Abstract
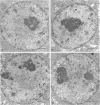
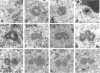
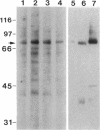
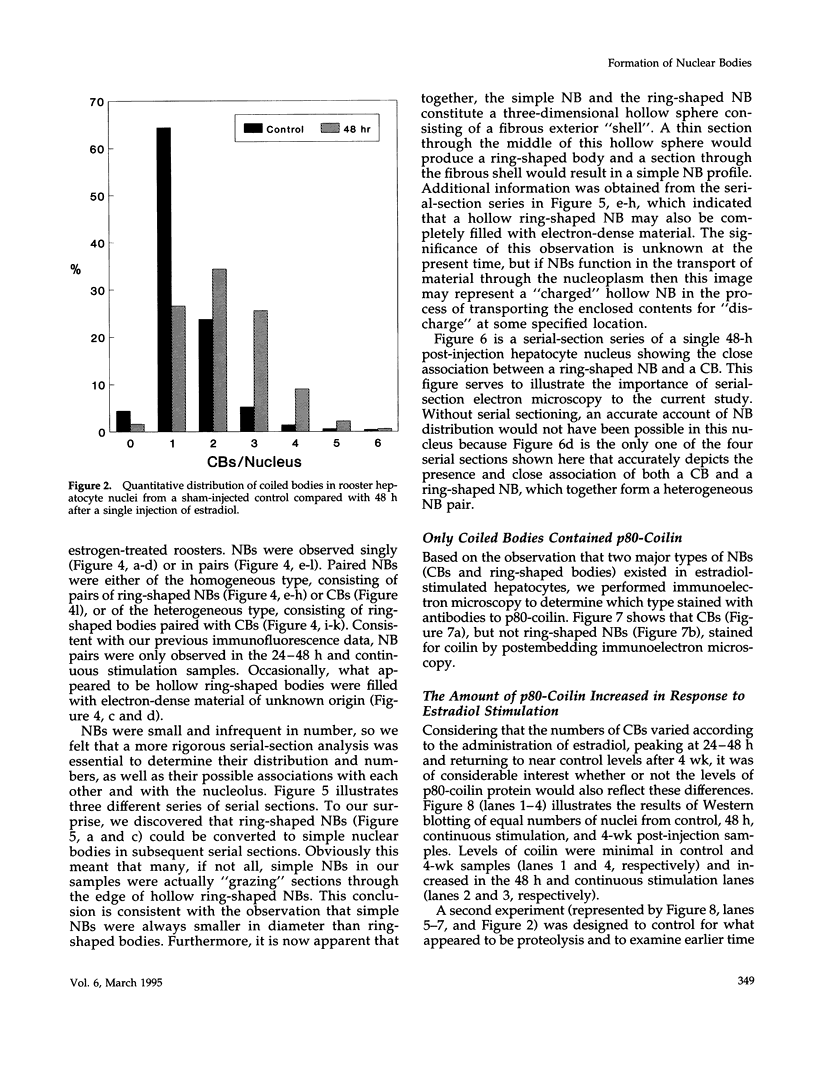
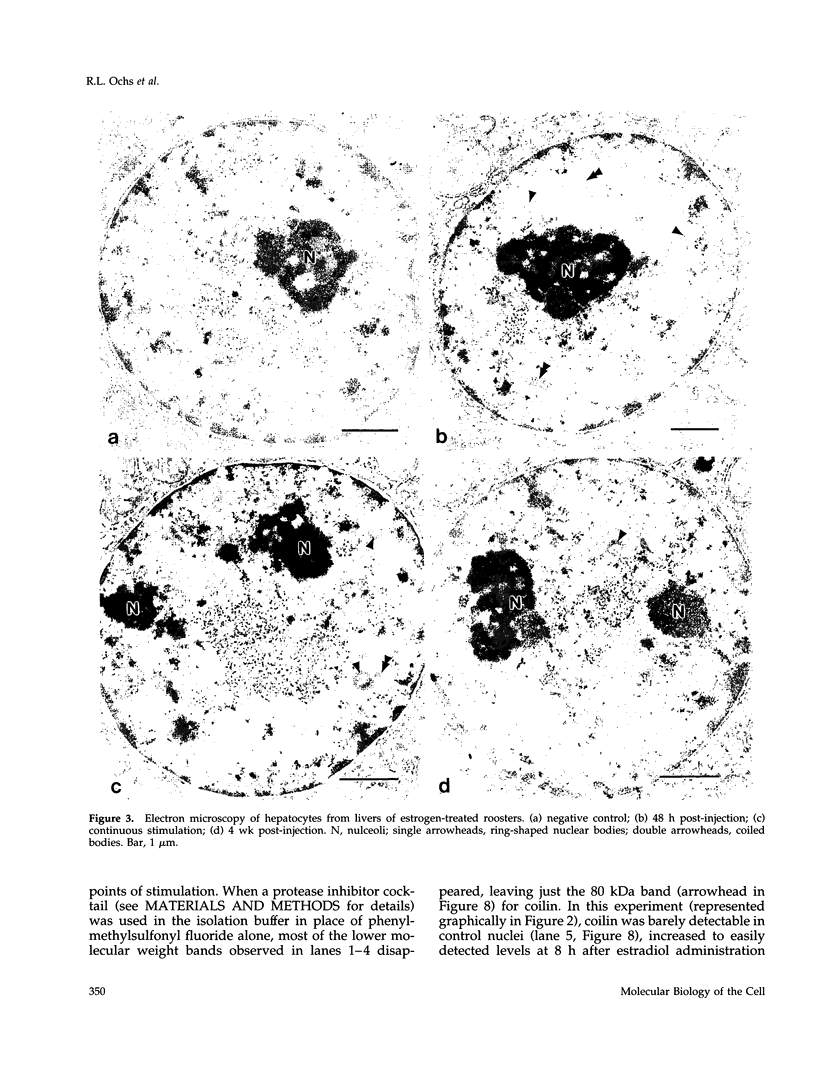
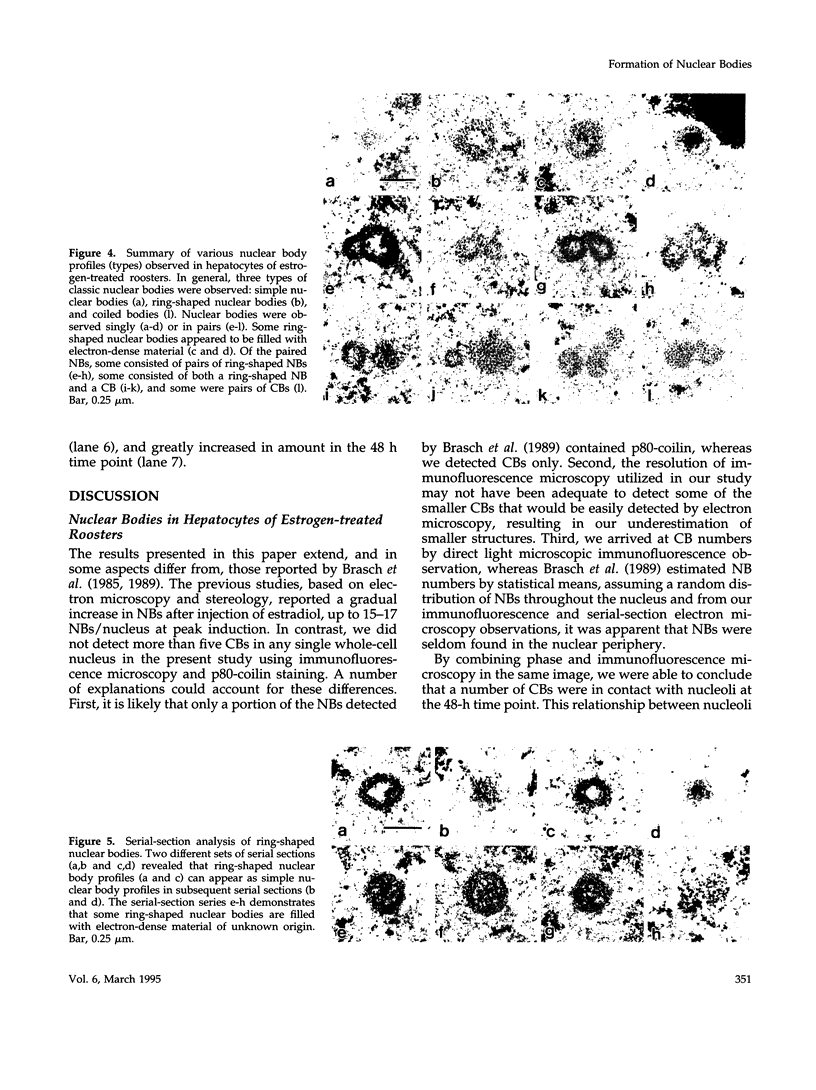
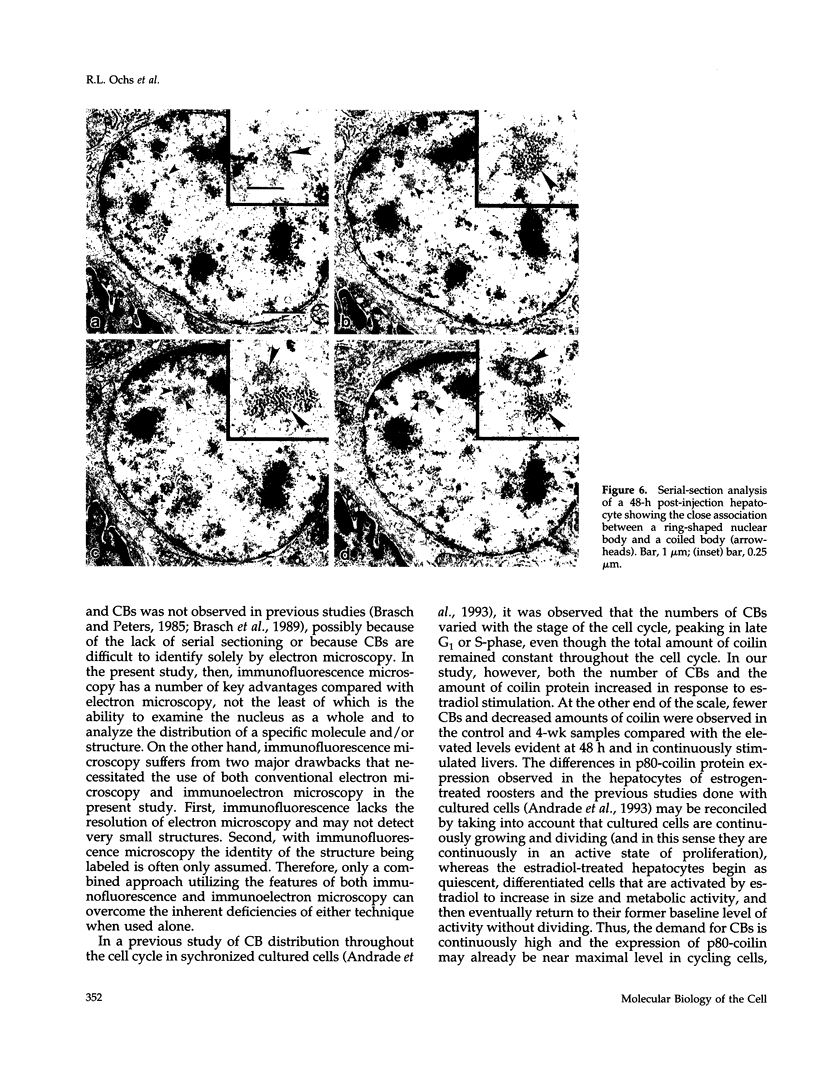
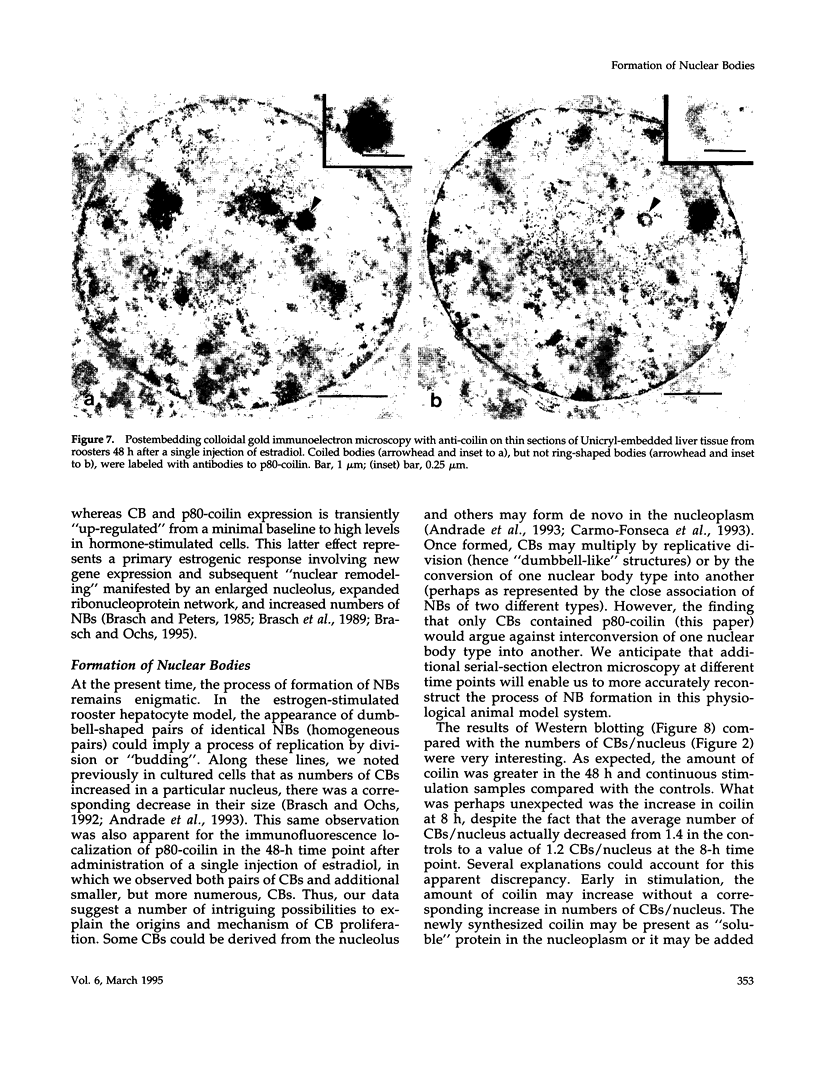
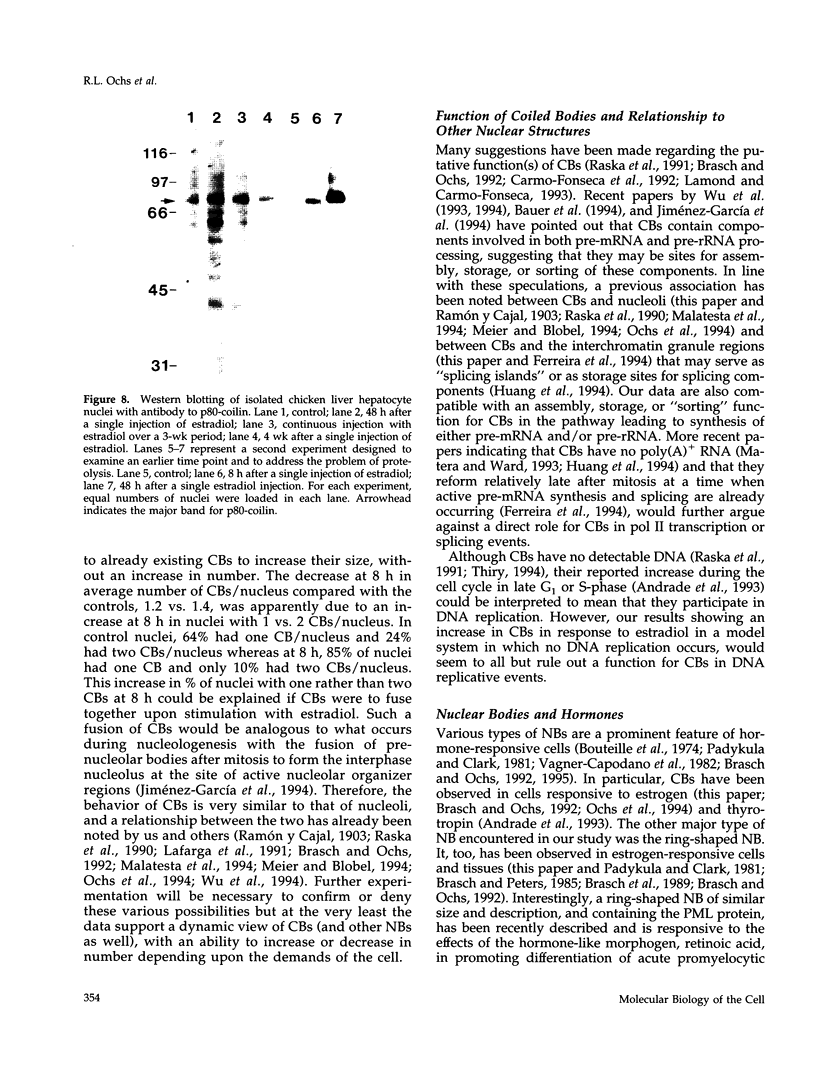
As a model for cellular growth and stimulation without accompanying proliferation, we have examined the induction and formation of nuclear bodies (NBs) in hepatocytes of estrogen-treated roosters. Four-week-old roosters were injected with a single intramuscular dose of estradiol and then killed at time points of 8 h, 48 h, and 4 wk post-injection. For immunofluorescence analyses, livers were excised and isolated hepatocyte nuclei were fixed and then labeled with antibody to the coiled body-specific protein, p80-coilin. In control animals (no estradiol) or in animals 8 h post-injection, each hepatocyte nucleus contained an average of 1.0 coiled body (CB), which appeared randomly distributed in the nucleoplasm. At 48 h post-injection, there were an average of 2.7 CBs/nucleus and many of these appeared to be in contact with the nucleolus. Pairs of CBs were also observed. By 4 wk post-injection an average of 1.5 CBs/nucleus were detected, with no apparent relationship to the nucleolus observed. By serial-section electron microscopy of intact livers, two different types of round NBs were observed, sometimes in close proximity to each other and to the expanded interchromatin granule region in maximally stimulated cells. One type of NB was a classical CB that averaged 0.35 microns in diameter and the other NB type was ring shaped, averaged 0.25 microns in diameter, was composed of a fibrous shell surrounding a hollow interior, and appeared as a simple NB when sectioned tangentially through its outer shell. Immunoelectron microscopy revealed that CBs were the only class of NBs that contained p80-coilin. From these data, we conclude that CBs proliferate in response to estrogen stimulation, possibly arising from the nucleolar surface and then increasing in number by replicative division.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Tan E. M., Chan E. K. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli C. A., Maul G. G. Identification of a novel nuclear domain. J Cell Biol. 1991 Mar;112(5):785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. W., Murphy C., Wu Z., Wu C. H., Gall J. G. In vitro assembly of coiled bodies in Xenopus egg extract. Mol Biol Cell. 1994 Jun;5(6):633–644. doi: 10.1091/mbc.5.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch K., Harrington S., Blake H. Isolation and analysis of nuclear bodies from estrogen-stimulated chick liver. Exp Cell Res. 1989 Jun;182(2):425–435. doi: 10.1016/0014-4827(89)90247-4. [DOI] [PubMed] [Google Scholar]
- Brasch K. Nuclear protein modifications in vitellogenic rooster liver. Cell Mol Biol. 1990;36(6):659–671. [PubMed] [Google Scholar]
- Brasch K., Ochs R. L. Nuclear bodies (NBs): a newly "rediscovered" organelle. Exp Cell Res. 1992 Oct;202(2):211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Brasch K., Ochs R. L. Nuclear remodeling in response to steroid hormone action. Int Rev Cytol. 1995;159:161–194. doi: 10.1016/s0074-7696(08)62107-5. [DOI] [PubMed] [Google Scholar]
- Brasch K., Peters K. E. Nuclear protein, matrix and structural changes in rooster liver after estrogenic induction of vitellogenesis. Biol Cell. 1985;54(2):109–121. doi: 10.1111/j.1768-322x.1985.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Kupfer D. Estrogenic action of DDT analogs. Am J Ind Med. 1983;4(1-2):163–173. [PubMed] [Google Scholar]
- Burch J. B., Evans M. I. Chromatin structural transitions and the phenomenon of vitellogenin gene memory in chickens. Mol Cell Biol. 1986 Jun;6(6):1886–1893. doi: 10.1128/mcb.6.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Ferreira J., Lamond A. I. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993 Feb;120(4):841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. I., O'Malley P. J., Krust A., Burch J. B. Developmental regulation of the estrogen receptor and the estrogen responsiveness of five yolk protein genes in the avian liver. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8493–8497. doi: 10.1073/pnas.84.23.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck F., Jr, Ricci A., Jr, Wolff M. S., Godbold J., Deckers P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Environ Health. 1992 Mar-Apr;47(2):143–146. [PubMed] [Google Scholar]
- Ferreira J. A., Carmo-Fonseca M., Lamond A. I. Differential interaction of splicing snRNPs with coiled bodies and interchromatin granules during mitosis and assembly of daughter cell nuclei. J Cell Biol. 1994 Jul;126(1):11–23. doi: 10.1083/jcb.126.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Deerinck T. J., Ellisman M. H., Spector D. L. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J Cell Biol. 1994 Aug;126(4):877–899. doi: 10.1083/jcb.126.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Segura-Valdez M. L., Ochs R. L., Rothblum L. I., Hannan R., Spector D. L. Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell. 1994 Sep;5(9):955–966. doi: 10.1091/mbc.5.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Moncharmont B., Jiricny J., Saluz H., Hertner T. In vitro secondary activation (memory effect) of avian vitellogenin II gene in isolated liver nuclei. Proc Natl Acad Sci U S A. 1986 Jan;83(1):43–47. doi: 10.1073/pnas.83.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M. H., Puvion-Dutilleul F., Guillemin M. C., Viron A., Linares-Cruz G., Stuurman N., de Jong L., Szostecki C., Calvo F., Chomienne C. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994 Mar 1;13(5):1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Andres M. A., Berciano M. T., Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol. 1991 Jun 15;308(3):329–339. doi: 10.1002/cne.903080302. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Carmo-Fonseca M. The coiled body. Trends Cell Biol. 1993 Jun;3(6):198–204. doi: 10.1016/0962-8924(93)90214-l. [DOI] [PubMed] [Google Scholar]
- Malatesta M., Zancanaro C., Martin T. E., Chan E. K., Amalric F., Lührmann R., Vogel P., Fakan S. Is the coiled body involved in nucleolar functions? Exp Cell Res. 1994 Apr;211(2):415–419. doi: 10.1006/excr.1994.1106. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Ward D. C. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J Cell Biol. 1993 May;121(4):715–727. doi: 10.1083/jcb.121.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994 Dec;127(6 Pt 1):1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Stein T. W., Jr, Tan E. M. Coiled bodies in the nucleolus of breast cancer cells. J Cell Sci. 1994 Feb;107(Pt 2):385–399. doi: 10.1242/jcs.107.2.385. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Andrade L. E., Chan E. K., Burlingame R., Peebles C., Gruol D., Tan E. M. Association between the nucleolus and the coiled body. J Struct Biol. 1990 Jul-Sep;104(1-3):120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Robison A. K., Sirbasku D. A., Stancel G. M. DDT supports the growth of an estrogen-responsive tumor. Toxicol Lett. 1985 Sep;27(1-3):109–113. doi: 10.1016/0378-4274(85)90127-4. [DOI] [PubMed] [Google Scholar]
- Shapiro D. Steroid hormone regulation of vitellogenin gene expression. CRC Crit Rev Biochem. 1982 Mar;12(3):187–203. doi: 10.3109/10409238209108706. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry M. Cytochemical and immunocytochemical study of coiled bodies in different cultured cell lines. Chromosoma. 1994 Jul;103(4):268–276. doi: 10.1007/BF00352251. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner-Capodano A. M., Bouteille M., Stahl A., Lissitzky S. Nucleolar ribonucleoprotein release into the nucleoplasm as nuclear bodies in cultured thyrotropin-stimulated thyroid cells: autoradiographic kinetics. J Ultrastruct Res. 1982 Jan;78(1):13–25. doi: 10.1016/s0022-5320(82)80010-5. [DOI] [PubMed] [Google Scholar]
- Weis K., Rambaud S., Lavau C., Jansen J., Carvalho T., Carmo-Fonseca M., Lamond A., Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994 Jan 28;76(2):345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Toniolo P. G., Lee E. W., Rivera M., Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993 Apr 21;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- Wu Z., Murphy C., Gall J. G. Human p80-coilin is targeted to sphere organelles in the amphibian germinal vesicle. Mol Biol Cell. 1994 Oct;5(10):1119–1127. doi: 10.1091/mbc.5.10.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Murphy C., Wu C. H., Tsvetkov A., Gall J. G. Snurposomes and coiled bodies. Cold Spring Harb Symp Quant Biol. 1993;58:747–754. doi: 10.1101/sqb.1993.058.01.082. [DOI] [PubMed] [Google Scholar]