Abstract
Although neuroendocrine tumors are rare, the more common types such as gastrointestinal and pancreatic carcinoids, medullary thyroid cancers, and small cell lung cancers have been studied in detail over the last few years. Published data thus far indicates that multiple signaling pathways are involved in these cancers. Recent focus has been on developing novel therapeutics by targeting specific signaling pathways.
This paper will detail several of the signaling mechanisms that have been discovered to play a role in the development and progression of neuroendocrine tumors. The therapeutic options developed to address the various pathways, including their specific mechanisms of actions will also be discussed.
Keywords: neuroendocrine tumor, carcinoid, medullary thyroid cancer, signaling pathways
Introduction
Neuroendocrine tumors (NETs) are rare, with an incidence of two to five per 100,000 people (1). These tumors are known to secrete hormones such as 5-hydroxytryptamine (serotonin (5-HT)), chromogranin A, neuron-specific enolase (NSE), and synaptophysin. These hormones can cause debilitating symptoms of carcinoid syndrome in patients with neuroendocrine malignancies, such as flushing, diarrhea, heart palpitations, and congestive heart failure. NETs also frequently metastasize to the liver long before they are diagnosed making curative resection unlikely. Unfortunately, traditional methods of cancer treatment, such as chemotherapy, have not been successful in the treatment of neuroendocrine tumors. Therefore, it is imperative that new therapies targeting the signaling pathways involved in neuroendocrine tumors are developed.
Many of the signaling pathways which are now known to play an important role in the development and progression of NETs were initially discovered and studied in other cancers. The PI3K-Akt pathway has been well characterized in ovarian cancer, breast cancer, melanoma, and colon cancer (2, 3). Inhibition of this pathway has been shown to suppress the growth of both small cell lung cancers and gastrointestinal carcinoids (4,5). Similarly, the Notch-1 signaling pathway was first studied as an oncogene in pancreatic cancer, colon cancer, non-small cell lung cancer, and various lymphomas (6, 7, 8). It was then discovered that Notch 1 plays the role of tumor suppressor in small cell lung cancer, pancreatic carcinoids, and medullary thyroid cancer (9, 10, 11). Interestingly, the Ras/Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway has also been reported to play an oncogenic role in colon cancer, lung cancer, and melanoma, but a tumor suppressive role in NETs, including small cell lung cancers, medullary thyroid cancer, and carcinoid tumors (12). Finally, the RET pathway, although shown to have a role in papillary thyroid cancer, breast cancer, and melanoma has been most extensively studied in medullary thyroid cancer (13, 14, 15).
PI3K-Akt pathway
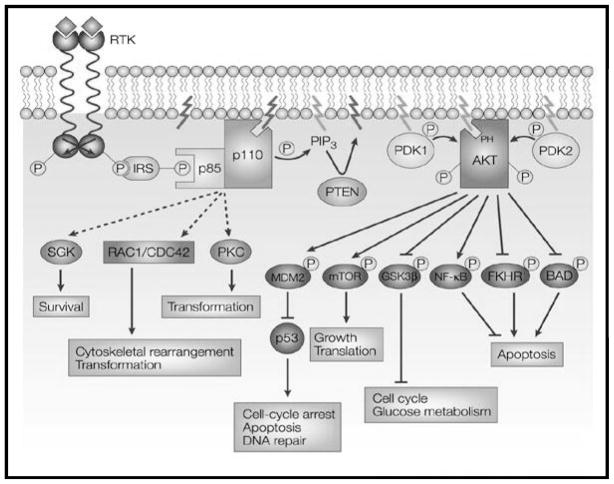
The phosphatidylinositol 3-kinase-Akt (PI3K-Akt) pathway has been shown to play a role in cell proliferation, survival, and motility (2). PI3Ks are heterodimer lipid kinases composed of two subunits, p85 and p110 that are activated by receptor tyrosine kinases (Figure 1). Once activated PI3Ks catalyze pophatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-triphosphate (PIP3) which can be converted back into PIP2 by 3′ phophatase PTEN. PIP3 in turn plays a role in the activation of the serine-threonine protein kinase Akt (Akt). There are three isoforms of Akt, but Akt1 is the isoform mainly studied in cancers, while Akt2 is found in tissues responding to insulin and Akt3 is found in the brain (16). Akt has been shown to activate and inhibit several target genes such as nuclear factor kappa B (NF-κB), mammalian target of rapamycin (mTOR), and glycogen synthase kinase-3β (GSK-3 β), all of which have been implicated in various cancers.
Figure 1.
The phosphatidylinositol 3-kinase-Akt (PI3K-Akt) pathway becomes activated via stimulation of receptor tyrosine kinases (RTKs) and the assembly of receptor–PI3K complexes. The complex then catalyses the conversion of PIP2 to PIP3. PIP3 then helps to activate AKT. Activated AKT then mediates the activation and inhibition of several targets, including GSK3β, glycogen synthase kinase-3β; mTOR, mammalian target of rapamycin; and NF-κB, nuclear factor of κB resulting in cellular growth, metabolism, and survival.
Reprinted by permission from Macmillan Publishers Ltd: Vivanco I, Sawyers C. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2(7):489–501.
Many of these cancers have been found to have either mutations in the p85 or p110 subunits or they exhibit a loss of function mutation in PTEN which then leads to unregulated activation of Akt (2, 3). Pulmonary carcinoid cells have been shown to contain high levels of phosphorylated Akt at baseline and when treated with an Akt specific siRNA, they demonstrated decreased growth (16). The same findings were seen in gastrointestinal carcinoid cells and these tumors have been shown to have a loss of PTEN function (5, 17, 18). Similarly, upregulation of the PI3K-Akt pathway via a loss of PTEN has been implicated in the development of up to 15% of small cell lung cancers (19, 20).
Due to the many steps involved in the PI3K-Akt pathway, it provides many approaches for the treatment of neuroendocrine malignancies. Two PI3K inhibitors, LY294002 and wortmannin, have been studied in human cancer cells, but LY294002 has been examined in detail as a potential therapy for gastrointestinal and pulmonary carcinoids as well as small cell lung cancers. These inhibitors specifically target the p110 subunit. In a study by Krystal and colleagues, they found that treatment with a PI3K inhibitor let to decreased growth and apoptosis of small cell lung cancer cells, but more importantly it increased the sensitivity of the cells to etoposide chemotherapy (4). In a pulmonary carcinoid cell line, treatment with LY294002 led to decreased growth of cancer cells, as well as decreased expression of the neuroendocrine tumor markers, ASCL-1 and CgA (16). Similar findings have been discovered in gastrointestinal carcinoid cells (5).
One of the downstream targets of both the PI3K-Akt pathway and the Ras/Raf/MEK/ERK pathway, the mammalian target of rapamycin (mTOR), has been a focus in treatment of neuroendocrine tumors. mTOR is a serine/threonine kinase that has been shown to regulate cell proliferation and apoptosis and treatment of carcinoid cells with the mTOR inhibitor, Rapamycin, has been shown to decrease tumor growth both in vitro and in vivo (21). Two Rapamycin derivatives, Temsirolimus and Everolimus, have been tested in multicenter, phase II clinical trials on patients with neuroendocrine tumors with some promising results. In initial studies published on Temsirolimus, 63.9% of patients had either a partial response to treatment or stable disease for at least two months (22). The Everolimus study had similar initial results with 92% of patients having stable disease or a partial response to treatment (23). Based on these finding, further clinical trials of these compounds are ongoing.
Another important downstream target of the PI3K-Akt and Ras/Raf/MEK/ERK pathways is glycogen synthase kinase-3β (GSK-3β), serine/threonine protein kinase, also found to regulate multiple cellular processes such as metabolism, proliferation, and survival (24). Studies have demonstrated that unlike most kinases, GSK-3β is active in the non-phosphorylated state and becomes inhibited when phosphorylated (25). Inhibition of GSK-3β has been shown to decrease tumor growth in several cancers, including pancreas, colon, and prostate cancers. Kunnimalaiyaan et al. first demonstrated that treatment with a GSK-3β inhibitor, such as Lithium chloride, an FDA approved medication in the treatment of bipolar disorder, reduced expression of ASCL-1 and CgA in medullary thyroid cancer cells (26). This study also suggested that Lithium induces cell growth inhibition in vitro and in vivo via cell cycle arrest. This led to a phase II trial of Lithium chloride for the treatment of medullary thyroid cancer are currently underway.
Notch-1 signaling pathway
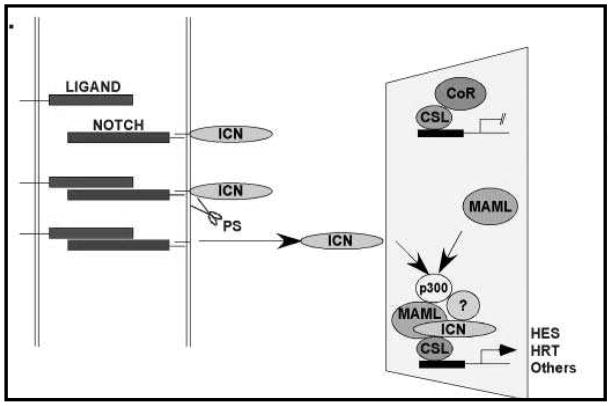
The Notch-1 signaling pathway is known to regulate cellular differentiation, proliferation, and cell survival. It has five ligands, referred to as Delta-like ligands (DLL-1, DLL-3, DLL-4, JAG-1, and JAG-2) (27). Notch-1 is a transmembrane receptor with an N terminal extracellular domain with epidermal growth factor (EGF)-like repeats that mediate ligand binding (Figure 2) (28). In the absence of ligand binding an area of three cysteine-rich Notch/Lin-12 (LN) repeats on the extracellular domain interacts to prevent signaling (28). Once a ligand binds to the Notch-1 receptor two proteolytic cleavages occur that release the Notch-1 intracellular domain (NICD) (29). The NICD translocates to the nucleus and binds to a transactivation complex known as DNA-binding protein complex CSL (C promoter-binding factor 1 [CBF-1], suppressor of hairless, and Lag-1). This results in the activation of multiple target genes such as hairy enhancer of split 1 (HES-1), which in turn controls the expression of Achaete Scute Complex-Like 1 (ASCL-1) (27). ASCL-1 has been shown to play a role in the development of pulmonary neuroendocrine cells, thyroid C cells, and adrenal chromaffin cells and is decreased when Notch signaling is active (30, 31).
Figure 2.
The Notch1 receptor is a transmembrane proteins that once bound to a ligand undergoes cleavage releasing the Notch intracellular domain (ICN). ICN migrates to the nucleus and forms a DNA-binding protein complex CSL, resulting in activation of multiple target genes including Hairy/Enhancer of Split (HES).
Adapted by permission from Elsevier Publishers: Maillard I, Pear W. Notch and cancer: best to avoid the ups and downs. Cancer Cell 2003;3(3):203–205.
The Notch-1 signaling pathway was initially identified as being oncogenic in human T-cell malignancies (32). It was then found to be upregulated in many different cancers including pancreatic cancer, colon cancer, cervical cancer, ovarian cancer, and renal cell carcinoma (7, 8). In contrast, researchers have demonstrated minimal or absent Notch-1 signaling in prostate cancer and in neuroendocrine tumors such as carcinoid cancers, small cell lung cancers, and medullary thyroid cancers suggesting its role as a tumor suppressor (9, 10, 11). As expected, with minimal Notch-1 signaling present, these cancers express high levels of ASCL-1, which make it useful as a neuroendocrine tumor marker. In a study from Nakakura et al, pancreatic carcinoid cells were treated with a Notch-1 viral vector (10). Following treatment, cellular growth was suppressed and there was increased expression of HES-1 and decreased expression of ASCL-1, chromogranin A, NSE, and synaptophysin. Similarly, treatment with a Notch-1 viral vector in a small cell lung cancer cell line led to a reduction in neuroendocrine tumor markers as well as growth suppression of these cells (33). In another study of medullary thyroid cancer by Kunnimalaiyaan et al., a medullary thyroid cancer cell line was treated with a doxycycline-inducible Notch-1 plasmid and HES-1 proteins levels were increased while ASCL-1 and calcitonin were decreased, correlating with the amount of Notch-1 present in these cells (34).
Although demonstrating Notch-1 as a tumor suppressor in NETs has been successful, the discovery of Notch-1 activating agents has been more difficult. In 2005, Stockhausen and colleagues demonstrated that valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, increased Notch-1 protein levels in neuroblastoma cells (35). Based on this work, studies were undertaken into the role VPA could play in both pulmonary and gastrointestinal carcinoid cells. VPA was successful in inhibiting NET cancer cell growth via G1 phase cell cycle arrest and suppressing expression of tumor markers both in vitro and in vivo (36). Other HDAC inhibitors such as suberoyl bis-hydroxamic acid (SBHA) have also demonstrated Notch-1 activation and neuroendocrine tumor suppression (37). Given these promising results, phase II trials are currently underway testing HDAC inhibitors as Notch-1 activating compounds.
Ras/Raf/MEK/ERK Pathway
The Ras/Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway begins with Ras, a G protein. Ras is activated upon phosphorylation of a GDP that then leads to activation of Raf, a family of three cytosolic kinases, of which Raf-1 is most important in cell differentiation (Figure 3) (12). Once Raf is activated it causes further downstream activation of MEK and ERK. This pathway plays an integral role in cell differentiation, growth, and survival (38, 39).
Figure 3.
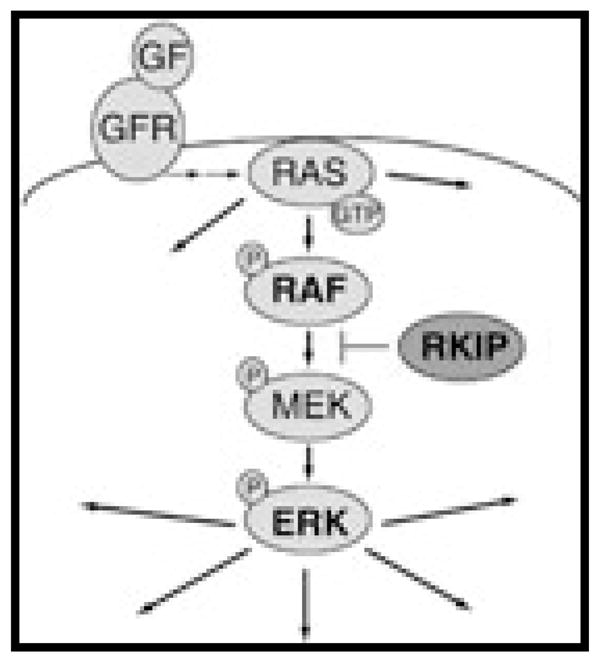
RAS, an intracellular G protein, is phosphorylated, leading to activation of RAF (depicted as BRAF). Once RAF is activated it in turn phosphorylates mitogen-activated protein kinase (MEK) which in turn phosphorylates and activates the extracellular signal-regulated kinase (ERK). Once activated, ERK phosphorylates cytoplasmic proteins and translocates into the nucleus, where it regulates transcription of genes involved in cell differentiation, proliferation, and survival.
Reprinted with permission from: Houben R, Michel B, Vetter-Kauczok C, et al. Absence of classical MAP kinase pathway signalling in Merkel cell carcinoma. J Invest Dermatol 2006;126(5):1135–1142.
Mutations in Ras and Raf lead to overexpression of this pathway and tumorigenesis in colon cancer, lung cancer, and many pancreatic cancers (40). Conversely, the Ras/Raf/MEK/ERK pathway has been shown to be minimally active or absent in neuroendocrine tumors such as small cell lung cancers, carcinoids, and medullary thyroid cancer (41, 42, 43). Small cell lung cancer cells transfected with a Raf-1 construct demonstrated increased Raf-1 activity and decreased cellular growth suggesting the Ras/Raf pathway as a tumor suppressor (41, 42). In similar experiments on pancreatic carcinoid cells, researchers were able to demonstrate a reduction in levels of serotonin and CgA with the activation of Raf-1 (43, 44). In medullary thyroid cancer, activation of Raf-1 has not only led to growth suppression and a reduction in the neuroendocrine hormones, serotonin and calcitonin, but also to reduced levels of the RET proto-oncogene (45, 11). This is evidence that the Ras/Raf/MEK/ERK pathway may play a role in neuroendocrine tumor suppression via auto activation, but also by interactions with other signaling pathways.
Given the decreased expression of the Ras/Raf/MEK/ERK pathway in neuroendocrine tumors and decrease in cell growth and hormone secretion with Raf-1 activation, this may serve as a potential therapeutic option. One compound, ZM336372, was initially found to cause Raf-1 inhibition, but demonstrated a significant increase in Raf-1 activation in vitro (46). When pancreatic and pulmonary carcinoid cells were treated with the agent, they exhibited an increase in Raf-1 and ERK phosphorylation and reduction in cell growth and hormone production (47). In vivo studies of ZM336372 have not yet been undertaken due to its insolubility at high doses. Therefore other Raf-1 activators such as Tautomycin, a potent and specific protein phosphatase inhibitor isolated from Streptomyces spiroverticillatus, have been studied. In 2008, Pinchot et al. reported that low doses of Tautomycin inhibited proliferation of carcinoid cells and suppressed CgA and ASCL-1 via cell cycle arrest (48). Further testing of Tautomycin in vivo is needed, but clinical trials of Streptozocin, a related compound, used to treat pancreatic islet cell tumors is underway in carcinoids (49). Leflunomide, an FDA approved rheumatoid arthritis medication, has also shown promise as a treatment for neuroendocrine tumors via Raf-1 activation. In a study by Cook et al., Leflunomide and its active metabolite, Teriflunomide, were shown to decrease the expression of neuroendocrine tumor markers and to inhibit in vitro and in vivo carcinoid cell proliferation (50). These studies demonstrate the potential of future treatments of neuroendocrine tumors via targeting of the Ras/Raf/MEK/ERK pathway.
RET pathway
The RET gene encodes a tyrosine kinase receptor, which is a single transmembrane receptor with a cysteine rich extracellular domain and two intracellular tyrosine kinase subdomains (51). Several previously discussed pathways such as PI3K-Akt and Ras/Raf/ERK/MEK have been known to interact with the RET pathway (52). Other downstream targets linked to the RET tyrosine kinase receptor include mitogen-activated protein kinase (MAPK), fibroblast growth factor receptor substrate 2 (FRS2), and phosphoinositide-dependent kinase 5 (CDK5) (53). Through all of these various targets, RET has been shown to play important roles in cell differentiation, growth, and survival.
Multiple mutations in the RET receptor are responsible for the development of medullary thyroid cancer. In familial medullary thyroid cancers associated with MEN 2A mutations causing the unpairing of cysteine residues in the extracellular domain are responsible for activation of the RET kinase (54). Conversely, a mutation of the intracellular domain is responsible for the development of MEN 2B (54). Somatic mutations of RET have also been discovered in sporadic medullary thyroid cancers.
Several tyrosine kinase inhibitors directed at RET kinase have been tested to treat medullary thyroid cancer. Vandetanib is a tyrosine kinase inhibitor that also inhibits vascular endothelial growth factor receptor 2 (VEGFR-2) (55). Vandetanib has been shown to block phosphorylation in the most common MEN2A and MEN2B mutations (56). A phase II clinical trial of Vandetanib in patients with metastatic familial medullary thyroid carcinoma demonstrated partial response or stable disease in 40% of patients at 24 weeks (57). Sunitinib is another promising tyrosine kinase inhibitors that has been shown to affect angiogenesis as well as direct inhibition of cellular proliferation via both the VEGF and the RET pathways (22, 58). Results in patients with advanced pancreatic neuroendocrine tumors treated with Sunitinib demonstrated increased median progression-free survival as well as an overall response rate of 9.3% with stable disease reported in 34.9% of patients (59). Further clinical trials of these and other RET tyrosine receptor kinases are ongoing.
Summary
The development, growth, and survival of neuroendocrine tumors depend on a variety of complex signaling mechanisms. Extensive research has begun to elucidate the details of the multiple pathways which play fundamental roles in carcinoids, small cell lung cancers, and medullary thyroid cancer and continued efforts into understanding the interactions of these pathways are imperative. Many therapeutic compounds have now shown promise in the treatment and palliation of neuroendocrine tumors, but further research into definitive medical therapies is needed.
Acknowledgments
This work was supported by the American College of Surgeons Research Fellowship Award (B.Z.); and National Institutes of Health T32 Training Grant (B.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim T, Grobmyer S, Liu C, et al. Primary presacral neuroendocrine tumor associated with imperforate anus. World J Surg Oncol. 2007;5:115. doi: 10.1186/1477-7819-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo J, Manning B, Cantley L. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers C. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Krystal G, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1(11):913–922. [PubMed] [Google Scholar]
- 5.Pitt S, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16(10):2936–2942. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murtaugh L, Stanger B, Kwan K, et al. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100(25):14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14(5):506–512. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3(10):756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G636–642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 10.Nakakura E, Sriuranpong V, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90(7):4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 11.Sippel R, Carpenter J, Kunnimalaiyaan M, et al. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134(6):866–871. doi: 10.1016/s0039-6060(03)00418-5. discussion 871-863. [DOI] [PubMed] [Google Scholar]
- 12.Kunnimalaiyaan M, Chen H. The Raf-1 pathway: a molecular target for treatment of select neuroendocrine tumors? Anticancer Drugs. 2006;17(2):139–142. doi: 10.1097/00001813-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Greco A, Borrello M, Miranda C, et al. Molecular pathology of differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009;53(5):440–453. [PubMed] [Google Scholar]
- 14.Walker G, Hayward N. Pathways to melanoma development: lessons from the mouse. J Invest Dermatol. 2002;119(4):783–792. doi: 10.1046/j.1523-1747.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 15.Zarebczan B, Chen H. Multi-targeted approach in the treatment of thyroid cancer. Minerva Chir. 2010;65(1):59–69. [PMC free article] [PubMed] [Google Scholar]
- 16.Pitt S, Chen H, Kunnimalaiyaan M. Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg. 2009;209(1):82–88. doi: 10.1016/j.jamcollsurg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Ignat A, Axiotis C. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10(2):139–146. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Shah T, Hochhauser D, Frow R, et al. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol. 2006;18(5):355–360. doi: 10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 19.Forgacs E, Biesterveld E, Sekido Y, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17(12):1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 20.Yokomizo A, Tindall D, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene. 1998;17(4):475–479. doi: 10.1038/sj.onc.1201956. [DOI] [PubMed] [Google Scholar]
- 21.Moreno A, Akcakanat A, Munsell M, et al. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15(1):257–266. doi: 10.1677/ERC-07-0202. [DOI] [PubMed] [Google Scholar]
- 22.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95(9):1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao J, Phan A, Chang D, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardt S, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90(10):1055–63. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 25.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 26.Kunnimalaiyaan M, Vaccaro A, Ndiaye M, et al. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6(3):1151–8. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 27.Cook M, Yu X, Chen H. Notch in the development of thyroid C-cells and the treatment of medullary thyroid cancer. Am J Transl Res. 2010;2(1):119–125. [PMC free article] [PubMed] [Google Scholar]
- 28.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12(5):535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 29.Allenspach E, Maillard I, Aster J, et al. Notch signaling in cancer. Cancer Biol Ther. 2002;1(5):466–476. doi: 10.4161/cbt.1.5.159. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Udaka N, Yazawa T, et al. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127(18):3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- 31.Lanigan T, DeRaad S, Russo A. Requirement of the MASH-1 transcription factor for neuroendocrine differentiation of thyroid C cells. J Neurobiol. 1998;34(2):126–134. [PubMed] [Google Scholar]
- 32.Ellisen L, Bird J, West D, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66(4):649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 33.Sriuranpong V, Borges M, Ravi R, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61(7):3200–3205. [PubMed] [Google Scholar]
- 34.Kunnimalaiyaan M, Vaccaro A, Ndiaye M, et al. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281(52):39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 35.Stockhausen M, Sjölund J, Manetopoulos C, et al. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92(4):751–759. doi: 10.1038/sj.bjc.6602309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenblatt D, Vaccaro A, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12(8):942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 37.Ning L, Greenblatt D, Kunnimalaiyaan M, et al. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13(2):98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Kunnimalaiyaan M, Van Gompel J. Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1. Thyroid. 2005;15(6):511–521. doi: 10.1089/thy.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 39.Dhillon A, Kolch W. Oncogenic B-Raf mutations: crystal clear at last. Cancer Cell. 2004;5(4):303–304. doi: 10.1016/s1535-6108(04)00087-x. [DOI] [PubMed] [Google Scholar]
- 40.Younes N, Fulton N, Tanaka R, et al. The presence of K-12 ras mutations in duodenal adenocarcinomas and the absence of ras mutations in other small bowel adenocarcinomas and carcinoid tumors. Cancer. 1997;79(9):1804–1808. [PubMed] [Google Scholar]
- 41.Ravi R, Thiagalingam A, Weber E, et al. Raf-1 causes growth suppression and alteration of neuroendocrine markers in DMS53 human small-cell lung cancer cells. Am J Respir Cell Mol Biol. 1999;20(4):543–549. doi: 10.1165/ajrcmb.20.4.3406. [DOI] [PubMed] [Google Scholar]
- 42.Ravi R, Weber E, McMahon M, et al. Activated Raf-1 causes growth arrest in human small cell lung cancer cells. J Clin Invest. 1998;101(1):153–159. doi: 10.1172/JCI831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sippel R, Carpenter J, Kunnimalaiyaan M, et al. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G245–254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 44.Sippel R, Chen H. Activation of the ras/raf-1 signal transduction pathway in carcinoid tumor cells results in morphologic transdifferentiation. Surgery. 2002;132(6):1035–1039. doi: 10.1067/msy.2002.128877. discussion 1039. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Strock C, Ball D, et al. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol Cell Biol. 2003;23(2):543–554. doi: 10.1128/MCB.23.2.543-554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall-Jackson C, Eyers P, Cohen P, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol. 1999;6(8):559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 47.Van Gompel J, Kunnimalaiyaan M, Holen K, et al. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4(6):910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 48.Pinchot S, Adler J, Luo Y, et al. Tautomycin suppresses growth and neuroendocrine hormone markers in carcinoid cells through activation of the Raf-1 pathway. Am J Surg. 2009;197(3):313–319. doi: 10.1016/j.amjsurg.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. [Accessed April 20, 2010];ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00602082?term=streptozocin&rank=2.
- 50.Cook M, Pinchot S, Jaskula-Sztul R, et al. Identification of a novel Raf-1 pathway activator that inhibits gastrointestinal carcinoid cell growth. Mol Cancer Ther. 2010;9(2):429–437. doi: 10.1158/1535-7163.MCT-09-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cakir M, Grossman A. Medullary thyroid cancer: molecular biology and novel molecular therapies. Neuroendocrinology. 2009;90(4):323–348. doi: 10.1159/000220827. [DOI] [PubMed] [Google Scholar]
- 52.de Groot J, Links T, Plukker J, et al. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27(5):535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 53.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J Biol Chem. 2000;275(5):3568–3576. doi: 10.1074/jbc.275.5.3568. [DOI] [PubMed] [Google Scholar]
- 54.Santoro M, Melillo R, Carlomagno F, et al. Molecular biology of the MEN2 gene. J Intern Med. 1998;243(6):505–8. doi: 10.1046/j.1365-2796.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 55.Herbst R, Heymach J, O’Reilly M, et al. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007;16(2):239–49. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 56.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–90. [PubMed] [Google Scholar]
- 57.Sherman S. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008;37(2):511–24. doi: 10.1016/j.ecl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Kulke M, Scherübl H. Accomplishments in 2008 in the management of gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2009;3(5 Supplement 2):S62–66. [PMC free article] [PubMed] [Google Scholar]
- 59.Dimou A, Syrigos K, Saif M. Neuroendocrine tumors of the pancreas: what’s new. JOP; Highlights from the “2010 ASCO Gastrointestinal Cancers Symposium”; Orlando, FL, USA. January 22–24, 2010; 2010. pp. 135–138. [PubMed] [Google Scholar]