Abstract
In this study we investigated the histological, biochemical, and integrative features of the neocartilage using swine auricular chondrocytes photoencapsulated into two poly(ethylene glycol) dimethacrylate (PEGDM) copolymer hydrogels of a different degradation profile: degradable (PEG-4,5LA-DM) and nondegradable (PEGDM) macromers in molar ratios of 60:40 and 70:30. Integration of the engineered tissue with existing native cartilage was examined using an articular cartilaginous ring model. Experimental group samples (total n = 96) were implanted subcutaneously into nude mice and harvested at 6, 12, and 18 weeks. Nonimplanted constructs (total n = 16) were used as controls for quantification of DNA, glycosaminoglycan, and hydroxyproline. Histologically, neocartilage resembled both the cellular population and composition of the extracellular matrix of the native swine auricular cartilage. DNA content demonstrated that the photoencapsulated chondrocytes were capable of survival and proliferation over time. Both glycosaminoglycan and hydroxyproline contents appeared higher in the neotissue, which was supported by less degradable PEGDM hydrogel. Integration of neocartilage with surrounding native cartilage improved with time, resulting in the development of tight integration interface. PEGDM copolymer hydrogels can support in vivo chondrogenesis by photoencapsulating auricular chondrocytes.
Introduction
The reconstruction of the external auricle continues to be a challenge in the field of plastic and reconstructive surgery.1 Two primary reconstructive approaches currently used are porous polyethylene prosthesis implantation and sculpted autologous cartilage grafts.2 Alloplastic implants provide a limitless off-the-shelf supply in predetermined shapes, and require shorter operative times.3,4 However, major concerns include increased susceptibility to infection, extrusion due to skin ischaemia, and uncertain long-term durability. The use of autologous costal cartilage graft is the most commonly used and preferred method for total external ear reconstruction, particularly in cases of congenital microtia. Good long-term durability and cosmetic results have been previously demonstrated.5,6 However, the sculpted shape and aesthetic result of the graft is less consistent, a longer operation time is required, and donor-site morbidity will most likely be a postoperative outcome. Additionally, both methods leave the patient with stiff appendages and less than optimal aesthetic results. Engineering tissue for reconstructing the external auricle could provide durable and flexible auricular cartilage with better aesthetic outcomes if suitable polymer scaffolds can be synthesized.
Previously, Cao et al. demonstrated engineering of auricular cartilage in vivo using a polyglycolic acid–polylactic acid copolymer fashioned in the shape of human ear and seeded with cultured bovine articular chondrocytes.7 Saim et al. reported the formation of mature elastic cartilage by isolated swine auricular chondrocytes, encapsulated into Pluronic F-127: a copolymer gel of poly(ethylene glycol) and poly (propylene oxide).8 However, a major disadvantage of this hydrogel is the maintenance of predetermined shape. Attempting to address this challenge, Arevalo-Silva et al. used various nonbiodegradable endoskeletal scaffolds to offer support to engineered cartilage.9 Isogai et al. reported that poly(L-lactic acid-ɛ-caprolactone) copolymer seeded with articular chondrocytes and implanted subcutaneously into athymic mice supported development and maintenance of cartilage in a human ear shape for up to 40 weeks.10 More recently, Kusuhara et al. tissue engineered a human ear model using bovine chondrocytes from different sources (articular, auricular, costal, and septal) on a poly(L-lactic acid) and poly(L-lactide-e-caprolactone) polymer.11 Not surprisingly, elastin was only found in the construct that used auricular chondrocytes as a cell source, whereas costal chondrocytes created calcifications and bony nodules throughout the construct.
Poly(ethylene glycol) (PEG) hydrogels have been used in numerous biomedical applications and have many favorable attributes for tissue engineering. PEG gels have been investigated for use as coatings on tissue surfaces, preventing thrombosis, or preventing postoperative adhesion formation.12,13 These hydrogels have also been employed as drug delivery depots or for coating biosensors.13,14 In the field of tissue engineering, PEG can be readily modified with photocrosslinkable endgroups like polyrotaxanes or methacrylates to form three-dimensional networks such as PEG dimethacrylate (PEGDM) hydrogels.15,16 Photocrosslinking of these macromers has been shown to be biocompatible with the unreacted dimethacrylates demonstrating only minimal cytotoxicity.17 Photopolymerization has several advantages in comparison with the conventional polymerization techniques, including the ability of in situ scaffold formation, spatial and temporal control over the progress of crosslinking, and more biocompatible conditions of polymerization.18
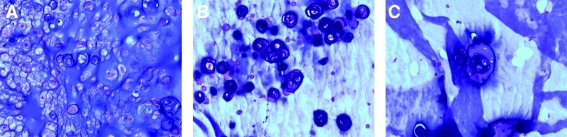
Several preliminary studies were performed in our laboratory investigating a variety of candidate experimental protocols to develop a novel PEGDM hydrogel that supported chondrocyte survival, growth, and matrix production. The basis of this study derived from our earlier work pertaining to the slow degradation kinetics of copolymer gels and the subsequent extracellular matrix (ECM)-producing potential of chondrocytes in the tissue-engineered cartilage.19 For this study, copolymers composed of degradable poly(ethylene glycol)-4,5 lactic acid dimethacrylate (PEG-LA-DM) and nondegradable PEGDM macromers in molar ratios that ranged from 90:10 to 50:50 were studied. Construct volume and histology (Fig. 1) of the engineered cartilage was investigated after 6 weeks in vivo, and concluded that the ratios of 60:40 and 70:30 degradable/nondegradable PEGDM macromers proved more optimal in maintaining the initial construct volume and supporting the formation of neotissue with improved histological features.
FIG. 1.
Hematoxylin and eosin-stained sections of the preliminary results demonstrated noncontiguous cartilage formation using 90:10 (A), 80:20 (B), and 50:50 (C) ratios. Original magnification, ×200 for all images. Color images available online at www.liebertonline.com/ten.
The aim of our study was to evaluate whether the combination of degradable and nondegradable PEGDM hydrogels can support the formation of engineered auricular cartilage by photoencapsulated swine auricular chondrocytes with improved histological and biochemical features. Also, we investigated if the neocartilage can integrate with existing native cartilage.
Materials and Methods
Overview
All procedures were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital. Chondrocytes were isolated from the ear cartilage of Yorkshire swine and encapsulated in photoreactive PEG gels. The gel-encapsulated cells were implanted into subcutaneous pockets on the backs of nude mice for 6, 12, and 18 weeks and evaluated for cartilage matrix formation.
Chondrocyte isolation
Both ears were harvested from 3-month-old Yorkshire swine and the skin and subcutaneous tissues removed. The perichondrium was separated from the auricular cartilage in a single piece using periosteal elevators. The auricular cartilage was minced into 1 mm3 chips and digested using 0.1% collagenase type 2 (Worthington Biochemical, Freehold, NJ) solution at 37°C for 14 h. Undigested tissue was removed by filtration of the cell suspension using a 100 μm sterile cell strainer (Becton Dickinson, Franklin Lakes, NJ). Cells were washed, centrifuged, and resuspended in phosphate-buffered saline. Cell number and viability were determined using a hemacytometer and trypan blue dye method.
Polymer preparation
The synthesis and in vitro data have been previously described.19 Briefly, PEG-LA-DM, a degradable PEG macromer, and PEGDM, a nondegradable form of PEG, were used in the polymer preparation. These macromers were dissolved in sterile phosphate-buffered saline to a final concentration of 10% (w/w) and mixed at molar ratios of 60:40 and 70:30 (PEG-LA-DM:PEGDM). The ultraviolet photoinitiator, 2-hydroxy-1[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (I2959), shown to be cytocompatible, was added to the co-macromer solution at a final concentration of 0.05% (w/w).
Chondrocyte photoencapsulation
The isolated chondrocytes were combined with the macromer/photoinitiator solution at a concentration of 60 × 106 cells/mL. Aliquots of 100 μL of nonpolymerized gel containing the cells were placed in cylindrical molds measuring 4.5 mm diameter × 6.5 mm in height. The gels were polymerized by irradiation of the macromer/photoinitiator/chondrocyte solutions using ultraviolet (365 nm) light at ∼10 mW/cm2 for 10 min. Thirty-two specimens were prepared for each polymer mixture.
Integration model assembly
Cartilaginous rings were used to study the integration of the neotissue with existing cartilage matrix.20 Disks of swine articular cartilage measuring 8 mm × 2 mm were made using an 8 mm punch biopsy. The center of the cartilage disk was removed using a 5 mm biopsy punch leaving a ring of native matrix. The cartilage rings were devitalized using five freeze–thaw cycles. The central cavities of the disks were filled with the gel containing cells and photopolymerized as described above.
Implantation and harvest
Using sterile technique, constructs were implanted subcutaneously into 5-week-old athymic male mice (nu/nu) (MGH, Boston, MA). Eight specimens from each group were harvested at 6, 12, and 18 weeks. Samples were evaluated macroscopically, weighed, and randomly selected for either histologic examination or biochemical evaluation through DNA, glycosaminoglycan (GAG) and hydroxyproline assays. Additional samples (n = 8) were prepared for each polymer mixture and used for assays at time zero. Specimens placed in cartilage rings to evaluate the integration of the neotissue with the native cartilage were grossly inspected and then processed for histology.
Specimen evaluation
At the time of harvest, the wet weight and overall dimensions of the samples were recorded. The specimens were cut in half with one-half submitted for histological processing and the remaining half used for biochemical assays. For the histological processing, the samples were fixed in 10% buffered formalin for 24 h and embedded in paraffin. Serial 5 μm sections were obtained, deparaffinized, and stained with hematoxylin and eosin, toluidine blue, and safranin O to evaluate tissue morphology and proteoglycan content. Additional sections were immunostained for collagen type I and II (Chondrex, Redmond WA). Briefly, slides were treated with 2% bovine testicular hyaluronidase at room temperature for 30 min, followed by a blocking reagent consisting of 0.3% hydrogen peroxide in methanol for another 30 min. Then, 10% goat serum was added to each slide for 30 min; on separate slides, antibodies for collagen type I and II were applied for 1 h. For negative control, N-Universal Negative Control was applied, and the secondary antibody was added for 20 min. Then, 3,3-diaminobenzidine was applied to each slide, and cell nuclei were counterstained with hematoxylin. Detection of elastin was performed using a Verhoeff's elastic stain kit (American Master Tech Scientific, Lodi, CA) by following the manufacturer's instructions. Briefly, Verhoeff's working elastic stain (5% alcoholic hematoxylin, 10% ferric chloride, and universal iodine solution) was placed on deparaffinized slides for 15 min and rinsed in tap water. Then, 2% ferric chloride differentiating solution was placed on the slide for 3 min and rinsed in tap water. Five percent sodium thiosulfate was applied for 1 min, and Van Gieson's stain was applied for 30 s. Slides were dehydrated through absolute alcohol and xylene and covered with a cover slip mounting media.
The experimental specimens as well as native auricular swine cartilage for biochemical evaluations were weighed to obtain the wet weight of the samples. The samples were lyophilized and weighed again to obtain the dry weight of the specimens. The difference in wet and dry weights gave the water content of the neotissue. The lyophilized specimens were enzymatically digested in papain type III solution (Sigma-Aldrich, St. Louis, MO) at 125 mg/mL at 60°C for 16–24 h. DNA content was determined using the PicoGreen dsDNA Quantitation Assay Kit (Molecular Probes, Eugene, Oregon). A five-point standard curve of Lambda DNA was plotted. The amount of DNA content was determined from the curve and used as indicator of the proliferative potential of the photoencapsulated chondrocytes. GAG content of the constructs was measured using the dimethylmethylene blue dye method.21 Chondroitin sulfate B (Sigma-Aldrich) was used as a standard in the interpretation of the data. The hydroxyproline content was quantified using the simplified hydroxyproline method as previously reported.22 L-4-Hydroxyproline (Fluka Biochemika, Steinleim, Switzerland) was used as standard in a seven point curve. All the biochemical values were normalized by wet tissue weight.
Statistical analysis
Statistical analysis was performed on the quantitative data of the study using a Student t-test. Level of statistical significance was set at 0.05 and all the values are reported as the mean ± standard deviation.
Results
Gross evaluation
An increase in wet weight was shown at 6 weeks in both hydrogel groups (p < 0.001) followed by a wet weight decrease at the 12-week time point (Table 1). This demonstrated a significant difference in the 70:30 hydrogel group (p < 0.001), compared with that at 6 weeks. Surprisingly, by 18 weeks, the wet weight of the samples appeared higher than initial one in both groups, particularly in the less degradable gel group (p < 0.001 for 60:40 hydrogel).
Table 1.
Summary of Specimen Dataa
| |
60/40 (PEG-LA-DM/PEGDM) |
70/30 (PEG-LA-DM/PEGDM) |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | 6 weeks | 12 weeks | 18 weeks | Control | 6 weeks | 12 weeks | 18 weeks | |
| Wet weight (mg) | 110.25 ± 3.75 | 130.40 ± 3.11 | 127.45 ± 3.75 | 122.00 ± 4.67 | 114.10 ± 6.36 | 128.60 ± 2.69 | 121.10 ± 3.39 | 116.35 ± 2.19 |
| Volume (mm3) | 94.28 ± 1.82 | 109.46 ± 3.95 | 106.55 ± 2.58 | 104.75 ± 3.23 | 94.54 ± 2.28 | 108.21 ± 1.77 | 103.16 ± 3.51 | 102.30 ± 1.41 |
| Water content (%) | 85.39 ± 3.79 | 89.25 ± 3.80 | 86.19 ± 3.10 | 87.15 ± 3.06 | 86.05 ± 2.57 | 89.40 ± 3.70 | 85.83 ± 1.95 | 86.58 ± 2.97 |
| DNA (% of initial) | 100 | 146.54 ± 10.32 | 203.75 ± 20.79 | 188.09 ± 31.23 | 100 | 143.48 ± 24.71 | 197.01 ± 13.77 | 163.85 ± 21.23 |
| GAG (μg/mg) | 9.87 ± 1.64 | 31.82 ± 3.84 | 40.73 ± 3.61 | 46.21 ± 10.36 | 10.44 ± 3.06 | 32.49 ± 2.94 | 35.07 ± 5.96 | 40.53 ± 9.71 |
| HYP (μg/mg) | 0.97 ± 0.47 | 4.96 ± 1.05 | 6.66 ± 1.06 | 10.19 ± 1.15 | 1.04 ± 0.27 | 4.25 ± 0.73 | 6.02 ± 0.92 | 8.88 ± 1.31 |
All the data are presented as mean value ± standard deviation. Data of DNA, GAG, and HYP assays were normalized by the wet weight of the samples. DNA content is shown as percentage of the initial one (that of control, nonimplanted samples).
PEG-LA-DM, poly(ethylene glycol)-4,5 lactic acid dimethacrylate; GAG, glycosaminoglycan; HYP, hydroxyproline.
At 6 weeks, the volume of the constructs increased significantly in both groups (p < 0.001) compared to control samples. As implantation time increased, the values showed slight decrease in volume. At the end of the final 18-week time point, all of the constructs from both groups appeared to have a higher volume than the controls. This was not statistically significant. After lyophilization of the samples and measurement of dry weight, calculation of water content followed. The water content of samples appeared increased at 18 weeks—but similar to the volume data—there was an absence of statistical significance among the study groups or harvest time points.
Macroscopically, the samples became increasingly opaque over time (Fig. 2). While the samples at 6 weeks still presented large areas of translucency due to the gel component, the samples at 18 weeks were completely opaque. The consistency of the samples at 6 weeks was relatively soft and gel-like, but as time increased the gel was replaced with new cartilage matrix and the nodules became stiffer. However, biomechanical testing was not performed in this study to confirm this change.
FIG. 2.
Macroscopic view of constructs over the implantation time. Color images available online at www.liebertonline.com/ten.
Biochemical evaluation
DNA content data was evaluated as percentage of the control sample DNA content values measured at time zero that was set at 100% (Fig. 3). In both groups, DNA content was increased throughout the study. At 18 weeks, the mean value of DNA content was 188.09% ± 31.23% and 163.85% ± 21.23% for 60:40 and 70:30 hydrogel groups, respectively. No significant difference was found among the study groups at any harvest time point.
FIG. 3.
Biochemical evaluation data. DNA, glycosaminoglycan (GAG), and hydroxyproline (HP) content. □, 0 week;  , 6 weeks;
, 6 weeks;  , 12 weeks;
, 12 weeks;  , 18 weeks;
, 18 weeks;  , native swine auricular cartilage. #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
, native swine auricular cartilage. #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
The GAG content of the neotissue (Fig. 3) demonstrated a very high increase (p < 0.001) during the first 6 weeks of implantation in both hydrogel groups. The amount of GAG content increased with time as well. At 18 weeks, the amount of GAG was 46.21 ± 10.36 μg/mg of wet tissue weight in the 60:40 group, whereas in the 70:30 group it was slightly lower, 40.53 ± 9.71 μg/mg. No statistically significant difference was observed. On the basis of data from our preliminary biochemical study of swine native auricular cartilage, the GAG content of engineered cartilage using 60:40 ratio of PEGDM hydrogels was 66.84% of that in native cartilage, whereas the GAG content of the neotissue made with 70:30 gel was 58.63% of the native ear cartilage.
In both groups, the hydroxyproline content (Fig. 3) also increased over time. At the final harvest time point the total collagen was of 10.19 ± 1.15 μg/mg of wet tissue weight in 60:40 hydrogel group, approximating 68.20% of that found in the native swine auricular cartilage. The amount of total collagen in the 70:30 hydrogel group was slightly lower, 8.88 ± 1.31 μg/mg or about 59.50% of the content measured in native cartilage. There was no significant difference between groups, although both groups only reached about two-thirds of the total collagen of native ear in the time frame of this study.
Histological evaluation
Tissue sections stained with hematoxylin and eosin revealed that the chondrocytes and ECM of the neocartilage resembled morphology of that observed in native swine ear cartilage. The presence of dispersed ovoid-shaped chondrocytes located within typical rounded lacunae and surrounded by a basophilic matrix was observed. Nonhomogeneous distribution of the encapsulated chondrocytes was observed in both study groups at 6 weeks, probably due to the incomplete degradation of the hydrolysable crosslinks of the polymer scaffold at that time point. In samples from later time points, more advanced degradation of the polymer scaffold allowed the cells to spread more homogeneously throughout the constructs. No visual difference was observed between the groups.
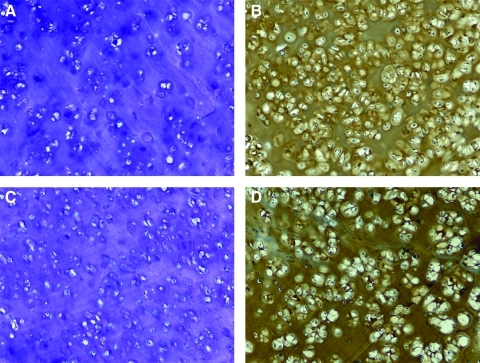
Toluidine blue staining confirmed the presence of negatively charged sulfated proteoglycans in the ECM of the neotissue (Fig. 4A, C). Synthesis of new cartilaginous matrix was demonstrated in all groups, irrespective of the PEGDM copolymer used. Both of the pericellular and extended pattern of deposition were demonstrated and no clear morphological difference was observed among the studied hydrogel groups.
FIG. 4.
Representative sections of 18-week samples. 60:40 copolymer hydrogel group, toluidine blue O (A) and immunostaining for collagen type II (B). 70:30 copolymer hydrogel group, toluidine blue (C) and immunostaining for collagen type II (D). Original magnification, ×100 for all images. Color images available online at www.liebertonline.com/ten.
Immunostaining for collagen type I and II demonstrated that the encapsulated chondrocytes maintained their phenotype and produced collagens typical of that found in native ear cartilage (Fig. 3B, D). Positive immunohistochemical staining for type I was observed mainly at the surfaces of the sections and was probably related to the remnants of reactive fibrous capsule. Intense positive staining for collagen type II was observed throughout the ECM of the constructs in both groups. In the 6-week specimens, the type II collagen was mainly confined to the immediate pericellular regions, presumably due to the incomplete degradation of the polymer. In later samples, the type II collagen staining pattern was more homogeneous throughout the specimens.
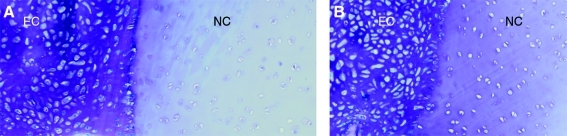
Results from the cartilage ring constructs demonstrated integration of the neotissue with the existing native cartilage in the ring. The cartilage rings were devitalized to eliminate the possibility of chondrocytes from the ring migrating into the repair interface and influencing the results. In samples from both groups at 6 weeks, integration of the neotissue with the apposing surfaces of the native articular cartilage was observed. However, void spaces were also observed in several sections at this early time point. By 18 weeks, the integration interface was extensive and involved almost all the surface of the surrounding native cartilage (Fig. 5). Attaching cells presented ovoid in shape and most often aligned perpendicular to the surface of the native cartilage. The engineered tissue appeared to closely follow the configuration of the devitalized cartilage surface and to fill irregularities of various depth and shape along the integration interface. No obvious morphological differences in the integration pattern were observed between the two copolymer groups studied.
FIG. 5.
Micrographs of integration interface of engineered cartilage (EC) with native articular cartilage (NC) at the 18-week timepoint. 60:40 hydrogel (A) and 70:30 hydrogel group (B) are shown. Stain: toluidine blue. Original magnification, ×100 for all images. Color images available online at www.liebertonline.com/ten.
The existence of elastin in the neocartilage was present, as expected, in native swine auricular cartilage. Additionally, samples harvested at the 12- and 18-week time point from both the 60:40 and 70:30 copolymer formulations demonstrated the existence of the pericellular elastic fibers (Fig. 6). No elastin was detected in samples harvested after 6 weeks.
FIG. 6.
Samples were stained for the identification of elastin. Elastic fibers from native swine auricular cartilage (A) and the 60:40 (B) and 70:30 (C) hydrogel copolymer formulations can be observed. Original magnification, ×200 for all images. Color images available online at www.liebertonline.com/ten.
Discussion
The goal of engineering auricular or nasal cartilage for craniofacial reconstruction will depend largely on the development of suitable polymer scaffolds to support chondrocyte growth and ECM production. Open fibrous scaffolds such as collagen or polyesters have been successful in promoting cartilage formation in immunocompromised animals, but having an open lattice network also permits invasion of inflammatory cells that can negatively affect matrix formation in immune competent animal models. Biomimetic gels can be used to encapsulate chondrocytes in a temporary pseudomatrix and provide a microenvironment similar to native cartilage during the period of new matrix formation. Nondegradable gels can be formulated that are resistant to erosion and maintain three-dimensional architecture, but do not permit cell-to-cell contact and interfere with ECM production. Degradable gels can be tailored to break down within predictable periods of time. Delicate timing of this process is necessary, however, to be able to maintain volume and three-dimensional shape. Employing photochemistry to polymerize the gels could allow molding the gels into predetermined shapes and permit cartilage formation in the desired form. The purpose of this study was to evaluate the cartilage formation capacity of a series of polymers made with a combination of degradable and nondegradable PEG macromers.
The data from this study show that new cartilage matrix was formed using mixtures of PEGDM and PEG-LA-DM polymers. Extensive study of fully degradable PEG-LA-DM was not performed because previously published in vitro data showed that the scaffold degraded too rapidly for cartilage matrix to form and pilot in vivo studies confirmed this as well. Although previous data have shown that fully nondegradable PEGDM gels permit pericellular matrix formation, the resilience of the nondegradabble polymer inhibits contiguous cartilage matrix formation. By combining the degradable with the nondegradable formulations, we hoped to exploit the advantages of both types of gels for forming auricular cartilage.
The experimental data showed increasing maturation of the cartilage matrix over time. The wet weight and volume of the specimens increased over the time periods studied when compared with the initial implantation values shown in Table 1. Histological evidence from the early time period showed pericellular matrix formation with intercellular regions filled with polymer scaffold using both formulations. In later time periods, cartilage matrix filled in the intercellular regions as the polymer was degraded and replaced. Biochemical changes paralleled the morphological observations over time. There was an increase in GAG and total collagen over the time periods studied reaching nearly two-thirds of the amounts measured in normal ear cartilage. The phenotype of the chondrocytes was preserved as evidenced by increased immunohistochemical staining of type II collagen over time. Native ear cartilage does not have significant levels of type I collagen and the engineered specimens similarly did not have significant amounts of collagen I. This finding agrees with the results of a study by Madsen et al., which concluded that the collagen types found in rabbit ear cartilage were identical with those present in hyaline cartilage.23 They demonstrated that type I collagen, in contrast with type II collagen, is not a significant constituent of ear cartilage matrix. Since auricular cartilage contains elastin, this could be assayed in future studies.
These findings are probably related with the chondrocyte source and are consistent with the conclusions of previous study by Xu et al., which investigated the effect of various chondrocyte sources on the construct mass and volume.24 Also, Isogai et al. compared four different chondrocyte sources—nasoseptal, articular, costal, and auricular—performing a preliminary investigation for fabricating a human ear model.25 They concluded that constructs seeded with auricular chondrocytes exhibited larger diameters compared to constructs seeded with the other cell types. Perhaps, each type of chondrocyte possesses a unique regulatory mechanism that determines the distribution pattern of ECM deposition in vivo. Additionally, the subcutaneous placement of the constructs in the present study provided an environment with closer approximation to their native growth conditions in contrast with other chondrocyte types.
Studying the integration of the engineered cartilage with existing native cartilage provides information on the capacity of the polymers to permit cartilage formation and, importantly, not inhibit the cells from interacting with the native matrix. In some settings where the engineered cartilage will be placed in proximity of existing cartilage, we believe that the new cartilage should attach firmly to the native cartilage and form a contiguous sheet of structural tissue. A weak interface could cause collapse of the tissue and loss of three-dimensional projection. Integration of neocartilage with recipient tissue appears to be dependent on viable cells at the interface of integration, even in only one of the two apposing surfaces.26,27 The adhesive strength of cartilage integration has been correlated with collagen synthesis and deposition.28 Investigating the integrative properties of the PEGDM-based neocartilage, we found that the engineered hydrogels can support the integration of the engineered cartilage with existing native cartilage. At the early 6-week time point the samples demonstrated only partial integration with the apposing surface of the native cartilage, but by week 12 and 18, an improvement of the integrative properties of the engineered cartilage was observed. The integration interface exhibited evidence of repopulation, deposition of matrix macromolecules, and tight adhesion of the engineered cartilage. The breakdown of the polymer scaffold over time could have influenced the properties of the neotissue, leading to improved integrative properties and a tight interface junction at the later times. This study used articular cartilage rings as opposed to native auricular cartilage because of tissue availability and ease of making cartilage rings. Future studies could use auricular cartilage rings.
The nude mouse, although an immune incompetent model, seems to pose several advantages for the study of engineering auricular cartilage. The skin of nude mouse is thin, hairless, and pliable, mimicking human ear skin. Also, the subcutaneous implantation offers a biological and biomechanical environment much like that of native human auricular cartilage. However, because of they lack a full complement of immune cells, nude mice cannot replicate the implantation of constructs into humans where there may be normal inflammatory response. Therefore, any inflammatory response to the engineered tissue by the host should be investigated using complete immunocompetent animal models.
Further modifications in hydrogel chemistry or photopolymerization mechanism can improve the scaffold properties of PEG derivatives for tissue engineering of auricular cartilage, particularly in terms of the intrinsic adhesive properties. Due to low protein absorption, PEG hydrogels require further modifications to facilitate the adhesion and spreading of the seeded cells into the polymer network. Attachment of various peptides to the surface of these hydrogels has been studied to address the cell adhesion. The Arg-Gly-Asp sequence, found in cell-binding domains of matrix macromolecules, is the most commonly researched adhesive peptide.29 Integrins, located on the surface of the cells, are capable of binding with this peptide sequence; thus, cells can adhere to normally nonadhesive surfaces. These modified hydrogels could allow more homogeneous cell encapsulation, resulting in improvement of biomechanical and integrative properties of the engineered articular cartilage.
In conclusion, this study demonstrated that copolymers, composed of degradable and nondegradable PEGDM macromers, can serve as scaffold for the engineering of auricular cartilage. The neocartilage resembled the native cartilage in terms of histological appearance and composition of ECM, and also exhibited a capacity for integrating with existing cartilage matrix. From our pilot data we ascertained that fully degradable PEG-LA-DM was unable to reliable form neocartilage because the polymer degraded much to fast, both in vitro and in vivo. On the other hand, 100% nondegradable PEGDM restricts the chondrocytes from cell–cell interactions and prevents contiguous cartilage formation. The molar ratios of 60:40 and 70:30 proved more optimal in the promoting of auricular chondrocyte phenotype in terms of GAG and collagen production. Because of the longer degradation time, it is reasonable to expect that this copolymer hydrogel provided higher mechanical support to engineered cartilage. The increased mechanical integrity over time could have influenced the biochemical properties of the neocartilage. Engineering auricular cartilage in the shape of human ear using 60:40 degradable/non degradable PEGDM copolymer will be the next step of our study, evaluating, additionally, the feasibility of the hydrogel in maintaining the initial construct shape and in supporting the development of improved biomechanical properties of the engineered auricular cartilage.
Acknowledgments
The authors would like to thank Mitun Ranka for supporting in preparation and implantation of the constructs and Arthur Foubert for assistance during the harvest of donor animal tissues. This work was supported by grants from the National Institutes of Health (R01DE12998) and Plastic Surgery Educational Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Park S.S. Hood R.J. Auricular reconstruction. Otolaryngol Clin North Am. 2001;34:713. doi: 10.1016/s0030-6665(05)70015-3. [DOI] [PubMed] [Google Scholar]
- 2.Thorne C.H. Brecht L.E. Bradley J.P. Levine J.P. Hammerschlag P. Longaker M.T. Auricular reconstruction: indications for autogenous and prosthetic techniques. Plast Reconstr Surg. 2001;107:1241. doi: 10.1097/00006534-200104150-00024. [DOI] [PubMed] [Google Scholar]
- 3.Han K. Son D. Osseointegrated alloplastic ear reconstruction with the implant-carrying plate system in children. Plast Reconstr Surg. 2002;109:496. doi: 10.1097/00006534-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Romo T. Presti P.M. Yalamanchili H.R. Medpor alternative for microtia repair. Facial Plast Surg Clin North Am. 2006;14:129. doi: 10.1016/j.fsc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Tanzer R.C. Microtia—a long-term follow-up of 44 reconstructed auricles. Plast Reconstr Surg. 1978;61:161. doi: 10.1097/00006534-197802000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Brent B. Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases. Clin Plast Surg. 2002;29:257. doi: 10.1016/s0094-1298(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y. Vacanti J.P. Paige K.T. Upton J. Vacanti C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Saim A.B. Cao Y. Weng Y. Chang C.N. Vacanti M.A. Vacanti C.A. Eavey R.D. Engineering autogenous cartilage in the shape of a helix using an injectable hydrogel scaffold. Laryngoscope. 2000;110:1694. doi: 10.1097/00005537-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Arévalo-Silva C.A. Eavey R.D. Cao Y. Vacanti M. Weng Y. Vacanti C.A. Internal support of tissue-engineered cartilage. Arch Otolaryngol. 2000;126:1448. doi: 10.1001/archotol.126.12.1448. [DOI] [PubMed] [Google Scholar]
- 10.Isogai N. Asamura S. Higashi T. Ikada Y. Morita S. Hillyer J. Jacquet R. Landis W.J. Tissue engineering of an auricular cartilage model utilizing cultured chondrocyte-poly(L-lactide-epsilon-caprolactone) scaffolds. Tissue Eng. 2004;10:673. doi: 10.1089/1076327041348527. [DOI] [PubMed] [Google Scholar]
- 11.Kusuhara H. Isogai N. Enjo M. Otani H. Ikada Y. Jacquet R. Lowder E. Landis W.J. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17:136. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill-West J.L. Chowdhury S.M. Slepian M.J. Hubbell J.A. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci U S A. 1994;91:5967. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawhney A.S. Pathak C.P. van Rensburg J.J. Dunn R.C. Hubbell J.A. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. J Biomed Mater Res. 1994;28:831. doi: 10.1002/jbm.820280710. [DOI] [PubMed] [Google Scholar]
- 14.Peppas N.a. Keys K.B. Torres-Lugo M. Lowman A.M. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 15.Quinn C.P. Pathak C.P. Heller A. Hubbell J. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16:389. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee W.K. Ichi T. Ooya T. Yamamoto T. Katoh M. Yui N. Novel poly(ethylene glycol) scaffolds crosslinked by hydrolyzable polyrotaxane for cartilage tissue engineering. J Biomed Mater Res A. 2003;67:1087. doi: 10.1002/jbm.a.10570. [DOI] [PubMed] [Google Scholar]
- 17.Lin-Gibson S. Bencherif S. Cooper J.A. Wetzel S.J. Antonucci J.M. Vogel B.M. Horkay F. Washburn N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen K. West J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 19.Rice M. Anseth K. Encapsulating chondrocytes in copolymer gels: bimodal degradation kinetics influence cell phenotype and extracellular matrix development. J Biomed Mater Res A. 2004;70:560. doi: 10.1002/jbm.a.30106. [DOI] [PubMed] [Google Scholar]
- 20.Tognana E. Chen F. Padera R.F. Leddy H.A. Christensen S.E. Guilak F. Vunjak-Novakovic G. Freed L.E. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthr Cartil. 2005;13:129. doi: 10.1016/j.joca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Taylor K.B. Jeffree G.M. A new basic metachromatic dye, I:9-dimethyl methylene blue. Histochem J. 1969;1:199. doi: 10.1007/BF01081408. [DOI] [PubMed] [Google Scholar]
- 22.Reddy G.K. Enwemeka C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 23.Madsen K. von Der Mark K. van Menxel M. Friberg U. Analysis of collagen types synthesized by rabbit ear cartilage chondrocytes in vivo and in vitro. Biochem J. 1984;221:189. doi: 10.1042/bj2210189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J.W. Zaporojan V. Peretti G.M. Roses R.E. Morse K.B. Roy A.K. Mesa J.M. Randolph M.A. Bonassar L.J. Yaremchuk M.J. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg. 2004;113:1361. doi: 10.1097/01.prs.0000111594.52661.29. [DOI] [PubMed] [Google Scholar]
- 25.Isogai N. Kusuhara H. Ikada Y. Ohtani H. Jacquet R. Hillyer J. Lowder E. Landis W.J. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 26.Obradovic B. Martin I. Padera R.F. Treppo S. Freed L.E. Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 27.Giurea A. DiMicco M.A. Akeson W.H. Sah R.L. Development-associated differences in integrative cartilage repair: roles of biosynthesis and matrix. J Orthop Res. 2002;20:1274. doi: 10.1016/S0736-0266(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 28.DiMicco M.A. Sah R.L. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19:1105. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 29.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]