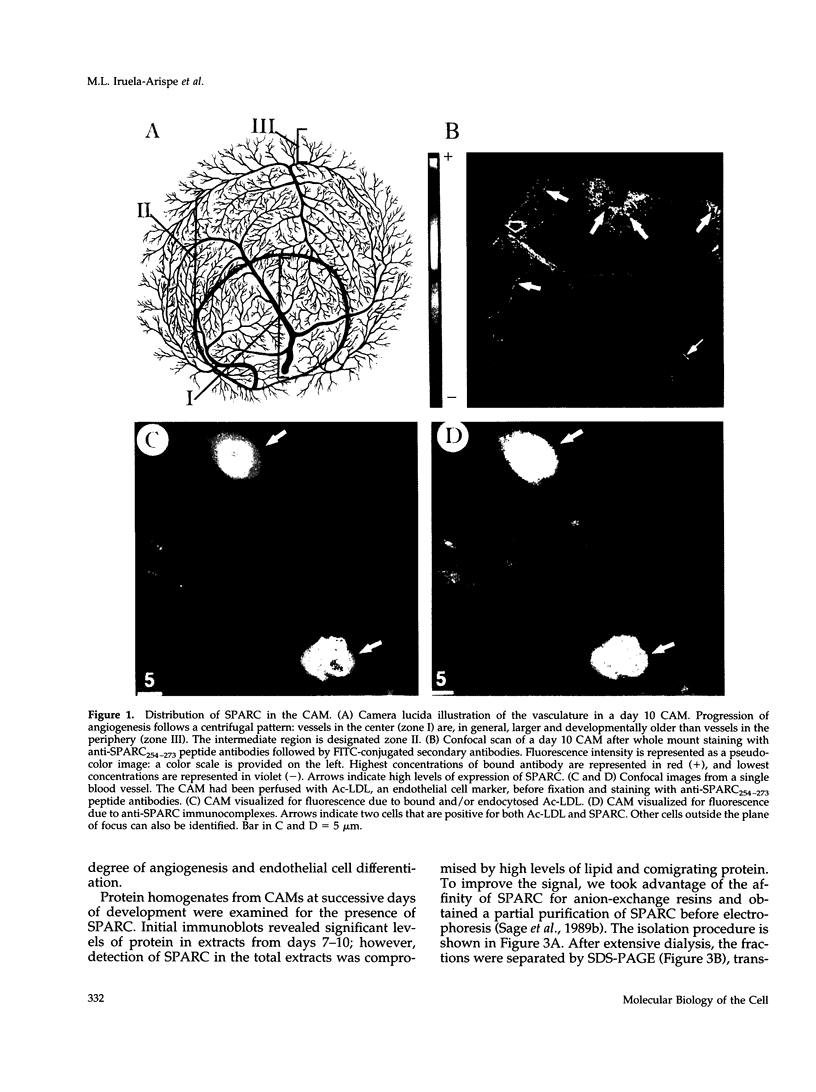
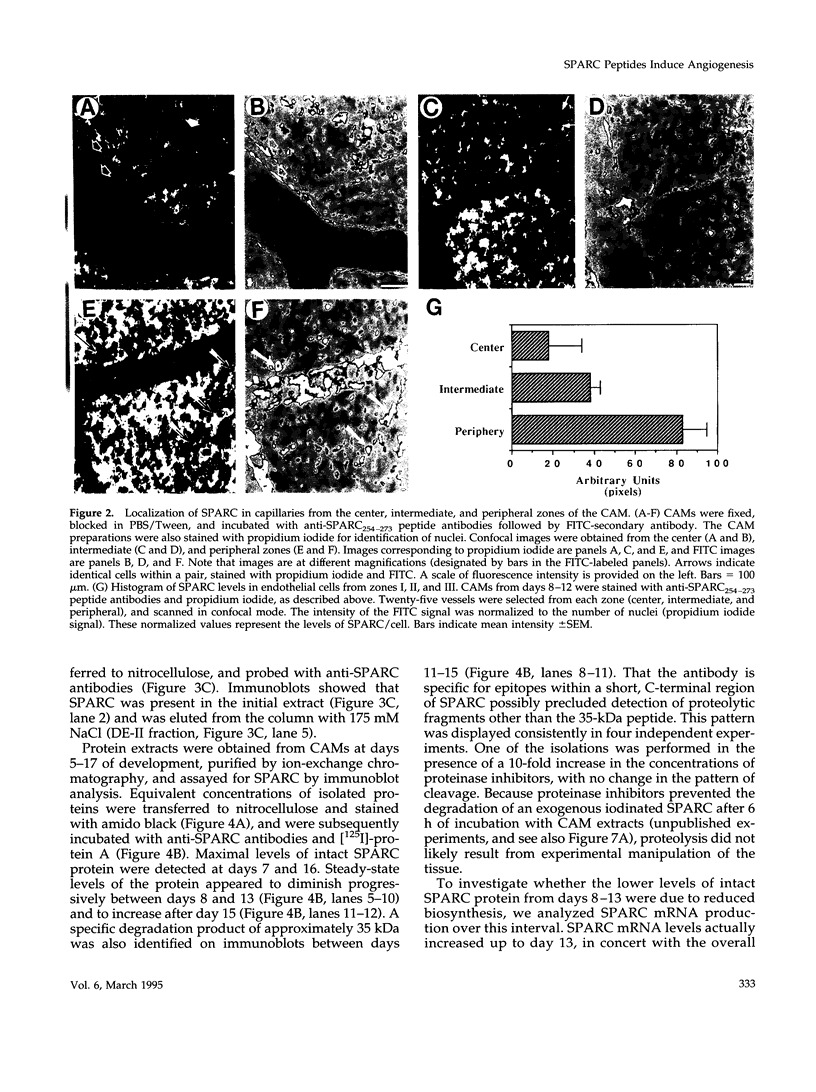
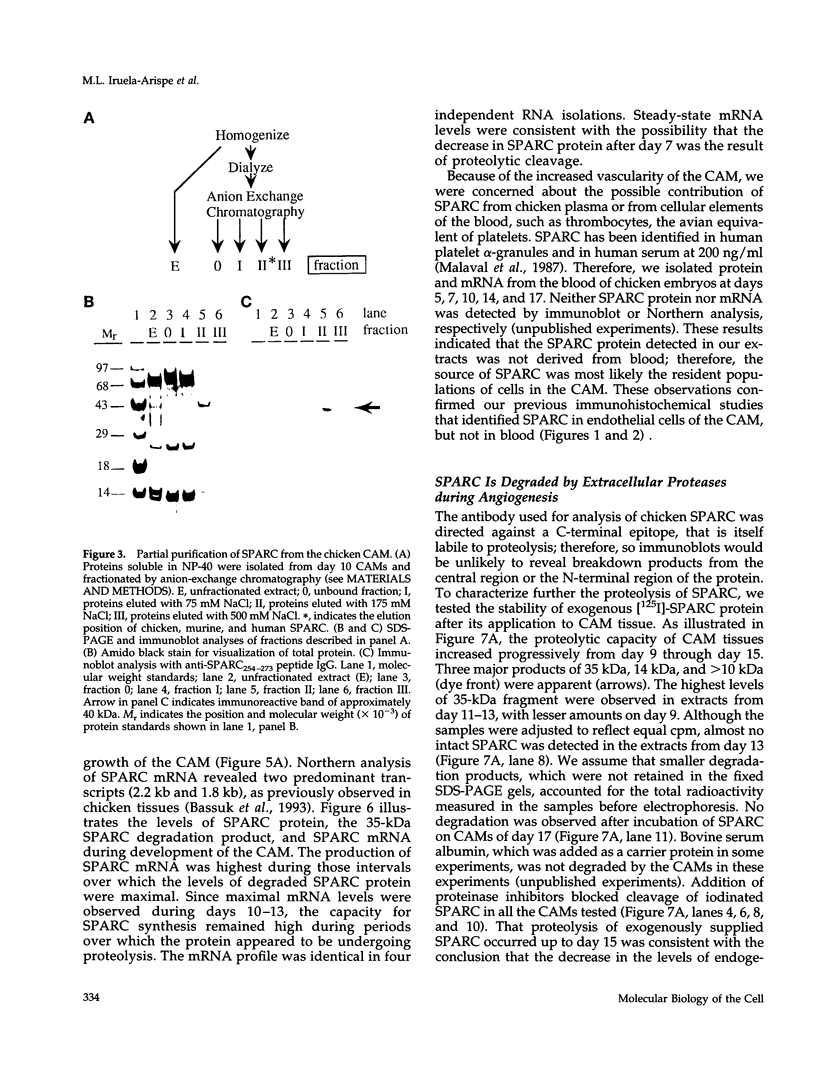
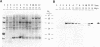Abstract
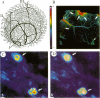
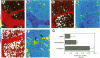
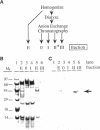
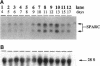
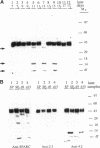
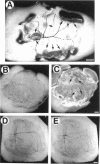
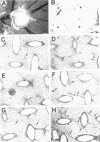
SPARC is a secreted glycoprotein that has been shown to disrupt focal adhesions and to regulate the proliferation of endothelial cells in vitro. Moreover, peptides resulting from the proteolysis of SPARC exhibit angiogenic activity. Here we describe the temporal synthesis, turnover, and angiogenic potential of SPARC in the chicken chorioallantoic membrane. Confocal immunofluorescence microscopy revealed specific expression of SPARC protein in endothelial cells, and significantly higher levels of SPARC were observed in smaller newly formed blood vessels in comparison to larger, developmentally older vessels. SPARC mRNA was detected at the earliest stages of chorioallantoic membrane morphogenesis and reached maximal levels at day 13 of embryonic development. Interestingly, steady-state levels of SPARC mRNA did not correlate directly with protein accumulation; moreover, the protein appeared to undergo limited degradation during days 10-15. Incubation of [125I]-SPARC with chorioallantoic membranes of different developmental ages confirmed that extracellular proteolysis occurred during days 9-15, but not at later stages (e.g., days 17-21). Comparison of peptides produced by incubation with chorioallantoic membranes with those generated by plasmin showed an identical pattern of proteolysis. Plasmin activity was present throughout development, and in situ zymography identified sites of plasminogen activator activity that corresponded to areas exhibiting high levels of SPARC expression. Synthetic peptides from a plasmin-sensitive region of SPARC, between amino acids 113-130, stimulated angiogenesis in the chorioallantoic membrane in a dose-dependent manner; in contrast, intact SPARC was inactive in similar assays. We have shown that SPARC is expressed in endothelial cells of newly formed blood vessels in a manner that is both temporally and spatially restricted. Between days 9 and 15 of chorioallantoic membrane development, the protein undergoes proteolytic cleavage that is mediated, in part, by plasmin. SPARC peptides released specifically by plasmin induce angiogenesis in vivo. We therefore propose that SPARC acts as an intrinsic regulator of angiogenesis in vivo.
Full text
PDF




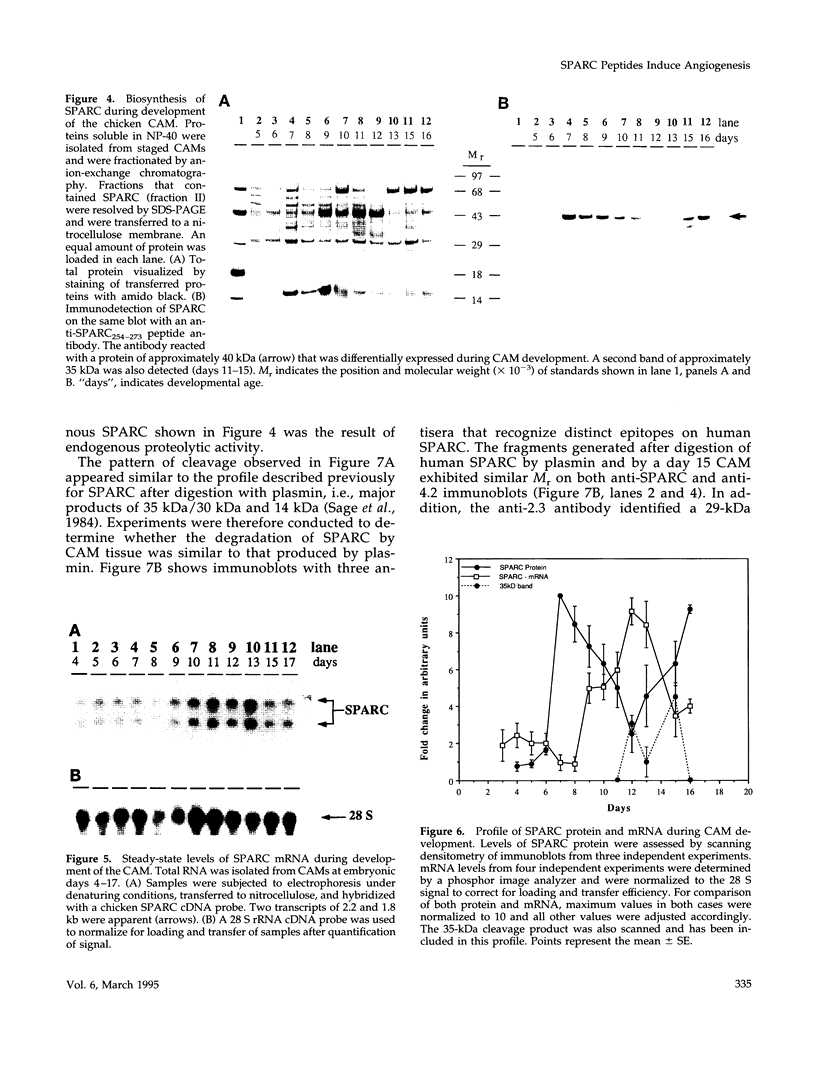
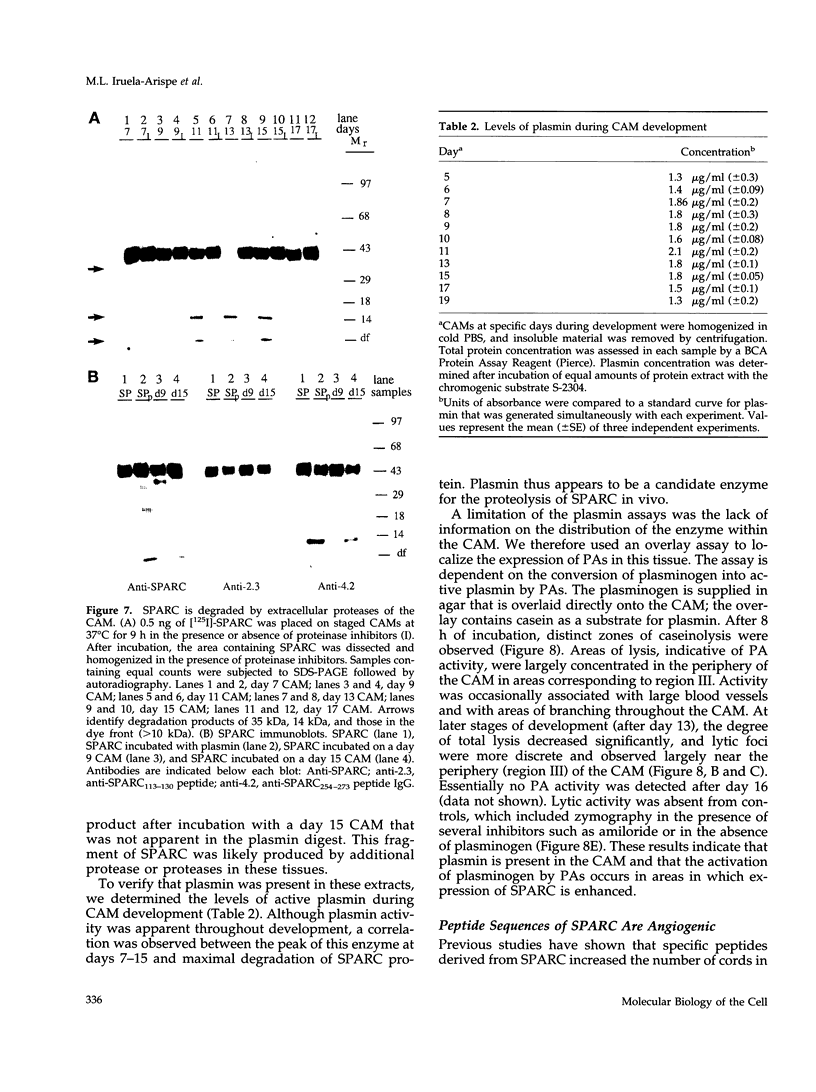
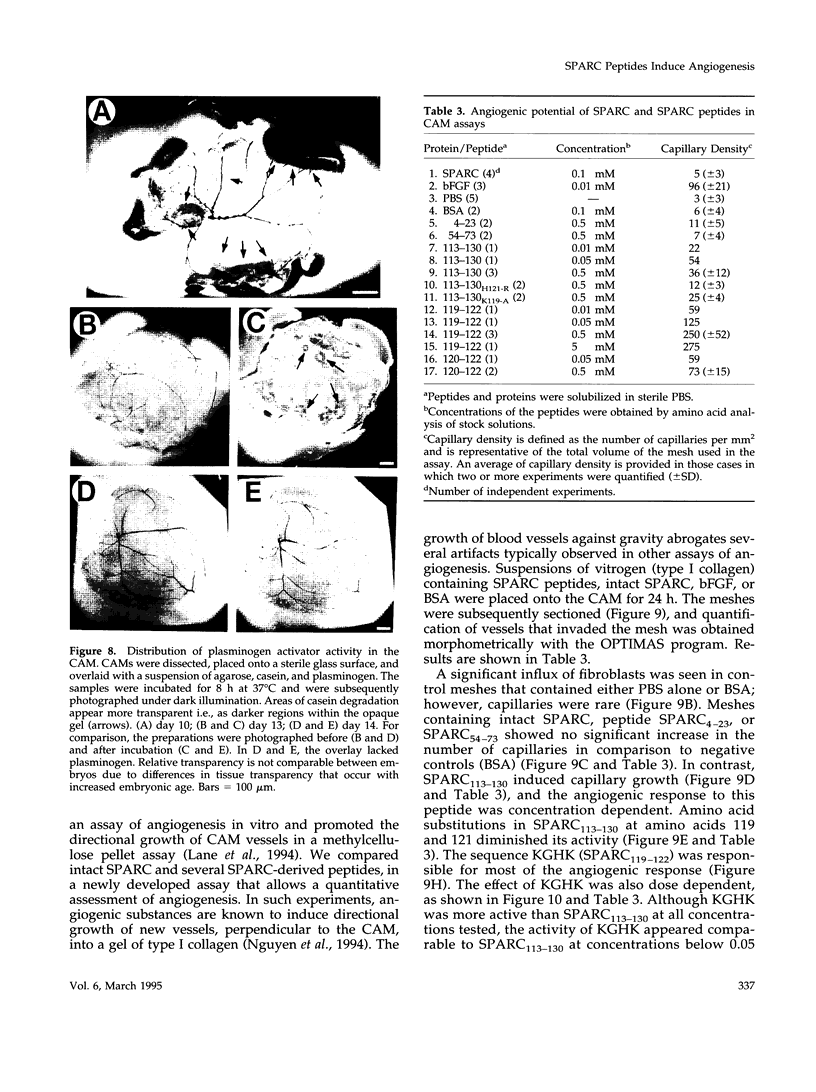
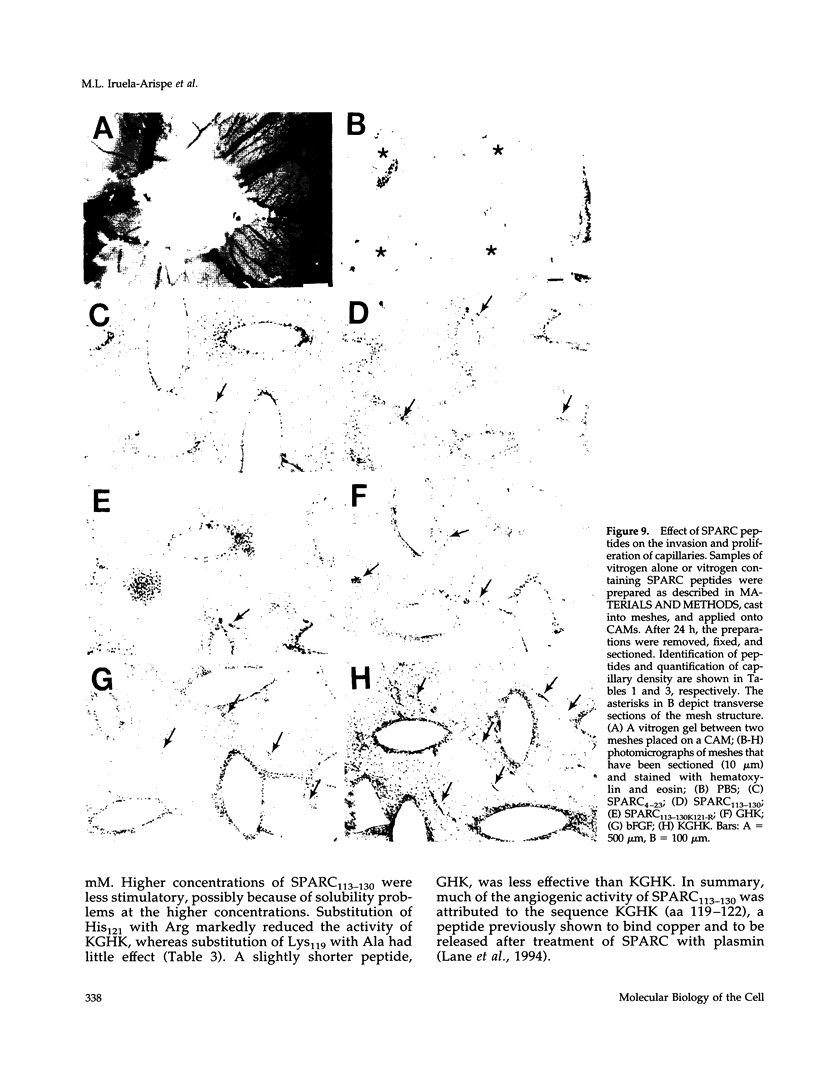




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelgot S., Coppey J., Fromentin A., Guille E., Poupon M. F., Roussel A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res. 1986 Mar-Apr;6(2):159–164. [PubMed] [Google Scholar]
- Auerbach R., Kubai L., Knighton D., Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974 Dec;41(2):391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Ausprunk D. H., Knighton D. R., Folkman J. Differentiation of vascular endothelium in the chick chorioallantois: a structural and autoradiographic study. Dev Biol. 1974 Jun;38(2):237–248. doi: 10.1016/0012-1606(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Bacharach E., Itin A., Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10686–10690. doi: 10.1073/pnas.89.22.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk J. A., Iruela-Arispe M. L., Lane T. F., Benson J. M., Berg R. A., Sage E. H. Molecular analysis of chicken embryo SPARC (osteonectin). Eur J Biochem. 1993 Nov 15;218(1):117–127. doi: 10.1111/j.1432-1033.1993.tb18358.x. [DOI] [PubMed] [Google Scholar]
- Bolender R. P., Charleston J. S. Software for counting cells and estimating structural volumes with the optical disector and fractionator. Microsc Res Tech. 1993 Jul 1;25(4):314–324. doi: 10.1002/jemt.1070250408. [DOI] [PubMed] [Google Scholar]
- Bolender R. P. Software for quantitative immunogold and in situ hybridization. Microsc Res Tech. 1993 Jul 1;25(4):304–313. doi: 10.1002/jemt.1070250407. [DOI] [PubMed] [Google Scholar]
- Braendgaard H., Evans S. M., Howard C. V., Gundersen H. J. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J Microsc. 1990 Mar;157(Pt 3):285–304. doi: 10.1111/j.1365-2818.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Brem S. S., Zagzag D., Tsanaclis A. M., Gately S., Elkouby M. P., Brien S. E. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol. 1990 Nov;137(5):1121–1142. [PMC free article] [PubMed] [Google Scholar]
- Clezardin P., Malaval L., Ehrensperger A. S., Delmas P. D., Dechavanne M., McGregor J. L. Complex formation of human thrombospondin with osteonectin. Eur J Biochem. 1988 Aug 1;175(2):275–284. doi: 10.1111/j.1432-1033.1988.tb14194.x. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D. B. Extracellular matrix regulation of growth factor and protease activity. Curr Opin Cell Biol. 1991 Oct;3(5):817–823. doi: 10.1016/0955-0674(91)90055-4. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D. B. The extracellular regulation of growth factor action. Mol Biol Cell. 1992 Oct;3(10):1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol. 1993 Jan;154(1):53–63. doi: 10.1002/jcp.1041540108. [DOI] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega D. S., Werz M. A., Macdonald R. L. Forskolin and phorbol esters reduce the same potassium conductance of mouse neurons in culture. Science. 1987 Jan 16;235(4786):345–348. doi: 10.1126/science.2432663. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bagger P., Bendtsen T. F., Evans S. M., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988 Oct;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan G. N., McAuslan B. R. Modulation of synthesis of specific proteins in endothelial cells by copper, cadmium, and disulfiram: an early response to an angiogenic inducer of cell migration. J Cell Physiol. 1982 May;111(2):207–212. doi: 10.1002/jcp.1041110213. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Diglio C. A., Sage E. H. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Hasselaar P., Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest. 1991 Feb;64(2):174–186. [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Liska D. J., Sage E. H., Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993 May;197(1):40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane T. F., Iruela-Arispe M. L., Sage E. H. Regulation of gene expression by SPARC during angiogenesis in vitro. Changes in fibronectin, thrombospondin-1, and plasminogen activator inhibitor-1. J Biol Chem. 1992 Aug 15;267(23):16736–16745. [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990 Dec;111(6 Pt 2):3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994 Feb;8(2):163–173. [PubMed] [Google Scholar]
- Lyons R. M., Gentry L. E., Purchio A. F., Moses H. L. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990 Apr;110(4):1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L., Fournier B., Delmas P. D. Radioimmunoassay for osteonectin. Concentrations in bone, nonmineralized tissues, and blood. J Bone Miner Res. 1987 Oct;2(5):457–465. doi: 10.1002/jbmr.5650020514. [DOI] [PubMed] [Google Scholar]
- Maquart F. X., Bellon G., Chaqour B., Wegrowski J., Patt L. M., Trachy R. E., Monboisse J. C., Chastang F., Birembaut P., Gillery P. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ in rat experimental wounds. J Clin Invest. 1993 Nov;92(5):2368–2376. doi: 10.1172/JCI116842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P., Mayer U., Bruch M., Jenö P., Mann K., Landwehr R., Engel J., Timpl R. High-affinity and low-affinity calcium binding and stability of the multidomain extracellular 40-kDa basement membrane glycoprotein (BM-40/SPARC/osteonectin). Eur J Biochem. 1992 Apr 1;205(1):233–240. doi: 10.1111/j.1432-1033.1992.tb16773.x. [DOI] [PubMed] [Google Scholar]
- Mayer U., Aumailley M., Mann K., Timpl R., Engel J. Calcium-dependent binding of basement membrane protein BM-40 (osteonectin, SPARC) to basement membrane collagen type IV. Eur J Biochem. 1991 May 23;198(1):141–150. doi: 10.1111/j.1432-1033.1991.tb15996.x. [DOI] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989 Jul;14(7):268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Shing Y., Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994 Jan;47(1):31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- Otsuka K., Yao K. L., Wasi S., Tung P. S., Aubin J. E., Sodek J., Termine J. D. Biosynthesis of osteonectin by fetal porcine calvarial cells in vitro. J Biol Chem. 1984 Aug 10;259(15):9805–9812. [PubMed] [Google Scholar]
- Page A. E., Hayman A. R., Andersson L. M., Chambers T. J., Warburton M. J. Degradation of bone matrix proteins by osteoclast cathepsins. Int J Biochem. 1993 Apr;25(4):545–550. doi: 10.1016/0020-711x(93)90662-x. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Montesano R. Proteolytic balance and capillary morphogenesis. Cell Differ Dev. 1990 Dec 2;32(3):319–327. doi: 10.1016/0922-3371(90)90046-y. [DOI] [PubMed] [Google Scholar]
- Pickart L., Lovejoy S. Biological activity of human plasma copper-binding growth factor glycyl-L-histidyl-L-lysine. Methods Enzymol. 1987;147:314–328. doi: 10.1016/0076-6879(87)47121-8. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Lane T. F., Iruela-Arispe M. L., Ross R., Sage E. H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. J., Puolakkainen P., Lane T. F., Dickerson D., Bornstein P., Sage E. H. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bassuk J. A., Yost J. C., Folkman M. J., Lane T. F. Inhibition of endothelial cell proliferation by SPARC is mediated through a Ca(2+)-binding EF-hand sequence. J Cell Biochem. 1995 Jan;57(1):127–140. doi: 10.1002/jcb.240570113. [DOI] [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Decker J., Funk S., Iruela-Arispe M. L. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989 Jun;37(6):819–829. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Vassalli J. D., Belin D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest. 1991 Mar;87(3):962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauk J. J., Norris K., Kerr J. M., Somerman M. J., Young M. F. Diverse forms of stress result in changes in cellular levels of osteonectin/SPARC without altering mRNA levels in osteoligament cells. Calcif Tissue Int. 1991 Jul;49(1):58–62. doi: 10.1007/BF02555904. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sleath P. R., Hendrickson R. C., Kronheim S. R., March C. J., Black R. A. Substrate specificity of the protease that processes human interleukin-1 beta. J Biol Chem. 1990 Aug 25;265(24):14526–14528. [PubMed] [Google Scholar]
- Stenner D. D., Tracy R. P., Riggs B. L., Mann K. G. Human platelets contain and secrete osteonectin, a major protein of mineralized bone. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6892–6896. doi: 10.1073/pnas.83.18.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Tyree B. The partial degradation of osteonectin by a bone-derived metalloprotease enhances binding to type I collagen. J Bone Miner Res. 1989 Dec;4(6):877–883. doi: 10.1002/jbmr.5650040612. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987 Apr 6;214(1):187–191. doi: 10.1016/0014-5793(87)80039-x. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Overall C. M., Sodek J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur J Biochem. 1991 Apr 23;197(2):519–528. doi: 10.1111/j.1432-1033.1991.tb15940.x. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. The emergence of the endothelial cell lineage in the chick embryo can be detected by uptake of acetylated low density lipoprotein and the presence of a von Willebrand-like factor. Dev Biol. 1989 Mar;132(1):230–240. doi: 10.1016/0012-1606(89)90219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost J. C., Sage E. H. Specific interaction of SPARC with endothelial cells is mediated through a carboxyl-terminal sequence containing a calcium-binding EF hand. J Biol Chem. 1993 Dec 5;268(34):25790–25796. [PubMed] [Google Scholar]
- Ziche M., Jones J., Gullino P. M. Role of prostaglandin E1 and copper in angiogenesis. J Natl Cancer Inst. 1982 Aug;69(2):475–482. [PubMed] [Google Scholar]