Abstract
This article describes a simple and rapid cell patterning method to form co-culture microarrays in commercially available Transwells. A thin poly(dimethylsiloxane) (PDMS) layer is printed on the underside of a Transwell using a PDMS stamp. Arbitrary cellular patterns are generated according to the geometric features of the thin PDMS layer through hydrodynamic forces that guide cells onto the membrane only over the PDMS-uncoated regions. Micropatterns of surface-adhered cells (we refer to this as two-dimensional) or non-surface-adhered clusters of cells (we refer to this as three-dimensional) can be generated depending on the surface treatment of the filter membrane. Additionally, co-cultures can be established by introducing different types of cells on the membrane or in the bottom chamber of the Transwell. We show that this co-culture method can evaluate mouse embryonic stem (mES) cell differentiation based on heterogeneous cell–cell interactions. Co-culture of mES cells and HepG2 cells decreased SOX17 expression of mES cells, and direct cell–cell contact further decreased SOX17 expression, indicating that co-culture with HepG2 cells inhibits endoderm differentiation through soluble factors and cell–cell contact. This method is simple and user-friendly and should be broadly useful to study cell shapes and cell–cell interactions.
Introduction
Micropatterning of multiple cell types in defined spatial patterns allows studies to evaluate effects of heterocellular interactions as well as facilitate engineering of tissue constructs and integration of cells into microdevices.1–6 Geometric features of cells and cell aggregates play important roles in regulating various cell behaviors, including cell growth,1 differentiation,1,5,7 polarity,8,9 and migration.10,11 Despite its biological importance, multiple cell type co-culture patterning systems are not as commonly used at least in part because of the often tedious device fabrication and cell patterning steps required. Here we describe a simple and rapid cell micropatterning method that can form co-culture cell arrays using commercially available Transwells with minimal fabrication and patterning steps. In addition to the accessibility of the procedure, cellular patterning on Transwells has a useful feature that nutrient and stimulation can be applied from the basal side, which is crucial for biological response of some cell types.12–15
We first explore the utility of the device to perform single-cell-type patterning and then demonstrate micropatterned co-cultures in (i) side-by-side patterning mode and (ii) above-and-below mode, where one type of cell is micropatterned on the upper side of the Tranwell membrane and the second cell type is cultured on the floor of the lower compartment of the Transwell. Biological effects of this heterocellular co-culture micropatterning system are shown by evaluating mouse embryonic stem (mES) cell differentiation based on different types of cell–cell interactions provided by the different modes of co-culture. The mES cells showed lower expression of SOX17 when they were co-cultured with HepG2 cells in the above-and-below mode compared with culture of mES cells alone. Even further decrease in SOX17 expression was observed upon co-culture with direct cell–cell contact using the side-by-side co-culture mode. These results suggest that HepG2 cells inhibit endoderm differentiation through soluble factors and perhaps also by direct cell–cell contact. This research also demonstrates the versatility of this Transwell-based micropatterned co-culture technology.
Experimental
Cell culture
Monkey kidney fibroblast cells (COS7 cell line; ATCC), human hepatocarcinoma cells (HepG2 cell line; ATCC), and human epithelial carcinoma cells16 (H9 cell line) were cultured in Dulbecco's modified Eagle's medium (11965; Invitrogen) containing 10% v/v fetal bovine serum (10082; Gibco), 100 U/mL penicillin, and 100 U/mL streptomycin. mES cells stably transfected with SOX17-enhanced green fluorescent protein (EGFP) (D3 cell line; provided by Dr. SJ Morrison, University of Michigan) were cultured in the complete medium containing Dulbecco's modified Eagle's medium comprising 15% v/v fetal bovine serum, 0.1 mM 2-mercaptoethanol, 0.02% v/v sodium pyruvate, 1% v/v nonessential amino acids, 100 U/mL penicillin, 100 U/mL streptomycin, and 1000 U/mL ESGRO, which contains leukemia inhibitory factor in a humidified incubator. When mES cells were introduced to differentiate, mES cells were co-cultured with HepG2 cells in the complete medium without leukemia inhibitory factor. Cells were stained with 1.5 μM CellTracker red CMTPX (Invitrogen), 10 μM CellTracker green CMFDA (Invitrogen), or 1 μM Calcein-AM (Invitrogen) for 1 h.
Fabrication of cell arrays on Transwell
The stamps were fabricated from poly(dimethylsiloxane) (PDMS) formed from prepolymer (Sylgard 184; Dow Corning) at a ratio of 1:10 base to curing agent using a soft lithography method. The relief features of the mold (50 μm in height) were composed of SU-8 (Microchem) patterns formed on a silicon wafer. A mixture of toluene and PDMS prepolymer with a volume ration of 3:2 was spin-coated (1500 rpm for 60 s) onto a glass slide to cover the slide with a thin layer of uncured PDMS (∼5 μm).17,18 A PDMS stamp was placed onto the coated glass slide for 5 s to transfer the uncured PDMS onto the surface of the stamp (see Fig. 1A). The coated stamp was put on a membrane of Transwell for a few seconds and peeled off to transfer the uncured PDMS pattern onto the membrane. The Transwell inserts were put into an oven at 60°C for 2 h to cure liquid PDMS. Before cell seeding, Transwell inserts were treated with Fibronectin solution (Invitrogen) at a concentration of 100 μg/mL in phosphate-buffered saline for 1 h or 1% solution of Pluronic F108 (BASF) for 1 h.
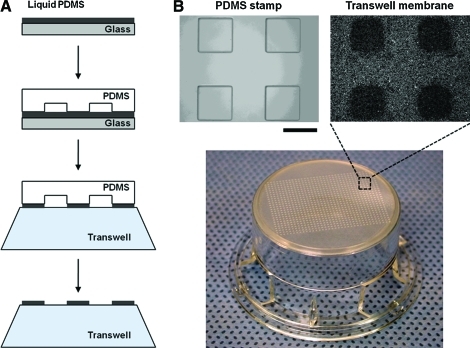
FIG. 1.
(A) Schematic illustration of fabrication procedure of a cell array. A thin layer of uncured PDMS is patterned on a Transwell membrane using a PDMS stamp. (B) Actual images of the PDMS stamp and the pattern formed on the Transwell membrane. Scale bar: 300 μm. PDMS, poly(dimethylsiloxane). Color images available online at www.liebertonline.com/ten.
Evaluation of mES cell differentiation
mES cell aggregates were imaged by fluorescence microscopy after 7 days in culture. Image analysis was carried out using MetaMorph software (Universal Imaging) to evaluate relative EGFP intensity of mES cell aggregates. Eighty cell aggregates in 5 wells were used to analyze EGFP expression. Obtained data were represented as mean ± standard deviation. The data were analyzed by analysis of variance followed by post hoc Tukey's multiple comparison test at a 99% confidence interval.
Results and Discussion
Formation of cell arrays on Transwell
Figure 1A shows a schematic illustration of the fabrication procedure of a patterned cell array. A thin layer of uncured PDMS17,18 is transferred onto the bottom of a Transwell membrane using a PDMS stamp having desired geometric features. Figure 1B shows actual images of the PDMS stamp, the Transwell membrane, and the Transwell. The black squares on the Transwell membrane are PDMS-uncoated regions. The PDMS pattern on the membrane is in good agreement with the PDMS stamp geometry.
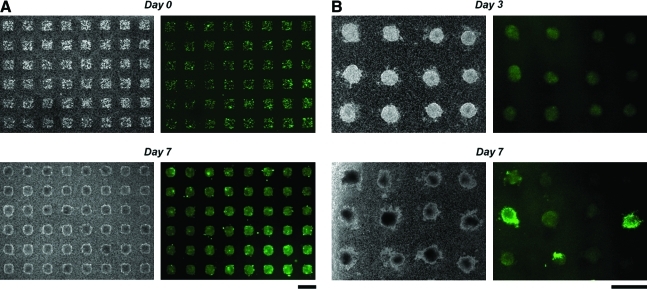
Figure 2A shows time-lapse images of an array of COS7 cells stained with Calcein-AM, which labels viable cells. Cells are organized into patterns on the membrane immediately after seeding because of hydrodynamically guided cell settling onto the non-PDMS-coated regions of the membrane. Here, the liquid level in the Transwell is maintained higher than the outside reservoir inducing gravity-driven flow of liquid through the Transwell membrane guiding sedimentation. Further, although the entire membrane was treated with Pluronic, cells were able to adhere somewhat on the membrane regions not stamped with PDMS, indicating that Pluronic is adsorbed primarily on PDMS, and less so on the hydrophilic polyester membrane itself. COS7 cells kept their pattern for 7 days and formed three-dimensional (3D) aggregates while maintaining their viability. Figure 2B shows time-lapse images of an array of mES cells stably transfected with SOX17-EGFP. mES cells formed 3D aggregates and their SOX17 expression increased, indicating that mES cells were gradually differentiating.
FIG. 2.
Formation of cell arrays on Transwells. (A) Optical and fluorescent images of an array of COS7 cells stained with Calcein-AM. Images were taken right after and 7 days after seeding the cells. (B) Optical and fluorescent images of an array of mES cells stably transfected with SOX17-EGFP on days 3 and 7. Scale bars: 500 μm. mES, mouse embryonic stem. EGFP, enhanced green fluorescent protein. Color images available online at www.liebertonline.com/ten.
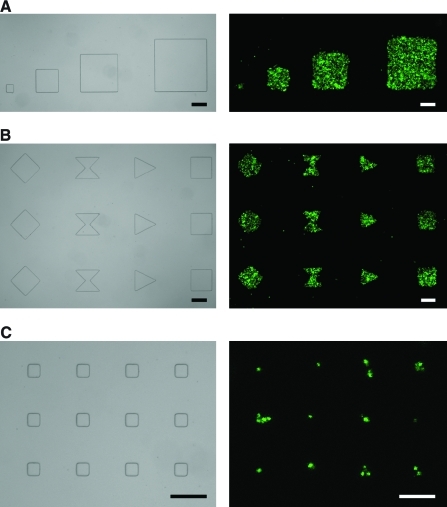
Cells can be controlled to organize into arbitrary geometries. Figure 3 shows images of various cell patterns formed after seeding. Sizes and shapes of cell patterns are in good agreement with the PDMS stamp geometry. As seen in Figure 3C, single-cell patterning is also possible when the size of squares is around 50 μm.
FIG. 3.
Arbitrary geometry control of cell patterning. (A–C) Optical images of PDMS stamps and fluorescent images of cells patterned on a membrane of Transwell. COS7 cells were labeled with Calcein-AM. The sizes of squares: (A) 100, 300, 500, 700 μm; (B) 300 μm; (C) 50 μm. Scale bars: 200 μm. Color images available online at www.liebertonline.com/ten.
Co-culture cell arrays on Transwell
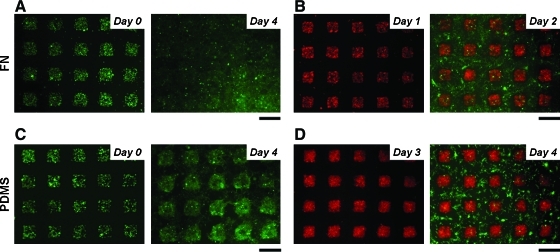
Figure 4 shows images of cellular patterning on membranes treated with (A, B) or without fibronectin (C, D). COS7 cells were labeled with CellTracker green. We note that cell adhesion occurs in patterns according to the PDMS features stamped on the membranes regardless of whether we uniformly apply a cell-adhesive fibronectin coating. This further indicates that the initial patterning is mainly hydrodynamic rather than based on differences in surface adhesiveness. Fluid flows from the insert through the membrane into the bottom chamber; thus, flow hydrodynamically focuses cells onto the PDMS-uncoated region of the membrane. COS7 cells patterned hydrodynamically on uniformly fibronectin-coated membrane immediately started to spread out beyond their initial pattern to fully cover the membrane by day 4 (Fig. 4A). On the other hand, COS7 cells patterned hydrodynamically on the membrane without fibronectin treatment kept their patterns for several days and started to spread out beyond their initial patterns later, on day 4, suggesting that COS7 cells start to adhere on PDMS regions after 3 days in culture (most likely promoted by protein absorption onto the PDMS19) (Fig. 4C). A key point to realize is that there are two factors that direct cell patterning. (1) Hydrodynamic patterning of initial cell positions. Here, the entire surface can be equally cell adhesive (fibronectin coated); yet, cells attachment will be patterned because where cells are seeded is patterned. (2) Surface chemistry-mediated cell patterning. Here, different parts of the surface have different ability to support cell adhesion. Thus, even if the cell seeding is uniform, attachment of cells will be patterned. (3) We can use a combination of hydrodynamic and surface-mediated effects to pattern cells. We conducted co-cultures on membranes with or without fibronectin using COS7 cells and HepG2 cells labeled with CellTracker red. Hepatocytes and fibroblasts are often used as a co-culture model because co-cultures help maintaining hepatic functions. Therefore, we used hepatic cancer cell line HepG2 and fibroblast cell line COS7 to demonstrate control of co-cultures. In the case of fibronectin-treated membranes, COS7 cells were introduced 1 day after patterning HepG2 cells. As seen in Figure 4B, HepG2 cells were on PDMS-uncoated square regions and COS7 cells adhered on PDMS-coated regions. The HepG2 cells were attached and spread on the membrane, thus presenting a two-dimensional (2D) mode of growth. In the case of nontreatment with fibronectin, COS7 cells were introduced 3 days after patterning HepG2 cells. As seen in Figure 4D, COS7 cells can still adhere and spread out on the PDMS-coated regions in a 2D growth mode, whereas HepG2 cells remain confined to PDMS-uncoated regions in a 3D cell aggregate mode. The combined use of hydrodynamic patterning together with different surface treatments and adhesive protein coatings provides versatility in co-culture patterning capabilities that should be applicable to a broad range of cell types.
FIG. 4.
Co-culture arrays on Transwell. The surface of the membrane was treated with (A, B) or without (C, D) fibronectin. COS7 cells were labeled with CellTracker green and HepG2 cells were labeled with CellTracker red. (A) Cells spread out over the PDMS pattered surface, and thus the cellular patterns disappeared on day 4. (B) COS7 cells were seeded 1 day after HepG2 cells had been patterned. (C, D) COS7 cells gradually spread out over the PDMS patterned surface without fibronectin coating after 4 days in culture, whereas HepG2 cells do not spread into the PDMS regions. (D) COS7 cells were seeded 3 days after HepG2 cells had been patterned. Scale bars: 300 μm. Color images available online at www.liebertonline.com/ten.
Local stimulation of a cell sheet
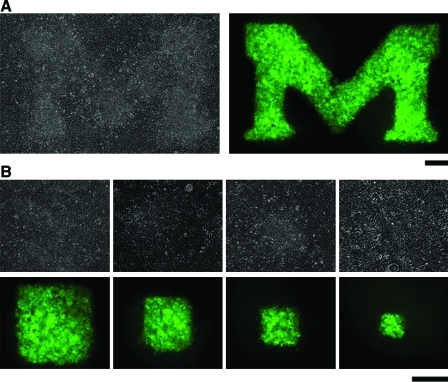
Our system also can apply local chemical stimulation to cells on the membrane from the basal side. Figure 5 shows optical and fluorescent images after local exposure of fluorescent dye. H9 cells were cultured on a membrane treated with fibronectin with minimization of hydrodynamic patterning by maintaining equal levels of liquid inside the Transwell as well as in the outside reservoir, so as to fully cover the surface of the membrane. Cell Tracker green was introduced into the bottom chamber of the Transwell and incubated for 10 min. Culture medium in the bottom chamber was replaced by phosphate-buffered saline before taking images. Cells were clearly stained only on the PDMS-uncoated regions. The size of stained cells was in good agreement with patterns produced by PDMS stamping. Thus, this system can be utilized for localized biochemical stimulation of cells from their basal side. This local stimulation should be useful for applications such as study of cell–cell communication in cellular networks.20
FIG. 5.
Localized staining of cell monolayer from the underside of a PDMS micropatterned Transwell. A confluent layer of H9 cells was formed on a PDMS patterned Transwell. M-shape (A) or different sizes of patterns (B) were generated on the Transwell membrane by PDMS stamps. (B) The sizes of square patterns on the membrane are 200, 300, 400, and 600 μm. CellTracker green was added into the bottom chamber of the Transwell. Scale bars: 300 μm. Color images available online at www.liebertonline.com/ten.
Evaluation of mES cellular differentiation in co-cultures
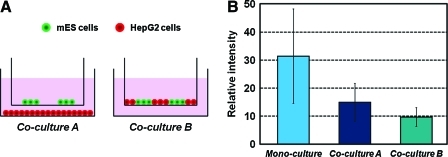
We applied this micropatterned Transwell system to evaluate ES cell differentiation based on different modes of heterocellular interactions: either with or without direct cell–cell contact. mES cells stably transfected with SOX17-EGFP were patterned hydrodynamically on a membrane without any surface treatment as an array and cultured for 3 days to form cell aggregates. HepG2 cells were introduced either onto the top side of the Transwell membrane (side-by-side mode with contact) or into a bottom chamber (above-and-below mode without contact), and they were co-cultured for 4 days (see Fig. 6A). SOX17 expression of mES cells was quantified by intensity of EGFP. The graph shows that co-culture with HepG2 cells decreased SOX17 expression in mES cells and that direct cell–cell contact decreased SOX17 expression even further. These results suggest that HepG2 cells inhibit endoderm differentiation of mES cells through soluble factors and that co-culturing with cell–cell contact further inhibits this process mostly likely by increasing concentrations of key secreted factors, likely also through direct contact effects, and likely by reducing cell proliferation rate of mES cells through HepG2 cell expressing Glypican-3.21 This corresponds to previous reports that a HepG2 cell-conditioned medium induces mesoderm formation and reduces formation of other lineages.22,23
FIG. 6.
Evaluation of mES cell differentiation. (A) Schematic illustration of experimental conditions. mES cells stably transfected with SOX17-EGFP were cultured in the top chamber for 3 days to form semi-spherical aggregates. HepG2 cells were then introduced into either the bottom chamber (Co-culture A, above-and-below mode) or the top chamber (Co-culture B, side-by-side mode) on day 3 and they were co-cultured for 4 days. (B) Relative EGFP intensity of mES cells. mES cells were cultured without (mono-culture) or with HepG2 cells for 7 days. Values represent the mean ± standard deviation. All three conditions are significantly different. p < 0.0001. Color images available online at www.liebertonline.com/ten.
Conclusion
A variety of recent work highlight the importance of controlling 2D and 3D size and shape of cellular niches to guide cell fate.24–32 For example, microwell systems have been utilized for ES cell studies to control the formation of ES cellular aggregates because the size of aggregates regulates ES cellular differentiation.33–35 Here, we present a simple and rapid cell patterning method to provide versatile control over the geometry of cellular niches formed using readily available Transwells. Depending on the type of surface treatment performed on the PDMS-stamped filter membrane, region-selectively sieved cells form micropatterns of cells that are firmly adhered and spread in 2D or just loosely attached and growing as 3D clusters of cells. Another important regulator of cellular niches is the type and geometry of heterocellular interactions.5,36–38 The patterned filter membrane of the Transwell, which allows convective flow of fluid to hydrodynamically guide cells during the initial cell seeding stage, also provides a means of diffusive chemical communication between cells in the upper and lower chambers during subsequent co-culture studies. A second cell type could alternatively be seeded on an existing pattern of cells in the upper chamber to form side-by-side co-cultures with direct contact between the different cell types. We demonstrated the usefulness of these capabilities by modulating mES cell differentiation. The methods are simple, user-friendly, and versatile and are envisioned to be broadly useful for engineering cellular niches comprised of various shapes, sizes, and heterocellular interactions.
Acknowledgments
Research was supported by NIH grants R01HL084370-05 and R01CA136829-02. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Dr. Sean J Morrison for SOX17-EGFP ES cells and the BASF Corporation for the Pluronic.
Disclosure Statement
No competing financial interests exist.
References
- 1.Shen C.J. Fu J. Chen C.S. Patterning cell and tissue function. Cell Mol Bioeng. 2008;1:15. [Google Scholar]
- 2.Torres A.J. Wu M. Holowka D. Baird B. Nanobiotechnology and cell biology: micro- and nanofabricated surfaces to investigate receptor-mediated signaling. Annu Rev Biophys. 2008;37:265. doi: 10.1146/annurev.biophys.36.040306.132651. [DOI] [PubMed] [Google Scholar]
- 3.Goubko C.A. Cao X. Patterning multiple cell types in co-cultures. Mater Sci Eng C. 2009;29:1855. [Google Scholar]
- 4.Nichol J.W. Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009;5:1312. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavana H. Mosadegh B. Takayama S. Direct printing of multiple heterocellular embryonic stem cell microenvironments. Adv Mater. 2010;22:2628. doi: 10.1002/adma.200904271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torisawa Y. Mosadegh B. Luker G.D. Morell M. O'Shea K.S. Takayama S. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr Biol. 2009;1:649. doi: 10.1039/b915965g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz S.A. Chen C.S. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson C.M. VanDuijn M.M. Inman J.L. Fletcher D.A. Bissell M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James J. Goluch E.D. Hu H. Liu C. Mrksich M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell Motil Cytoskeleton. 2008;65:841. doi: 10.1002/cm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X. Bruzewicz D.A. Wong A.P. Piel M. Whitesides G.M. Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci USA. 2005;102:975. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmud G., et al. Directing cell motions on micropatterned ratchets. Nat Phys. 2009;5:606. [Google Scholar]
- 12.Wang J. Loberg R. Taichman R.S. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25:573. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 13.Christopher M.J. Liu F. Hilton M.J. Long F. Link D.C. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J.W., et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS ONE. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang K.-J. Suh K.-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 16.Ho M., et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 17.Wu H. Huang B. Zare R.N. Construction of microfluidic chips using polydimethylsiloxane for adhesive bonding. Lab Chip. 2005;5:1393. doi: 10.1039/b510494g. [DOI] [PubMed] [Google Scholar]
- 18.Chueh B. Huh D. Kyrtsos C.R. Houssin T. Futai N. Takayama S. Leakage-free bonding of porous membranes into layer microfluidic array systems. Anal Chem. 2007;79:3504. doi: 10.1021/ac062118p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson C.M. Raghavan S. Tan J.L. Chen C.S. Degradation of micropatterned surfaces by cell-dependent and -independent processes. Langmuir. 2003;19:1493. [Google Scholar]
- 20.Takoh K. Ishibashi T. Matsue T. Nishizawa M. Localized chemical stimulation of cellular micropatterns using a porous membrane-based culture substrate. Sens Actuat B. 2005;108:683. [Google Scholar]
- 21.Zittermann S.I. Capurro M.I. Shi W. Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126:1291. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 22.Rathjen J. Lake J.-A. Bettess M.D. Washington J.M. Chapman G. Rathjen P.D. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112:601. doi: 10.1242/jcs.112.5.601. [DOI] [PubMed] [Google Scholar]
- 23.Hwang Y.-S. Randle W.L. Bielby R.C. Polak J.M. Mantalaris A. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng. 2006;12:1381. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 24.Griffith L.G. Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K.M. Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Folch A. Toner M. Microengineering of cellular interactions. Annu Rev Biomed Eng. 2000;2:227. doi: 10.1146/annurev.bioeng.2.1.227. [DOI] [PubMed] [Google Scholar]
- 27.Charnley M. Textor M. Khademhosseini A. Lutolf M.P. Integration column: microwell arrays for mammalian cell culture. Integr Biol. 2009;1:625. doi: 10.1039/b918172p. [DOI] [PubMed] [Google Scholar]
- 28.Mohr J.C. de Pablo J.J. Palecek S.P. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda J., et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 30.Khetani S.R. Bhatia S.N. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 31.Tan C.P. Seo B.R. Brooks D.J. Chandler E.M. Craighead H.G. Fischbach C. Parylene peel-off arrays to probe the role of cell–cell interactions in tumour angiogenesis. Integr Biol. 2009;1:587. doi: 10.1039/b908036h. [DOI] [PubMed] [Google Scholar]
- 32.Gallego-Perez D., et al. High throughput assembly of spatially controlled 3D cell clusters on a micro/nanoplatform. Lab Chip. 2010;10:775. doi: 10.1039/b919475d. [DOI] [PubMed] [Google Scholar]
- 33.Park J., et al. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 2007;7:1018. doi: 10.1039/b704739h. [DOI] [PubMed] [Google Scholar]
- 34.Bauwens C.L., et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 35.Hwang Y.-S. Chung B.G. Ortmann D. Hattori N. Moeller H.-C. Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci USA. 2009;106:16978. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao A.Y. Torisawa Y. Tung Y.-C. Sud S. Taichman R.S. Pienta K.J. Takayama S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30:3020. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.Y. Tuleuova N. Jones C.N. Ramanculov E. Zern M.A. Revzin A. Directing hepatic differentiation of embryonic stem cells with protein microarray-based co-cultures. Integr Biol. 2009;1:460. doi: 10.1039/b905757a. [DOI] [PubMed] [Google Scholar]
- 38.Cho C.H., et al. Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells: applications for the treatment of liver failure. FASEB J. 2008;22:898. doi: 10.1096/fj.06-7764com. [DOI] [PubMed] [Google Scholar]