Abstract
Soybean flour and wheat germ are the two most important protein components of wheat germ-based insect artificial diets. The effect of modifying the proportion of these two ingredients in a Noctuidae-specific diet was investigated utilizing the tobacco budworm Heliothis virescens (F.) (Lepidoptera: Noctuidae), with the goal of developing a suboptimal diet that, without drastically affecting this insect's growth and reproductive rates, could manifest subtle negative effects in this insect. The original diet formula contained 2.51% protein. When the proportions of soybean flour and wheat germ were changed to 2.15% protein the net reproductive rate of the first generation was significantly lower. In the second generation, the net reproductive rate, development time, percent female survivorship, fertility, intrinsic rate of increase, finite rate of increase and female longevity were significantly lower in both the 2.15% and 2.26% protein diets. The survival rate of immatures to the adult stage was 1% in the 2.05% protein diet in the first generation. Interestingly, females exposed to these suboptimal diets produced a significantly higher number of eggs but the survival of their larvae was significantly reduced. It is evident from these results that modifications to the protein content and the nutrient composition profile of the original wheat germ-based insect artificial formula can be used to produce subtle negative effects on the growth of tobacco budworm.
Keywords: tobacco budworm, intrinsic rate of increase, development time, fertility, longevity, diet preparation and cost
Introduction
Insect artificial diets are essential tools for arthropod research. There are a wide range of commercial diets as well as published formulations. These diets have been developed to maximize insect growth and reproduction, by meeting or surpassing their minimum nutritional requirements. Therefore, these diets may mask potential developmental negative effects when they are used for reproductive studies that would be apparent only when the insects are exposed to sub-optimal diets. For example, insects reared on plant tissue might not develop as well as when they are reared on artificial diet because the plants' secondary chemistry and or lower nutritional value might arrest their optimal development (Blanco et al. 2008).
Soybean-flour/wheat-germ diets are commonly used to rear Noctuidae (Brewer and Martin 1976; Brewer and Tidwell 1978; Brewer and King 1979; Brewer 1981). The Shaver and Raulston (1971) formulation, an economical medium to produce large quantities of Lepidoptera, has been the basis for rearing multiple noctuid species in the USDA Agricultural Research Service facility in Stoneville, Mississippi for over 30 years. Over time, its original formula has been modified to meet different needs and to optimize costs.
Protein is an essential ingredient of the wheat germ-based diets and a component that can be easily manipulated without having considerable extra effort or expense because soybean flour and wheat germ ingredients amount to only ≈ 10% of the diet's cost. Soybean flour and wheat germ are commercially available in large quantities that, under proper storage, can produce consistent batches of diet through time. The appropriate protein content for rearing the tobacco budworm is ≈ 3% (Guerra and Bhuiya 1977). NutriSoy® flour is the main source of protein in this diet's formula as compared to wheat germ. By adjusting the proportion of NutriSoy and wheat germ the total protein of the diet can be reduced and this manipulation might result in small negative developmental and reproductive effects on Heliothis virescens (F.) (Lepidoptera:Noctuidae). In this way, a suboptimal diet could be developed that might stress the insect's development as compared to a diet composed entirely of plant tissue, that could allow the identification of physiological and reproductive effects caused by suboptimal nutrition for ecological and reproductive biology studies.
This study had the goal of lowering the protein content of the Shaver and Raulston (1971) formula, to decrease the reproductive potential of the tobacco budworm, such that artificial diet might more accurately reflect this insect's development on its plant hosts. Our hypothesis was that reduction of protein content would result in a decline in biological fitness of this species thus unmasking the effects of “over-nutrition” and allowing nonnutritionally-based fitness problems to be expressed.
Materials and Methods
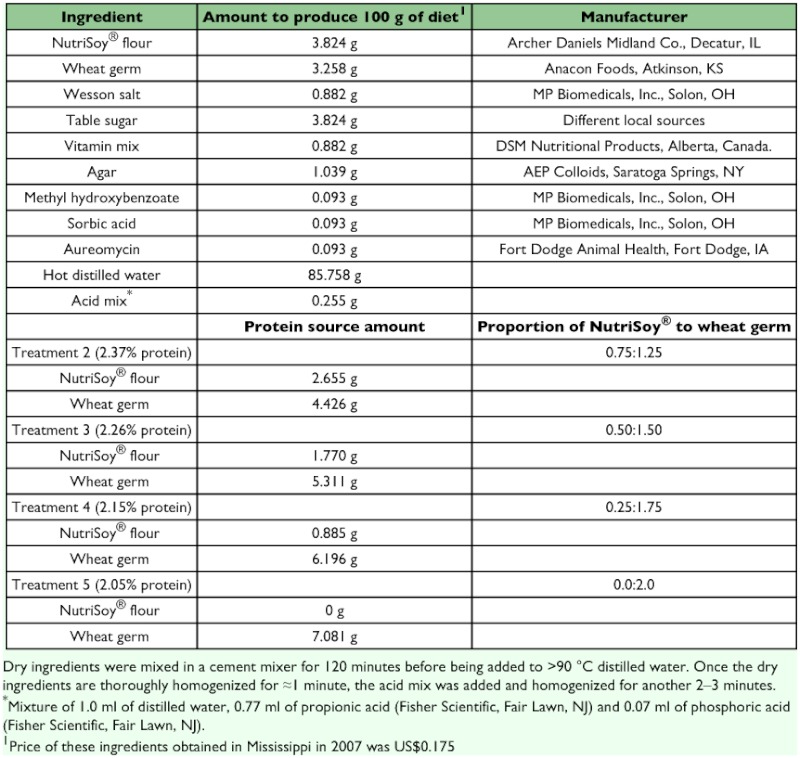
This study was conducted using the H. virescens colony of the USDA Agricultural Research Service of Stoneville, Mississippi, a colony that was initiated in 1971 from larval collection from wild host plants. Neonates (300 ≤ 16 hold) from the same cohort (P0 generation) were placed individually in plastic cups (37-ml [T-125 Solo® plastic soufflés, www.solocup.com]) containing ≈15 ml of one of the diets described in Table 1. These diets reduced the proportion of protein by reducing the amount of NutriSoy® (which contains 40–42% protein) and increasing the amount of wheat germ (which contains 29% protein). Cups were closed with cardboard lids and kept in an incubator at 27 (± 0.4) °C, 75 ± 10 % RH and a 14:10 L:D photoperiod. Larval developmental time, immature mortality (eggs, larvae, pupae), and adult eclosion and sex were recorded for the parental (P0), F1 and F2 generations on a daily basis.
Table 1.
List of ingredients and amounts of the modified Shaver and Raulston's diet.
A sample of recently emerged (≤ 72 h) F1 and F2 moths was used to set-up 18 pairs (replicates) from each treatment, except treatment 5 that had only 3 moths on the P0 generation. Individual pairs were held in 500 ml containers (Model 42505LY, Consolidated® Plastic Co., www.consolidatedplastics.com) with free access to 10% sucrose solution, capped with Batist cloth (Zweigart®, www.zweigart.com) and maintained in incubators as previously described. Cloths with eggs were replaced daily form each pair, the number of eggs was estimated visually and placed in sandwich bags (Ziploc® 94600, www.Ziploc.com) in incubators set as previously described for larval hatching. Egg visual estimation was previously determined based on the comparison of the following three eggs counts: a) experienced personnel made visual estimations of eggs on cloth, b) eggs were counted on 20% of the area of the same egg cloth samples under magnification with a microscope and counts were extrapolated to total area (65.03 cm2 ), and c) eggs on 20% of the area of the same egg cloth samples were counted using the software Image Pro-Plus for Windows and extrapolating to total area. The three different egg-counting methods did not differ statistically (F = 0.06, P = 0.94, df = 2, 24). Using the personnel's visual estimation departed 20.04 ± SEM 4.1% from the absolute counted area mean.
A set of 300 ≤ 16-h old neonates obtained simultaneously from the 18 pairs from the P0 and F1 generations was used to set-up the next generation for each treatment. Moth pairs were maintained in their containers until death to assess longevity. The position of the moth containers inside the incubators was arranged daily in a different pattern.
Demographic Rates of Increase
The net reproductive rate R0, represents the mean number of female offspring produced by each female during its entire lifetime (Carey 1993). It was calculated as:
The generation time (T), which is the mean interval separating the birth of one generation from the birth of the next, was calculated as:
Where survival (lx) is the probability of an individual attaining age x, and net fertility (mx) is the mean number of individual females produced per female of age x. The mx value was determined by multiplying the mean number of eggs produced per female at age x by their sex ratio.
The intrinsic rate of increase (rm), is a special case of a crude growth rate, where its exact r value was determined from survival and reproduction by substituting trial values of r based on life fecundity tables constructed using Lotka's equation (Carey 1993; Krebs 2001).
The intrinsic rate of increase was then used to calculate the finite rate of increase (λ), denoted as the population increment (female and male individuals/female/generation based on r value), and the doubling time (dt), which is the expression for geometrical increase.
Statistical Analysis
The net reproductive rate, developmental time, doubling time, gross and net fecundity were calculated by constructing individual life tables for each pair (1 ♀: 1 ♂ / replicate) per treatment per generation. Therefore, for the individual pair tables lx= 1 for all values of x, so R0 has the same value as mean age of gross fecundity (Mx) (mean number of individual females and males produced per female of age x) (Baker et al. 1992). To obtain more accurate results the values of the 300-unit sample size used to calculate the mortality of immature stages and sex ratio were incorporated into the life tables to estimate the lx and mx parameters (Portilla et al. 2000). Data (means ± SE) of demographic parameters presented in table 2 were analyzed using a one-way analysis of variance (ANOVA) by the general lineal model (GLM) procedure of SAS (9.1). Differences between least square means for all variables for each treatment and for each generation (calculated by the GLM procedure) were evaluated by t-test in SAS (9.1). Regression analyses were used to determine the correlation between net reproductive rate (R0) and soybean flour/wheat germ proportions (treatments).
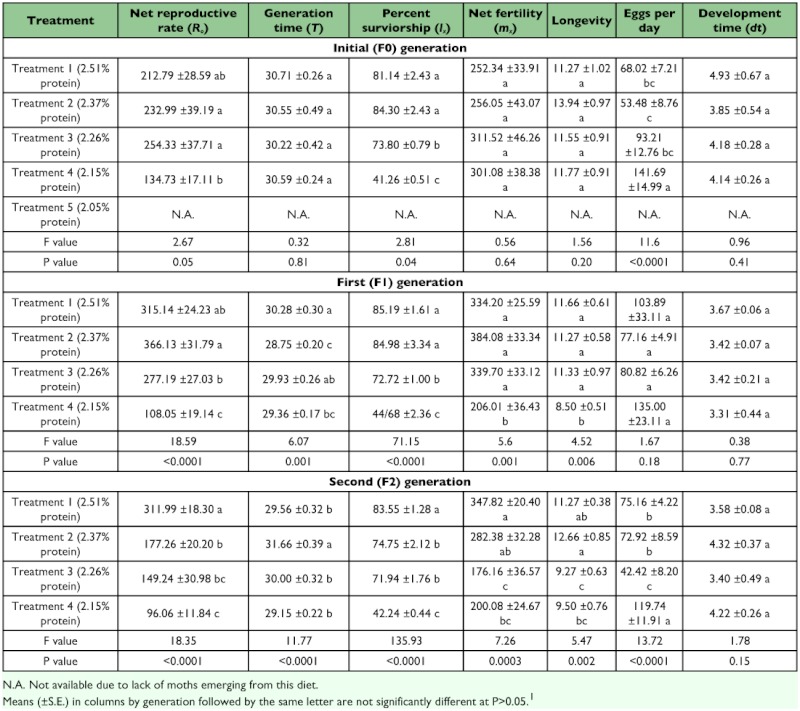
Table 2.
Effect of different diet's protein content on the development of three sequential generations of Heliothis virescens.
Results
Wheat germ alone (treatment 5, 2.05% protein, 0% soybean flour: 100% wheat germ content) could not maintain this tobacco budworm colony. Although a small proportion (1.3%) of larvae reached pupation, the resulting moths (1 ♀ and 2♂) did not produce progeny. The negative effect of this treatment was so drastic that its use would not result in the desired subtle effects for reproductive biology studies. Protein content manipulation might not be the only factor behind these results. The addition of extra amounts of wheat germ changed the nutritional composition profile of the diet due to the fact that wheat germ contains antinutrients and digestive enzyme inhibitors (Cohen 2004).
The net reproductive rate (R0), of larvae was negatively affected in the initial (P0) generation for treatments 2 (2.37% protein) and 4 (2.15% protein), and in the subsequent (F1) generation for treatment 3 (2.26% protein). Thus, a reduction in protein of ≥ 5.5% had a negative effect on reproduction. An interesting phenomenon was noticed with moths emerging from treatment 4. Those females laid a significantly higher number of eggs but larval survivorship (lx) was negatively affected (Table 2). In the first generation, the net reproductive rate (R0), development time, percent immature survivorship, net fertility (mx), intrinsic rate of increase (rm), finite rate of increase (λ) and female longevity were also significantly reduced with treatment 4, and some of these effects were also noticeable in treatment 3 as well (Tables 2 and 3). The same reproductive and developmental parameters were significantly affected in the second (F2) generation, with the exception of development time, further corroborating the effect of lower protein levels on development of H. virescens.
Table 3.
Effect of protein content on finite rate of natural increase and finite rate of increase of three sequential generations of Heliothis virescens.
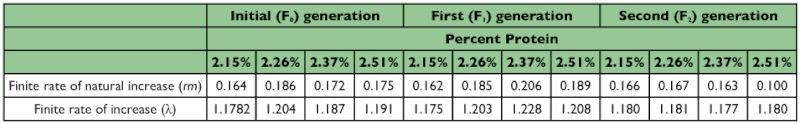
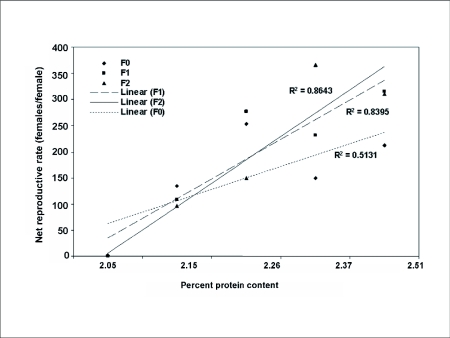
In the second generation, the effects on some of the above mentioned parameters began to be apparent in H. virescens fed treatment 2 (2.37% protein) (Table 2 and 3). Net reproductive rate, net fertility and longevity were negatively affected, while eggs per day significantly increased for treatment 3 (2.26% protein) following the same pattern previously found on the first generation with treatment 4 (2.15% protein). Regression analysis performed with net reproductive rate (R0) values made these trends also noticeable (Figure 1). The first generation was not greatly affected by the treatments (R2 = 0.51), but the subsequent generations due to the effect of suboptimal treatments (≤ 2.26% protein), produced a clear effect on net reproductive rate (R2 = 0.83 for F1 and R2 = 0.86 for F2). Similar tendencies were also noticed with two other important parameters, cumulative gross fecundity and immature survival that were negatively affected by some of the treatments when H. virescens were exposed for more than two generations to a suboptimal diet.
Figure 1.
Correlation between Net reproductive Rate (R0) of Heliothis virescens and the percent of protein content in insect artificial diets.
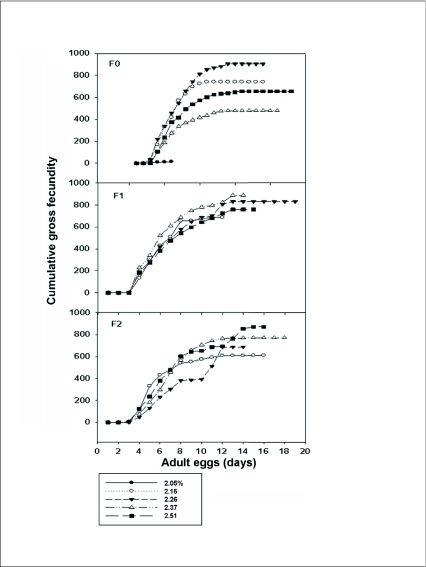
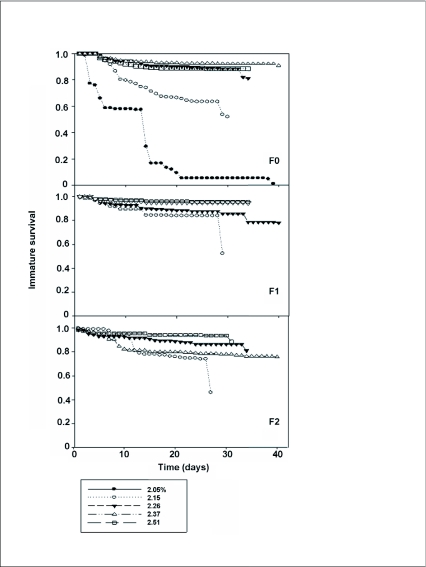
Figure 2 shows similar cumulative gross fecundity patterns for all treatments and generations where egg production rapidly reached its peak by day 11. The highest cumulative gross fecundity value was recorded for treatment 1 (2.51% protein) in the initial generation (654.62 individuals/female). Immature survival decreased considerably on larvae fed treatment 5 (2.05% protein) after the first day and became more pronounced after 14 days, whereas in treatment 4 for all generations the decrease was delayed until after the 25th day. The highest immature survival was obtained on individuals fed treatment 1 (all generations) and treatment 2 (initial and first generation) (Figure 3).
Figure 2.
Cumulative gross fecundity of Heliothis virescens affected by percent protein content in insect artificial diets throughout three generations.
Figure 3.
Immature survival of Heliothis virescens affected by the percent protein content in diets throughout three generations.
Discussion
Subtle differences between diet components can have an effect on noctuids' feeding preferences (Moore 1986) or on their development. An insect artificial diet that can produce suboptimal growth may reflect more accurately the development pattern of noctuids been fed certain plant tissue. Because most of the noctuids plant hosts contain some type of ‘noxious phytochemicals’ capable of interfering with proper nutrient assimilation on insects (Whittaker and Feeny 1971), a suboptimal insect artificial diet may simulate insect growth on plant tissue. Additions of naturally-occurring plant components such as gossypol (Gunasena et al. 1988) or nicotine (Gunasena et al. 1990) to insect artificial diet can also achieve lower developmental rates on certain insects as well, such as it is the case of the tobacco budworm. However, adding these plant components can be expensive or the specific chemicals might be difficult to obtain. Since wheat germ is an essential ingredient in many diets providing ≈30% less protein than soybean flour (Cohen 2004), an elevated proportion of this component, that provides ≥ 68% of the total protein content of the diet, can produce easilymeasured negative effects on the development of tobacco budworm such as percent survivorship (lx). Other easyto-document parameters such as longevity and egg production were not constant in all generations or appeared only after one generation (longevity), but are important quantifiable parameters that their differences can be noticeable with these suboptimal diets.
Manipulations of the diet's protein ingredients as described in this study might be one way to notice developmental and reproductive parameters that otherwise cannot be noticeable when insects are fed with diets that surpass all the minimum developmental requirements. One of the advantages of producing a suboptimal insect artificial diet that can simulate some of the effects found on H. virescens when fed on plant tissue is that an artificial diet may be more homogenous in its nutritional contents compared with the potential nutritional and/or chemical variation that the environment can produce on fieldgrown plants. The development of H. virescens on 2.05% protein closely simulated its development on garbanzo beans (Cicer arietinum) and white clover (Trifolium repens) (Blanco et al. 2008).
The interpretation of these results could be mitigated by the fact that the H. virescens colony used has been strongly selected to feed on the Sharver and Raulston (1971) diet for hundreds of generations.
Acknowledgments
The authors wish to thank Arnetrius Ellis, Gloria Patterson, Chad Roberts, Ada Peterson and Essanya Winder for their help conducting this study, and to Henry Hagedorn, Guadalupe Rojas, Antonio Terán-Vargas, Allen C. Cohen and three anonymous reviewers for their comments on the manuscript.
The views expressed in this article are those of the individual authors and do not necessarily reflect the views or policies of the U.S. Department of Agriculture. Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- Baker PS, Barrera JF, Rivas A. Life-history studies of the coffee berry borer (Hypothenemus hampei, Scolytidae) on coffee trees in southern Mexico. Journal of Applied Ecology. 1992;29:656–662. [Google Scholar]
- Blanco CA, Terán-Vargas AP, Abel CA, Portilla M, Rojas MG, Morales-Ramos JA, Snodgrass GL. Plant host effect on the development of Heliothis virescens (F.) (Lepidoptera: Noctuidae). Environmental Entomology. 2008;37 doi: 10.1603/0046-225x-37.6.1538. in press. [DOI] [PubMed] [Google Scholar]
- Brewer FD, Martin DF. Substitutes for agar in wheat germ diet used to rear the corn earworm and the sugarcane borer. Annals of the Entomological Society of America. 1976;69:255–256. [Google Scholar]
- Brewer FD, Tidwell AL. Evaluation of wheat, proflo, oats and seven soybean materials as a source of protein in a wheat germbased diet for Heliothis zea. Journal of the Georgia Entomological Society. 1978;13:7–12. [Google Scholar]
- Brewer FD, King EG. Consumption and utilization of soyflourwheat germ diets by Heliothis spp. Annals of the Entomological Society of America. 1979;72:415–417. [Google Scholar]
- Brewer FD. Development of Heliothis virescens and Diatraea saccharalis on a soyflour-corn oil diet. Annals of the Entomological Society of America. 1981;74:320–323. [Google Scholar]
- Carey FG. Applied demographic for biologists with special emphasis on insects. Oxford University Press; 1993. [Google Scholar]
- Cohen AC. Insect diets: science and technology. CRC Press LLC; 2004. [Google Scholar]
- Guerra AA, Bhuiya AD. Nutrition of the tobacco budworm: an economical larval diet for rearing. Journal of Economic Entomology. 1977;70:568–570. [Google Scholar]
- Gunasena GH, Vinson SB, Williams HJ, Stipanovic RD. Effects of caryophyllene, caryophyllene oxide, and their interaction with gossypol on the growth and development of Heliothis virescens (F.) (Lepidoptera: Noctuidae. Journal of Economic Entomology. 1988;81:93–97. [Google Scholar]
- Gunasena GH, Vinson SB, Williams Effects of nicotine on growth, development, and survival of the tobacco budworm (Lepidoptera: Noctuidae) and the parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Journal of Economic Entomology. 1990;83:1777–1782. [Google Scholar]
- Krebs CJ. Ecology: The experimental analysis of distribution and abundance. 5 edition. Wesley Longman; 2001. [Google Scholar]
- Moore RF. Feeding preferences and utilization studies as tools in developing an optimum diet for Heliothis zea (Lepidoptera: Noctuidae). Journal of Economic Entomology. 1986;79:1707–1710. [Google Scholar]
- Portilla M, Mumford J, Baker P. Reproductive potential response of continuous rearing of Hypothenemus hampei (Coleoptera: Scolytidae) developed using Cenobroca artificial diet. Revista Colombiana de Entomologia. 2000;26:29–105. [Google Scholar]
- SAS Statistical Analysis Software 2001. Version 9.1. SAS Institute; Cary, NC: [Google Scholar]
- Shaver TN, Raulston JR. A soybean-wheat germ diet for rearing the tobacco budworm. Annals of the Entomological Society of America. 1971;64:1077–1079. [Google Scholar]
- Whittaker RH, Feeny PP. Allelochemicals: chemical interactions between species. Science. 1971;171:757–770. doi: 10.1126/science.171.3973.757. [DOI] [PubMed] [Google Scholar]