBS-5% BSA and stored overnight at 4 °C in this buffer. After overnight storage midguts were brought to room temperature and a 1:200 dilution of HRP conjugated goat anti-mouse (Sigma) was added. After incubation with secondary antibody for 2 hr at room temperature, midguts were washed 5X with TBS-5% BSA and 3X with TBS. A 4-chloro-l-naphthol based color development reagent (BioRad, www.biorad.com), or ImmPACT DAB (Vector Laboritories, www.vectorlabs.com), was added and the peroxidase reaction proceeded until distinct labeling was detected. The midguts were then washed with PBS and mounted in 50% glycerol for microscopic examination.
Measurement of cell and nuclear area
To estimate the size of the anterior midgut, an area bounded by 10 adjacent cells, having the largest nuclei, was determined. An example is shown in Figure 1c. Multiple measurements per midgut were taken by measuring several 10 cell regions that overlapped by two cells. The number of small and intermediate sized nuclei, and the area encompassed by their cells, within the region bounded by the 10 large cells, was measured. These cells were designated the interstitial cells and had nuclei of various sizes, all smaller than the nuclei of the largest cells. To measure nuclear size, the areas of sharply focused, DAPI stained nuclei were determined. Cell area, as well as nuclear area, was estimated from digital images taken at the same magnification, using ImageJ software (http://rsb.info.nih.gov/ij/).
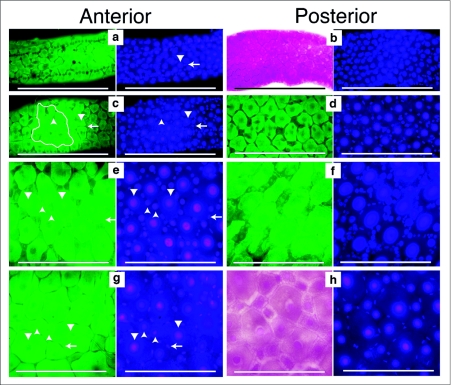
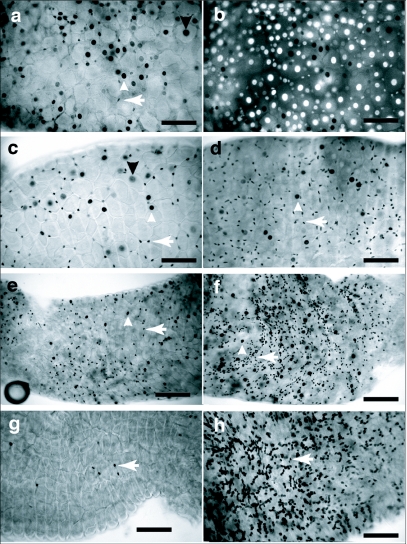
Figure 1.
Growth and development of the Aedes aegypti larval midgut. Midguts from 1st through 4th instars were dissected, fixed and stained with Hematoxylin and DAPI. First through 3rd instar midguts were dissected approximately 12 hours after the molt. Fourth instar midguts were dissected 24 hours after the molt. The left side image of each pair was taken using autofluorescence (green) or bright field (red), to emphasize the boundaries of the cells. The right side image is the same field of view showing DAPI stained nuclei. All images were taken at the same magnification and the scale bar represents 200 µm. Large nuclei are indicated by downward pointing arrow heads. Intermediate sized nuclei are indicated by upward pointing arrow heads. Small oval nuclei are indicated by left pointing arrows, (a) 1st instar anterior midgut had uniformly spaced large nuclei with interspersed small nuclei, (b) 1st instar posterior midgut had uniformly spaced large nuclei with interspersed small nuclei, (c) 2nd instar anterior midgut had uniformly spaced large nuclei and more interspersed smaller and intermediate sized nuclei, (d) 2nd instar posterior midgut had uniformly spaced large nuclei and numerous interspersed smaller and intermediate sized nuclei, (e) 3rd instar anterior midgut had uniformly spaced large nuclei that were surrounded by numerous intermediate and small sized nuclei, (f) 3rd instar posterior midgut had uniformly spaced large nuclei and many intermediate and small sized nuclei surrounding the large nuclei, (g) 4th instar anterior midgut had large and intermediate sized nuclei surrounded by small elongate nuclei, (h) 4th instar posterior midgut also had large and intermediate sized nuclei surrounded by elongate small nuclei.
Results
Cytology of Ae. aegypti larval midgut growth
Anterior and posterior regions of 1st instar midguts had uniformly distributed large nuclei of relatively equal size. There were also a few small nuclei, some of which were interspersed among the large nuclei and others arranged in long chain-like arrays (Figure 1a, b). Phalloidin staining indicated that the small nuclei arranged in long chain-like arrays may be those of muscle cells that form the contractile network along the entire midgut (data not shown). Midguts of 2nd instars also had uniformly distributed large nuclei of relatively equal size in both the anterior and posterior regions (Figure 1c, d). There appeared to be more small elongate and round nuclei interspersed among the largest nuclei. Also visible were small nuclei arranged in a linear chain-like array. Third instar anterior and posterior midguts had very pronounced and numerous intermediate sized nuclei that surrounded the uniformly spaced larger nuclei (Figure 1e, f). The elongate, and smallest round nuclei, were arranged like those in 2nd and 1st instars. Fourth instar midguts had a mixture of large cells with large nuclei, intermediate size cells with nuclei of many different sizes, and very small nuclei surrounding the largest nuclei (Figure 1g, h).
The cytological results suggested that larval midgut growth in Ae. aegypti was associated with an increase in number of intermediate sized cells as well as increased cell size. The smallest nuclei, that were interspersed between the largest nuclei, were often found in pairs and mitotic figures were observed (data not shown), suggesting that the smallest cells divided during the larval stages.
Cytology of Cx. pipiens larval midgut growth
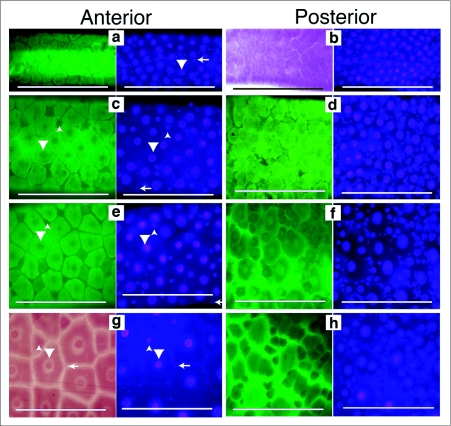
Our previous studies indicated that, unlike Ae. aegypti, the Cx. pipiens 4th instar anterior midgut had few small and intermediate sized endoreplicating cells (Nishiura and Smouse 2000), suggesting that the pathway of midgut growth may differ among mosquito species. To further investigate this, midgut growth during Cx. pipiens larval development was cytologically investigated. Cx. pipiens 1st instar anterior and posterior midguts had large nuclei of relatively uniform size and distribution. Smaller nuclei were either interspersed among the large nuclei or arranged in a chain-like array (Figure 2 a, b). Anterior and posterior midguts of 2nd instars also had evenly distributed large nuclei. However, posterior midguts of 2nd instars appeared to have many more of the smallest nuclei than did the anterior midgut (Figure 2 c, d). Third instar anterior midguts looked very different than posterior midguts. The anterior midgut had many large cells, of relatively uniform size with large nuclei, and interspersed among them were a few intermediate sized nuclei. However, posterior midguts of 3rd instars had many intermediate size nuclei, heterogeneous in size, surrounding the largest nuclei (Figure 2 e, f). The anterior midgut of Cx. pipiens 4th instars had mainly large cells with few widely separated cells of intermediate size. The posterior midgut of 4th instars was similar to that of the 3rd instar posterior midgut. Comparisons of larval midguts from Cx. pipiens and Ae. aegypti suggested that the posterior midguts of the two species grew in a very similar manner. Anterior midgut growth appeared very different in the two species.
Figure 2.
Growth and development of the Culex pipiens larval midgut. Midguts from 1st through 4th instars were dissected, fixed and stained with Hematoxylin and DAPI. First through 3rd instar midguts were dissected approximately 12 hours after the molt. Fourth instar midguts were dissected 24 hours after the molt. Each subfigure has a left side image, taken using autofluorescence (green) or bright field (red), to emphasize the boundaries of the cells. The right side image is the same field of view to show DAPI stained nuclei. Large nuclei are indicated by downward pointing arrow heads. Intermediate sized nuclei are indicated by upward pointing arrow heads. Small oval nuclei are indicated by left pointing arrows. All images are taken at the same magnification and the scale bar represents 200 µm. (a) 1st instar anterior midgut have evenly spaced large nuclei with a few small nuclei between them, (b) 1st instar posterior midgut has evenly spaced large nuclei with more small nuclei between them, (c) 2nd instar anterior midgut has evenly spaced large nuclei and a few intermediate sized nuclei between them, (d) 2nd instar posterior midgut has evenly spaced large nuclei with many small and intermediate sized nuclei between them, (e) 3rd instar anterior midgut has evenly spaced large nuclei with a few intermediate sized nuclei between them, (f) 3rd instar posterior midgut has evenly spaced large nuclei with many intermediate and small sized nuclei between them, (g) 4th instar anterior midgut has evenly spaced large nuclei, a few intermediate sized and small nuclei between them, (h) 4th instar posterior midgut has many small and intermediate sized nuclei surrounding the large nuclei.
Measurement of A. aegypti and Cx. pipiens midgut growth
To estimate midgut growth only anterior midgut cells were measured since 3rd instar posterior midgut cells were too numerous and closely packed to accurately determine their number. Areas of anterior midguts bounded by 10 clearly defined adjacent cells having the largest nuclei were measured (Figure 1c). It was assumed that the largest cells, with the largest nuclei, would be representative of the oldest cells of the midgut. Multiple measurements from a midgut were taken by measuring several large cell regions that overlapped by two cells. The contribution of small and intermediate sized cells to midgut growth was estimated by counting the number of intermediate and small nuclei within the areas delineated by 10 large cells. The areas encompassed by the cells having these small and intermediate sized nuclei were also measured. Nuclei were classified into four groups: (1) muscle cell nuclei that were identified by their arrangement in chain-like arrays; (2) small irregular or oval shaped nuclei, approximately equal in size to the muscle cell nuclei but interspersed between the larger nuclei; (3) intermediate sized nuclei, circular in shape but larger than the muscle cell nuclei and interspersed among the larger cells; and finally (4) the largest nuclei that were surrounded by the other nuclei.
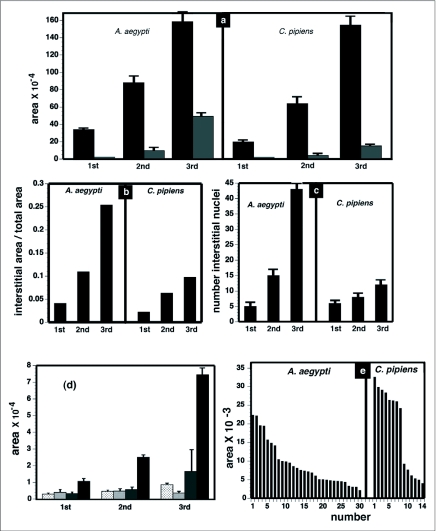
Measurements of larval anterior midgut growth were taken in 1st, 2nd and 3rd instars. During this time period, the areas bounded by 10 adjacent large cells increased approximately 5 fold in Ae. aegypti (Figure 3a) and approximately 4 fold in Cx. pipiens (Figure 3 a). The areas encompassed by the small and intermediate sized cells, within the boundaries of 10 large cells, increased approximately 33 fold in Ae. aegypti (Figure 3 a) and 10 fold in Cx. pipiens (Figure 3a). The number of small and intermediate sized nuclei, within the boundaries of 10 large cells, increased approximately 8 fold in Ae. aegypti (Figure 3c) and 2 fold in Cx. pipiens (Figure 3c). The results indicated that, in 3rd instars, the intermediate and small sized cells contributed approximately 25% of the Ae. aegypti anterior midgut area (Figure 3b) and approximately 10% of the Cx. pipiens anterior midgut area (Figure 3b).
Figure 3.
Measurements of Aedes aegypti and Culex pipiens midgut growth. Bright field and fluorescence images of midguts were used to measure growth in 1st through 3rd instar midguts that were dissected 12 hours after the molt. The areas of anterior midgut regions bounded by 10 adjacent cells with large nuclei were measured (black bars). An example is shown in Figure 1c. The areas of interstitial small and intermediate sized cells within the 10 cell regions were measured (grey bars). The number of interstitial small and intermediate sized nuclei within the 10 cell regions were counted. Areas are measured in pixels using ImageJ software, (a) Growth of anterior midgut from 1st to 3rd instars. Ten cell area (black bars); interstitial small and intermediate sized cell area (grey bars), (b) Fraction of anterior midgut growth due to interstitial small and intermediate sized cells, (c) Number of interstitial small and intermediate cell nuclei, (d) Sizes of nuclei in Ae. aegypti 1st through 3rd instars. Nuclei identified by their chain-like arrangement and circular shape (stippled bars). Small oval nuclei interspersed between larger nuclei (crosshatched bars). Intermediate sized nuclei, round in shape, and interspersed between largest nuclei (grey bars). Largest nuclei surrounded by small and intermediate sized nuclei (black bars), (e) Distribution of cell sizes in anterior midguts of Ae. aegypti and Cx. pipiens 4th instars. Squares with the dimensions of 0.3mm long by 0.3mm wide were marked in autofluorescence images of Ae. aegypti and Cx. pipiens 24 hour postmolt 4th instar anterior midguts and the sizes of the cells within these squares were measured.
In Ae. aegypti, nuclei arranged in long chains, and small oval shaped nuclei interspersed among the large nuclei, remained relatively constant in size from the 1st to 3rd instars (Figure 3d). The average size of the intermediate sized nuclei increased approximately 3 fold during this time period. However there was a large variation in sizes, ranging from 2 to 10 times the size of the smallest cell nuclei. The average size of the largest nuclei increased approximately 7 fold during this time period. In 3rd instars there was about a 2 fold variation in size of the largest nuclei (Figure 3d). The increase in number and area of intermediate sized cells during the interval between the 1st to 3rd instars suggested that they differentiated and grew during this time period.
Anterior midguts of Ae. aegypti 4th instars appeared to be a mosaic of cells of different sizes (Figure 1g) while anterior midguts of Cx. pipiens 4 instars appeared to be mainly composed of large cells with intermediate sized cells interspersed among them (Figure 2g). To get an estimate of the distribution of cell sizes in the anterior midguts, areas of equal size in the anterior midguts of both species were outlined and the size distribution of cells within these areas was determined (Figure 3e). There was approximately a 5 fold difference in cell sizes in Ae. aegypti anterior midgut and the distribution of sizes was relatively continuous. In C. pipiens anterior midguts there was approximately a 6 fold difference in cell sizes which appeared to be arranged into two groups: a large sized cell group and an intermediate sized group.
BrdU incorporation in Ae. aegypti 2nd and 3rd instars
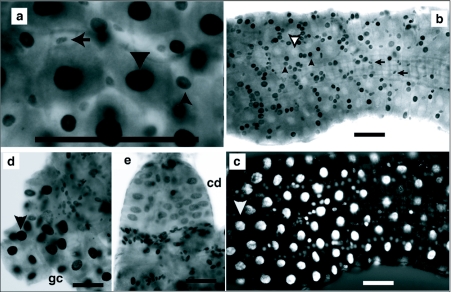
Cells with intermediate size nuclei may have differentiated from mitotically dividing regenerative diploid cells. To determine if small, intermediate and large cells replicated their DNA during the larval stages, BrdU incorporation experiments were performed. First, 2nd and 3rd instars were exposed to BrdU for 24 hours, during which time some of each instar molted. Labeling in this manner should have exposed premolt, intermolt, and newly molted larvae to BrdU and allowed the detection of DNA synthesis even if it only occurred at specific intervals during an instar. The incorporation data indicated that in 2nd instars the very smallest, as well as large and intermediate size cells incorporated BrdU (Figure 4a). In some 3rd instars, the largest nuclei were unlabeled, while the surrounding intermediate size nuclei and smallest nuclei were labeled (Figure 4b, c). In other midguts the largest nuclei were labeled but the surrounding intermediate size nuclei were mostly unlabeled (data not shown). These patterns suggest that DNA replication in the largest and intermediate size cells did not occur during the same time periods. Extensive DNA synthesis was also detected in the gastric ceacae and cardia (Figure 4d, e).
Figure 4.
Endoreplicating and diploid cells of 2nd and 3rd instars incorporate BrdU. Aedes aegypti 1st through 3rd instars were exposed to 100µg/ml BrdU for 24h. BrdU labeled large nuclei are indicated by solid downward pointing arrow head. Large nuclei not labeled with BrdU are indicated by open downward pointing arrowhead. Intermediate sized nuclei are indicated by upward pointing arrow head. Small oval nuclei are indicated by left pointing arrow. Scale bar represents 100 µm. (a) Anterior midgut of 2nd instar showing small oval, intermediate and large sized nuclei were BrdU labeled, (b) Posterior midgut of 3rd instar showing small oval and intermediate sized nuclei were labeled by BrdU. Large nuclei were not labeled, (c) Same field of view as presented in Figure 4b illuminated to reveal DAPI stained nuclei. Large nuclei are brightly fluorescent but unlabeled by BrdU. (d) Gastric caecum (gc) of 3rd instar showing labeled large nuclei and labeled diploid nuclei at the base of the gastric ceacum. (e) Cardia (cd) of 3rd instar showing labeled large nuclei and small nuclei at the base of the cardia.
DNA synthesis during Ae. aegypti midgut metamorphosis
Hormonal changes occur in 4th instars that signal the end of the larval phase and initiate metamorphic midgut remodeling, which includes division of diploid cells that will form the adult midgut epithelium. BrdU incorporation was used to determine when diploid cells replicated during the 4th instar. During the first 24 hours after the molt to 4th instars, both endoreplicating and diploid cells incorporated BrdU (Figure 5a, b, c, d). Among the endoreplicating cells, incorporation occurred mainly in those with intermediate sized nuclei. Between 24 and 36 hours after the molt the number of labeled diploid cells increased in the posterior and anterior midgut while a few endoreplicating cells incorporated BrdU (Figure 5e, f). Between 38 and 48 hours after the molt BrdU incorporation by posterior midgut diploid cells was wide spread while incorporation by anterior midgut diploid cells was sparse (Figure 5g, h). Incorporation by posterior midgut diploid cells continued until pupation, which occurred between 62 and 72 hours after the last larval-larval molt and past the time with larvae reach their maximum weight and size (data not shown).
Figure 5.
BrdU incorporation during midgut metamorphosis in Aedes aegypti 4th instars exposed to 100 µg/ml BrdU for the time intervals indicated below. Staining was performed as described in the Methods. BrdU labeled large nuclei are indicated by a downward pointing arrow head. Intermediate sized nuclei are indicated by an upward pointing arrow head. Small oval nuclei are indicated by left pointing arrow. All images were taken at the same magnification and scale bar represents 100 µm. (a) Anterior midgut of 4th instar exposed to BrdU from the time of molt to 14 hours after the molt, showing large, intermediate sized and small oval nuclei incorporated BrdU. (b) Same field as in Figure 4a but illuminated to detect DAPI staining. Most large nuclei were brightly fluorescent but not BrdU labeled, (c) Anterior midgut of 4th instar exposed to BrdU from 14 to 24 hours after the molt. Large, intermediate sized and small oval nuclei incorporated BrdU. (d) Posterior midgut of 4th instar exposed to BrdU from 14 to 24 hours after the molt. Incorporation by small oval nuclei greater than in Figure 5c. A few intermediate sized nuclei incorporated BrdU. (e) Anterior midgut of 4th instar exposed to BrdU from 24 to 36 hours after the molt. BrdU incorporation was predominately in small oval nuclei, (f) Posterior midgut of 4th instar exposed to BrdU from 24 to 36 hours after the molt. Labeled small oval nuclei were more numerous but a few intermediate sized cells were labeled, (g) Anterior midgut of 4th instar exposed to BrdU from 38 to 48 hours after the molt showed very little incorporation, (h) Posterior midgut of 4th instar exposed to BrdU from 38 to 48 hours after the molt showed many labeled diploid nuclei and no labeled large nuclei.
Discussion
Growth of the mosquito larval midgut
The mosquito early 4th instar midgut is a mixture of cells of different sizes with nuclei of different sizes (Trager 1937; Richins 1938; Richins 1945; Clements 1992). By cytological analysis, the nuclei of the larger endoreplicating cells are reported to have polytene chromosomes (Trager 1937; Sutton 1942; Gillham 1957; Clements 1992). The smallest cells are considered to have diploid nuclei because of their size as well as being associated with mitotic figures (Trager 1937). The midgut diploid cells are termed regenerative and differentiate into endoreplicating cells replacing those that were damaged and lost (Trager 1937; Richins 1938; Richins 1945). By measuring the cells of the anterior midgut in 1st through 4th instars, the increased size of the midgut was attributed mainly to increased cell size rather than increased cell number (Trager 1937). Our observations suggest that a substantial number of new endoreplicating cells appear during Ae. aegypti larval development and these postembryonic endoreplicating cells significantly contributed to the size of the midgut. Most of the growth of the Cx. pipiens larval anterior midgut occured by endoreplicating cell enlargement rather than by the formation of postembryonic polytene cells. However, growth of the Cx. pipiens posterior midgut was associated with both increased cell number and size.
Our conclusions were derived mainly from cytological observations and measurements of cells and nuclei in midgut whole mounts. A combination of hematoxylin staining and fluorescence microscopy provided a clear distinction between anterior midgut cells having the largest nuclei and cells having smaller endoreplicating or diploid nuclei that were found between the largest cells (Figure 1). Several complications had to be considered when trying to measure parameters of midgut growth during the larval phase. Whole mount microscopy of midguts provided images in which only limited regions in any one field of view were clearly focused. Measurements were restricted to the clearly focused areas and this precluded measurements from an entire anterior midgut. Although anterior and posterior midguts were unambiguously identifiable, transition areas between them were not. In 4th instars the largest cells had endoreplicating nuclei that were approximately the same size as those of some smaller cells (data not shown). Thus, in Ae. aegypti 4th instars, it was not possible to unambiguously differentiate the largest cells from some intermediate size cells by the criteria of nuclear size. The measurements were therefore limited to 1st, 2nd and 3rd instars. Measurements of 3rd instar posterior midgut cells and nuclei could not be made because they were too numerous and crowded to get accurate counts. However, in Ae. aegypti posterior midguts, changes in number, and area, of intermediate sized cells appeared to be similar to those that occurred in anterior midguts (Figure 1).
Ae. aegypti 1st instar anterior and posterior midgut endoreplicating cells were relatively uniform in size and distribution (Figure 1a, b). We assume that these cells uniformly grew and were the largest cells in 2nd and 3rd instars (Figure 1c–g). Thus an estimate of midgut growth was made by measuring the area bounded by 10 adjacent large cells. By this method it was estimated that Ae. aegypti midguts grew 5 fold between the 1st and 3rd instars (Figure 3a). Trager (1937) estimated that Ae. aegypti midguts and larvae increased in size from 1 relative unit in 1st instars to 5 relative units in 3rd instars.
During Ae. aegypti larval development there was an increase in the number of cells having small and intermediate sized nuclei that surrounded the largest cells (Figure 3c). As the area bounded by 10 adjacent large cells increased during the 1st to 3rd instars, the fraction of this area attributable to the small and intermediate sized cells increased from 3% in 1st instars to to about 25% in 3rd instars (Figure 3b). Since these cells did not appear to be present in 1st instar midguts, the results suggest that a substantial fraction of larval midgut growth is attributable to an increase in cell number during the larval phase.
There was both interspecific variation, as well as midgut region variations, in the formation of intermediate sized endoreplicating cells. Using the same methods of analysis as was used with Ae. aegypti, it was estimated that Cx. pipiens anterior midguts grew approximately 4 fold between the 1st to 3rd instars (Figure 3a). However, the number of small and intermediate sized cells increased 2 fold during this period and the fraction of midgut area attributable to these cells was only 9% in 3rd instars (Figure 3b, c). This suggests that anterior midgut growth in Cx. pipiens was primarily from cell enlargement. In both species the increase in intermediate sized endoreplicating cells in posterior midguts appeared to be far greater than the increase in the anterior midgut. The 4th instar anterior midguts of the two species appeared very different but the posterior midguts were very similar (Figures 1h, 2h).
To explain the increased number of smaller endoreplicating cells that appeared during the larval stages we hypothesize that midgut regenerative diploid cells divide, and gave rise to mitotic diploid cells, as well as endoreplicating cells. Alternatively, 1st instars may have had a population of diploid cells that were committed to the endoreplication cycle but only did so during the latter instars. It is unlikely that such cells resided in the 1st instar midgut since not enough cells were observed there to account for all of the smaller endoreplicating cells in 3rd or 4th instar midguts. However, it is possible that new endoreplicating cells migrated into the midgut during larval development.
This hypothesis requires that midgut diploid cells regularly replicated during the early larval stages. BrdU incorporation studies showed that diploid and endoreplicating cells underwent DNA replication in 2nd, 3rd and 4th instars. The BrdU labeling pattern also suggested that all cells did not undergo DNA replication in the same time period (Figure 4). Although our results do suggest that diploid cells replicate during early instars they do not directly show that daughter diploid cells differentiate into endoreplicating cells.
Growth of the larval midgut in other insects
The differentiation of diploid regenerative cells into midgut epithelial cells during larval development has been described in other insects. Before each molt of the apterous Lepisma saccharina (Zygentoma), clusters of regenerative cells give rise to columnar epithelial cells that are subsequently destroyed at the molt (Rost-Roszkowska et al. 2007). In 5th instar midguts of the hemimetabolous Locusta migratoria there are niches of stem cells that differentiate into columnar epithelial cells and endocrine cells (Illa-Bochaca and Montuenga 2006). In the holometabolous Manduca sexta larval midgut stem cells divide at each molt and differentiate into epithelial cells, so that by the end of the larval phase, the dividing stem cells have given rise to a 200 fold increase in the number of larval epithelial cells (Baldwin and Hakim 1991). The arrangement of midgut imaginai histoblasts and polyploid cells of the brachycerus dipteran Drosophila melanogaster is very different than that of the more basal nematocerus dipteran Ae. aegypti. In D. melanogaster, midgut diploid cells begin endoreplication during embryogensis. In the 1st, 2nd and much of the 3rd instars, the midgut epithelium is composed of endoreplicating cells that are of uniform size and evenly spaced. The imaginai histoblasts are arranged in clusters, and do not replicate until just prior to pupariation. In addition, the number of endoreplicating polyploid cells does not increase during the larva phase. Metamorphic remodeling of the midgut occurs from 18 hours before pupariation to 12 hours after pupariation and involves division of midgut imaginai histoblast and autophagy of the endoreplicating cells (Madhavan and Schneiderman 1977; Zaffran et al. 1998).
DNA synthesis during mosquito metamorphosis
Mosquito metamorphosis starts in 4th instar and is completed in the pupa (Richins 1938; Richins 1945). A substantial amount of larval growth also occurs during this period (Trager 1937; Nishiura et al. 2007) so in mosquitoes there is an overlap between metamorphic changes and larval growth. Midgut metamorphosis is a remodeling process in which diploid cells divide giving rise to the adult epithelium, and the larval endoreplicating cells undergo programmed cell death. To gain a better understanding of the timing of these events and the factors that control midgut growth and remodeling, BrdU labeling was used to measure DNA replication in 4th instar.
When Ae. aegypti 4th instars were continuously fed, both endoreplicating and diploid cells synthesized DNA during the first 24 hours after the molts (Figure 5a, b, c, d). BrdU incorporation was widespread in the smaller endoreplicating cells but almost absent in the largest endoreplicating cells. Between 24 and 48 hours after the last larval-larval molt, BrdU incorporation by endoreplicating cells largely ceased while incorporation by diploid cells continued (Figure 5e, f, g, h). Posterior midgut diploid cell DNA synthesis continued in prepupae (56 hours post-molt) and the BrdU-labeled larval diploid cells were found in posterior pupal midguts (data not shown). Ae. aegypti larvae attain critical weight by 24 hours after the last larval-larval molt (Chambers and Klowden 1990; Nishiura et al. 2007; Telang et al. 2007). The attainment of the critical weight has been, in other insects, correlated with a drop in juvenile hormone concentration (Nijhout et al. 2006). The coincidence between the time when endoreplicating cell DNA synthesis ceased and attainment of critical weight suggests that endoreplicating cell DNA synthesis may be dependent upon the continued presence of juvenile hormone, although juvenile hormone concentrations during the mosquito larval period have not yet been determined. Diploid cell division accelerated in the posterior midgut approximately 48 hours after the molt. This time period corresponds to the time when the major ecdysone pulse occurs (Jenkins et al. 1992; Lan and Grier 2004; Telang et al. 2007).
Growth and metamorphosis of the mosquito larval midgut
The results presented here lead to the following working hypothesis concerning Ae. aegypti midgut growth, development and metamorphosis. During embryonic development midgut epithelial cells begin to endocycle (Clements 1992). However, some diploid stem cells may also populate the midgut. During larval stages, we hypothesize that the diploid cells divide and some of them enter an endocycling pattern giving rise to new, postembryonic endoreplicating epithelial cells of sizes intermediate to those of the smallest diploid cells and largest endoreplicating cells. These intermediate sized cells may substantially contribute to the size and function of the larval midgut. In 4th instars, we hypothesize that both the synthesis of DNA by endoreplicating cells, and the differentiation of diploid cells to endoreplicating cells, cease. Although diploid cell DNA synthesis appears to occur in 4th instars of all ages, we hypothesize that the increase in ecdysone concentration that induces metamorphosis stimulates diploid cell division.
Acknowledgments
This work was supported by NIH grant S06 GM060654 and the NSF Louis Stokes Alliances for Minority Participation
Abbreviations
- BrdU:
5-bromo-2-deoxyuridine
References
- Baldwin KM, Hakim RS. Growth and differentiation of the larval midgut epithelium during molting in the moth, Manduca sexta. . Tissue and Cell. 1991;23:411–422. doi: 10.1016/0040-8166(91)90058-2. [DOI] [PubMed] [Google Scholar]
- Berger CA. Cytology of metamorphosis in the Culininae. Nature. 1938;141:834–835. [Google Scholar]
- Bernick EP, Moffett SB, Moffett DF. Organization, ultrastructure, and development of midgut visceral muscle in larval Aedes aegypti. . Tissue and Cell. 2007;39:277–292. doi: 10.1016/j.tice.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. Journal of Medical Entomology. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- Briegel H. Physiological basis of mosquito ecology. Journal of Vector Ecology. 2003;28:1–11. [PubMed] [Google Scholar]
- Brown MR, Cao C. Distribution of ovary edysteroidogenic hormone I in the nervous system and gut of mosquitoes. Journal of Insect Science 1: 1. 2001. Available online at: http://insectscience.org./1.1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Crim JW, Lea AO. FMRFamide- and pancreatic polypeptide-like immunoreactivity of endocrine cells in the midgut of a mosquito. Tissue and Cell. 1986;18:419–428. doi: 10.1016/0040-8166(86)90061-3. [DOI] [PubMed] [Google Scholar]
- Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell and Tissue Research. 2001;304:317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Klowden MJ. Correlation of nutritional reserves with a critical weight for pupation in larval Aedes aegypti mosquitoes. Journal of the American Mosquito Control Association. 1990;6:394–399. [PubMed] [Google Scholar]
- Chambers GM, Klowden MJ. Nutritional reserves of autogenous and anautogenous selected strains of Aedes albopictus (Diptera: Culicidae). Journal Medical Entomology. 1994;31:554–560. doi: 10.1093/jmedent/31.4.554. [DOI] [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L). The Yellow Fever Mosquito. Cambridge University Press; 1960. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman & Hall; 1992. [Google Scholar]
- Corena MP, Seron TJ, Lehman HK, Ochrietor JD, Kohn A, Tu C, Linse PJ. Carbonic anhydrase in the midgut of larval Aedes aegypti: cloning, localization and inhibition. Journal of Experimental Biology. 2002;205:591–602. doi: 10.1242/jeb.205.5.591. [DOI] [PubMed] [Google Scholar]
- Gillham NW. Genetic studies in Aedes. I. The distribution of polytene chromosomes in Aedes aegypti. . The American Naturalist. 1957;91:265–268. [Google Scholar]
- Illa-Bochaca I, Montuenga LM. The regenerative nidi of the locust midgut as a model to study epithelial cell differentiation from stem cells. Journal of Experimental Biology. 2006;209:2215–2223. doi: 10.1242/jeb.02249. [DOI] [PubMed] [Google Scholar]
- Jenkins SP, Brown MR, Lea AO. Inactive prothoracic glands in larvae and pupae of Aedes aegypti: ecdysteriod release by tissues in the thorax and abdomen. Insect Biochemistry and Molecular Biology. 1992;22:555–559. [Google Scholar]
- Lan Q, Grier CA. Critical period for pupal commitment in the yellow fever mosquito, Aedes aegypti. . Journal of Insect Physiology. 2004;50:667–676. doi: 10.1016/j.jinsphys.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Madhavan MM, Schneiderman HA. Histological Analysis of the Dynamics of Growth of Imaginai Discs and Histoblast Nests During the Larval Development of Drosophila melanogaster. . Wilhelm Roux's Archives. 1977;183:269–305. doi: 10.1007/BF00848459. [DOI] [PubMed] [Google Scholar]
- Margam VM, Gelman DB, Palli SR. Ecdysteroid titers and developmental expression of ecdysteroid-regulated genes during metamorphosis of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). Journal of Insect Physiology. 2006;52:558–568. doi: 10.1016/j.jinsphys.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Moffett SB, Moffett DF. Comparison of immunoreactivity to serotonin, FMRMamide and SCPb in the gut and visceral nervous system of larvae, pupae and adults of the yellow fever mosquito Aedes aegypti. . Journal of Insect Science. 2005;5(20):1–12. doi: 10.1093/jis/5.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. . Journal of Biology. 2006;5:16–23. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura JT. Coordinated Morphological changes in midgut, imaginai discs and respiratory trumpets during metamorphosis of Aedes aegypti (Diptera: Culicidae). Annals of the Entomological Society of America. 2002;95:498–504. [Google Scholar]
- Nishiura JT, Burgos C, Aya S, Goryacheva Y, Lo W. Modulation of larval nutrition affects midgut neutral lipid storage and temporal pattern of transcription factor expression during mosquito metamorphosis. Journal of Insect Physiology. 2007;53:47–58. doi: 10.1016/j.jinsphys.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Ho P, Ray K. Methoprene interferes with mosquito midgut remodeling during metamorphosis. Journal of Medical Entomology. 2003;40:498–507. doi: 10.1603/0022-2585-40.4.498. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Ray K, Murray J. Expression of nuclear receptor-transcription factor genes during Aedes aegypti midgut metamorphosis and the effect of methoprene on expression. Insect Biochemistry and Molecular Biology. 2005;35:561–573. doi: 10.1016/j.ibmb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Smouse D. Nuclear and cytoplsmic changes in the Culex pipiens (Diptera:Culicidae) alimentary canal during metamorphosis and their relationship to programmed cell death. Annals of the Entomological Society of America. 2000;93:282–290. [Google Scholar]
- Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochemistry and Molecular Biology. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- O'Brien JF. Development of the muscular network of the midgut in the larval stages of the mosquito, Aedes aegypti Linnaeus. Annals of the New York Entomological Society. 1965;73:226–231. [Google Scholar]
- Parthasarathy R, Palli SR. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti. . Journal of Insect Physiology. 2007;53:216–229. doi: 10.1016/j.jinsphys.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Service MW, Birley MH. Size variation and reproductive success in the mosquito Aedes cantons. . Medical and Veterinary Entomology. 1994;8:179–186. doi: 10.1111/j.1365-2915.1994.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Richins CA. The metamorpphosis of the digestive tract of Aedes dorsalis Miegen. Annals of the Entomological Society of America. 1938;31:78–84. [Google Scholar]
- Richins CA. The development of the midgut in the larva of Aedes dorsalis Miegen. Annals of the Entomological Society of America. 1945;31:74–87. [Google Scholar]
- Rost-Roszkowska MM, Pilka M, Szymska R, Klag J. Ultrastructural studies of midgut epithelium formation in Lepisma saccharina L. (Insecta, Zygentoma). Journal of Morphology. 2007;268:224–231. doi: 10.1002/jmor.10513. [DOI] [PubMed] [Google Scholar]
- Seron TJ, Hill J, Linser PJ. A GPI-linked carbonic anhydrase expressed in the larval mosquito midgut. Journal of Experimental Biology. 2004;207:4559–4572. doi: 10.1242/jeb.01287. [DOI] [PubMed] [Google Scholar]
- Soliman MA, Seif AI, Hassan AN, Abdel-Hamid ME, Mansour MA, Gad AM. Nutritional reserves in autogenous and anautogenous populations of Culex pipiens and Aedes caspius (Diptera: Culicidae). . Journal of Egyptian Society Parasitology. 1995;25:499–507. [PubMed] [Google Scholar]
- Sutton E. Salivary gland type chromosomes in mosquitoes. Proceeding of the National Academy of Sciences. 1942;28:268–272. doi: 10.1073/pnas.28.7.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang A, Frame L, Brown MR. Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). . Journal of Experimental Biology. 2007;210:854–864. doi: 10.1242/jeb.02715. [DOI] [PubMed] [Google Scholar]
- Telang A, Li Y, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. Journal of Experimental Biology. 2006;209:645–655. doi: 10.1242/jeb.02026. [DOI] [PubMed] [Google Scholar]
- Telang A, Wells MA. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. Journal of Insect Physiology. 2004;50:677–685. doi: 10.1016/j.jinsphys.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tkatchenki AV. Whole-mount BrdU staining of proliferating cells by DNase treatment: application of postnatal mammalian retina. BioTechniques. 2006;40:29–32. doi: 10.2144/000112094. [DOI] [PubMed] [Google Scholar]
- Trager W. Cell size in relation to the growth and metamorphosis of the mosquito, Aedes aegypti. . The Journal of Experimental Zoology. 1937;76:467–489. [Google Scholar]
- Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. . Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. The storage of protein, fat, glycogen and uric acid in the fat body and other tissues of mosquito larvae. Journal of Experimental Biology. 1942;19:56–77. [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mechanism of Development. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Chartier A, Gallant P, Astier M, Arquier N, Doherty D, Gratecos D, Semeriva M. A Drosophila RNA helicase gene, pitchounce, is required for cell growth and proliferation is a potential target of d-Myc. Development. 1998;125:3571–3584. doi: 10.1242/dev.125.18.3571. [DOI] [PubMed] [Google Scholar]
- Zhou G, Pennington JE, Wells MA. Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochemistry and Molecular Biology. 2004;34:919–925. doi: 10.1016/j.ibmb.2004.05.009. [DOI] [PubMed] [Google Scholar]