Abstract
Dopamine (DA) and serotonin (5-HT) transporter availability in heroin users and healthy controls was measured using [123I]β-CIT and SPECT imaging. Heroin users had statistically similar striatal DA and brainstem and diencephalon 5-HT transporter availability compared to controls. No associations between transporter availability and heroin use characteristics were found.
Keywords: heroin, dopamine transporter, serotonin transporter
1. Introduction
A core feature of acute administration of opioid drugs of abuse is their activation of brain dopaminergic neurons and increase in striatal dopamine (DA) release (Di Chiara and Imperato 1988; Wise et al. 1995). With more chronic administration, compensatory changes begin to occur, such as downregulation of DA receptors, transporters and function (Simantov 1993; Spampinato et al. 1988; Wang et al. 2008) that may promote continued use of opioid drugs. Lower DA transporter availability has been reported in imaging studies in recently detoxified heroin users (Jia et al. 2005) and former heroin users with prolonged abstinence (Shi et al. 2008) compared to controls. However, a postmortem study found no difference in striatal DA transporter levels between actively using heroin users and controls (Kish et al. 2001).
The effects of heroin on serotonin (5-HT) function are not as well researched. Acute opiate administration enhances 5-HT activity (Desole et al. 1996; Spampinato et al. 1985); however, the effects of prolonged opiate use on 5-HT activity are not clear. In postmortem brain of chronic heroin users, striatal levels of 5-HT were slightly elevated, whereas levels of the serotonin metabolite 5-hydroxyindoleacetic acid were significantly decreased compared to controls (Kish et al. 2001), suggesting 5-HT activity may be reduced after prolonged use.
In this study, we used [123I]β-CIT and single photon emission computed tomography (SPECT) brain imaging to measure DA and 5-HT transporter availability in chronic heroin users and healthy controls. [123I] β-CIT binds with high affinity to the presynaptic striatal DA and brainstem and diencephalon 5-HT transporters (Laruelle et al. 1993) with high test-retest reliability (Seibyl et al. 1996; Seibyl et al. 1997). We hypothesized lower DA and 5-HT transporter availability in subjects with chronic heroin dependence vs. healthy controls.
2. Methods
2.1. Subjects
Eight heroin dependent subjects (37.0 ± 9.1 years; age range 23–47; 7 men, 1 woman; 6 Caucasian, 2 Hispanic) and 8 healthy controls (38.0 ± 9.4 years; age range 26–54; 6 men, 2 women; 8 Caucasian) provided informed consent to participate in the study. Groups were matched for age and smoking status (2 heroin and 2 control subjects were nonsmokers). Eligibility was determined as follows: no use of psychotropic drugs within 3 months prior to the study, no lifetime use of the designer drug “ecstasy”(MDMA), and no significant psychiatric, neurological, or medical problems as determined by physical and psychiatric examinations. Heroin dependent subjects met the DSM-III-R diagnostic criteria for heroin dependence, had at least a 1-year history of heroin use (subjects reported intravenous and intranasal use), tested positive for heroin on the day of their intake, and no dependence on any drug other than heroin or nicotine. Within the group of heroin dependent subjects 1 was diagnosed with alcohol abuse, 1 with cocaine abuse, and 1 subject tested positive for methadone. Heroin dependent subjects were currently using and tested positive for heroin use with toxicology on the day of their intake evaluation; however, their last use of heroin relative to their SPECT scan was not recorded. All women had a negative pregnancy test prior to radiotracer injection. Heroin use characteristics, including total number of years of heroin use and quantity used per day, were documented during the initial intake.
2.2. [123I]β-CIT SPECT and Magnetic Resonance Imaging
[123I]β-CIT was synthesized as described previously (Baldwin et al. 1993), with a radiochemical purity of > 97%. On day 1 of the SPECT study, subjects were given a Lugol’s solution approximately 30 min prior to radiotracer administration, to block thyroid uptake of [123I]iodide. [123I]β-CIT was administered by intravenous bolus injection to subjects with chronic heroin use (213.9 ± 35.6 MBq) and healthy controls (223.9 ± 1.3 MBq). SPECT scans (one 24-min emission scan and one 15-min simultaneous transmission and emission scan) were performed on a Picker PRISM 3000 three-headed camera (Phillips, Cleveland, OH) equipped with a low-energy, ultrahigh resolution fan beam collimator (photopeak window 159 keV ± 10%; matrix 128 × 128) with a standardized sensitivity across the field of view. The axial resolution (full width at half maximum) of the camera was 12.2 mm. Blood samples were obtained before radiotracer injection and 24 h after injection to determine blood measures including plasma [123I]β-CIT and protein binding, expressed as free fraction fP (Baldwin et al. 1993; Gandelman et al. 1994). Subjects were scanned approximately 24h after radiotracer injection (Laruelle et al. 1994). Magnetic resonance imaging (MRI) studies were performed on a 1.5 Tesla GE Signa device (TR = 25 ms, TE = 5ms, number of excitations = 2, matrix = 256 × 256 pixels, and field of view = 24 cm).
2.3. Image analysis
SPECT data were reconstructed and attenuation corrected as previously described (Staley et al. 2001). MRIs were coregistered to the SPECT images to provide an anatomical guide for placement of the regions of interest using Medx (version 3.4) software (Medical Numerics, Inc., MD). [123I]β-CIT labels DA transporter availability in the striatum and 5-HT transporter availability in the diencephalon and brainstem. The ROIs were: right and left striatum (average of caudate and putamen), diencephalon, brainstem, and right and left cerebellum. Regional brain activities are reported as the average value of right and left hemispheres. The primary outcome measure for regional brain uptake is the binding potential (BPND,) which is proportional to the receptor concentration (Bmax/KD) at equilibrium. BPND is defined as the ratio of specific to nonspecific binding with the cerebellum as the reference region. The cerebellum has no detectable levels of DA transporters and very low levels of 5-HT transporters (Backstrom et al. 1989; Laruelle and Maloteaux 1989; Laruelle et al. 1988) that are not measured reliably in vivo (Laruelle et al. 1993). BPP (ratio of specific binding to total plasma parent) was also evaluated as an outcome measure, but because results were similar between BPND and BPP, only BPND results are described.
2.4. Statistical Analysis
DA and 5-HT transporter availability were analyzed independently. Striatal DA transporter availability was compared between chronic heroin users and controls using independent t-tests. A linear mixed model was used to compare diencephalon and brainstem 5-HT transporter availability (within-subjects factor) between groups (between-subjects factor). The best fitting variance-covariance structure was determined by information criteria. Confidence intervals on the difference between the means were also calculated. Potential associations between brain and heroin use characteristics including total number of years of heroin use and quantity used per day were assessed using correlation analysis. Analyses were performed using SAS, version 9.1 (Cary, NC). Due to the preliminary nature of this report, all significance levels are uncorrected at the p≤0.05 level.
3. Results
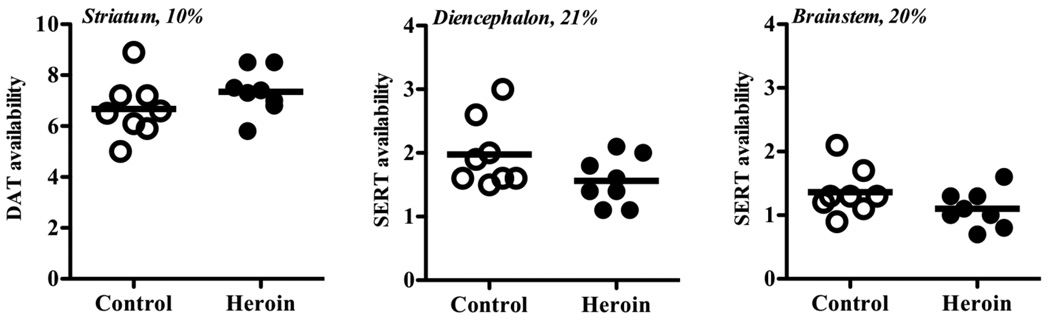
Chronic heroin users had been using heroin for 8.3±8.6 years (mean±SD; range 1–24 years). They reported using 11.6±5.3 bags of heroin per day (range 6–18 bags/day). There were no differences between groups in blood measures, e.g., total plasma parent or free fraction. There was no significant difference in striatal DA transporter availability between chronic heroin users (7.4±0.90) and controls (6.7±1.15) [t=−1.33, df=14, p=0.20, 95% CIdiff −1.80, 0.42] (Figures 1 and 2). There was no significant difference in diencephalon (t=2.02, df=14, p=0.06, 95% CI −0.03, 0.85) or brainstem (t=1.33, df=14, p=0.21, 95% CI −0.17, 0.71) 5-HT transporter availability between chronic heroin users compared to controls (Figures 1 and 2). There were no significant associations between DA or 5-HT transporter availability and heroin use characteristics including total number of years of heroin use and quantity used per day.
Figure 1.
Striatal DA transporter availability (BPND) (left panel), and diencephalons (middle panel) and brainstem (right panel) 5-HT transporter availability (BPND) in control subjects (open circles) and chronic heroin users (filled circles). The percent difference reflects the difference between heroin users and controls in each brain region.
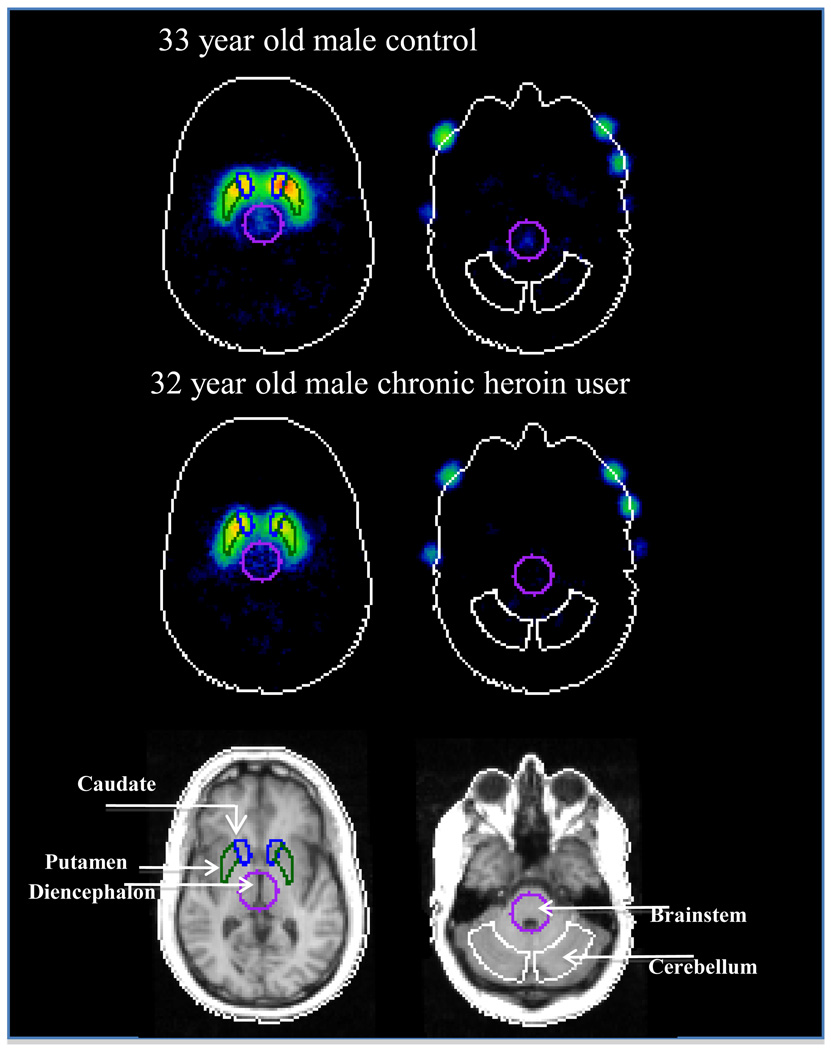
Figure 2.
[123I]beta-CIT image for a representative control subject (upper panel) and age- and sex-matched chronic heroin user (middle panel) and the corresponding MRI for the chronic heroin user (lower panel). Regions-of-interest shown are for DA transporter availability, e.g., striatum (caudate and putamen), and 5-HT transporter availability, e.g., diencephalon and brainstem, and for the cerebellum, which is the reference region.
4. Discussion
In this preliminary study, we report statistically similar DA and a trend for lower 5-HT transporter availability in chronic heroin users compared to healthy controls. We did not find associations between DA or 5-HT transporter availability and chronicity of heroin use or amount of heroin used per day in this small sample.
Our finding of similar striatal DA transporter availability in chronic heroin users compared to controls is consistent with a rodent study that reported chronic morphine administration reduced dopamine transporter density in the anterior basal forebrain but not striatum (Simantov 1993) and with a human postmortem study that reported no difference in striatal DA transporters in heroin users compared to controls (Kish et al. 2001). Other imaging studies have reported lower DA transporter availability in chronic heroin users (Jia et al. 2005) and in former heroin users on or off methadone maintenance (Shi et al. 2008). Notably, in both previous imaging studies reporting lower striatal dopamine transporters in heroin dependent subjects vs. controls (Jia et al. 2005; Shi et al. 2008) the heroin dependent subjects were detoxified for a minimum of 10 days, whereas in the current study and the postmortem study (Kish et al. 2001) reporting no difference, the heroin dependent subjects were actively using heroin.
We also report statistically similar 5-HT transporter availability in living chronic heroin users compared to controls. In previous studies, there is evidence for altered 5-HT function in chronic heroin users. Specifically, in postmortem brain of heroin users, striatal levels of 5-HT were normal or elevated, and striatal concentrations of 5-hyroxyindoleacetic acid, a serotonin metabolite, were significantly decreased (Kish et al. 2001). Previous studies report elevated levels of platelet 5-HT in chronic heroin users that returned to normal after 8 days of abstinence (Schmidt et al. 1997), a dysfunctional response to d-fenfluramine in abstinent heroin users (Gerra et al. 2003) and a genetic link between heroin addiction and the serotonin system (Levran et al. 2008; Saiz et al. 2008). Taken together with the present results, this suggests that while serotonergic dysfunction may be a consequence of heroin dependence, the availability of 5-HT transporters is not different from controls.
This study is limited by the small sample size which precludes a correction for potential sex differences shown to be important in the serotonin system (Staley et al. 2001). Additionally, [123I]β-CIT is limited because it is not selective for the serotonin transporters in the diencephalon and brainstem, but also measures dopamine and noradrenergic transporters. In summary, our findings do not support a difference in DA or 5-HT transporter availability in heroin users compared to controls. This suggests other neurochemical systems are likely involved in modulating active heroin use.
Acknowledgments
We would like to thank Dr. Stephanie O’Malley for helpful comments on the manuscript. This study was supported by NIH grants (KO1 DA20651, KO1 AA00288; P50-DA18197; K05 DA454).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backstrom I, Bergstrom M, Marcusson J. High affinity [3H]paroxetine binding to serotonin uptake sites in human brain tissue. Brain Res. 1989;486:261–268. doi: 10.1016/0006-8993(89)90511-8. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin R, Zea-Ponce Y, Zoghbi S, et al. Evaluation of the monoamine uptake site ligand [123I]methyl 3β-(4-iodophenyl)-tropane-2β-carboxylate ([123I]β-CIT) in non-human primates: pharmacokinetics, biodistribution and SPECT brain imaging coregistered with MRI. Nucl Med Biol. 1993;20:597–606. doi: 10.1016/0969-8051(93)90028-s. [DOI] [PubMed] [Google Scholar]
- 3.Desole M, Esposito G, Fresu L, et al. Effects of morphine treatment and withdrawal on striatal and limbic monoaminergic activity and ascorbic acid oxidation in the rat. Brain Res. 1996;723:154–161. doi: 10.1016/0006-8993(96)00235-1. [DOI] [PubMed] [Google Scholar]
- 4.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandelman M, Balswin R, Zoghbi S, et al. Evaluation of ultrafiltration for the free fraction determination of single photon emission computed tomography (SPECT) tracers: β-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 6.Gerra G, Zaimovic A, Moi G, et al. Neuroendocrine correlates of antisocial personality disorder in abstinent heroin-dependent subjects. Addict Biol. 2003;8(1):23–32. doi: 10.1080/1355621031000069846. [DOI] [PubMed] [Google Scholar]
- 7.Jia SW, Wang W, Liu Y, et al. Neuroimaging studies of brain corpus striatum changes among heroin-dependent patients treated with herbal medicine, U'finer capsule. Addict Biol. 2005;10(3):293–297. doi: 10.1080/13556210500222456. [DOI] [PubMed] [Google Scholar]
- 8.Kish S, Kalasinsky K, Derkach P, et al. Striatal Dopaminergic and Serotonergic Markers in Human Heroin Users. 2001;24:561–567. doi: 10.1016/S0893-133X(00)00209-8. [DOI] [PubMed] [Google Scholar]
- 9.Laruelle M, Baldwin R, Malison R, et al. SPECT imaging of dopamine and serotonin transporters with [123I]β–CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993;13:295–309. doi: 10.1002/syn.890130402. [DOI] [PubMed] [Google Scholar]
- 10.Laruelle M, Maloteaux J. Regional distribution of serotonergic pre- and postsynaptic markers in human brain. Acta psychiatr scand. 1989;80:56–59. doi: 10.1111/j.1600-0447.1989.tb07175.x. [DOI] [PubMed] [Google Scholar]
- 11.Laruelle M, Vanisberg M, Maloteaux J. Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry. 1988;24:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- 12.Laruelle M, Wallace E, Seibyl J, et al. Graphical, kinetic, and equilibrium analyses of in vivo [123I]β-CIT binding to dopamine transporters in healthy human subjects. J Cerebral Blood Flow & Metab. 1994;14:982–994. doi: 10.1038/jcbfm.1994.131. [DOI] [PubMed] [Google Scholar]
- 13.Levran O, Londono D, O'Hara K, et al. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7(7):720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saiz PA, Garcia-Portilla MP, Arango C, et al. Association between heroin dependence and 5-HT2A receptor gene polymorphisms. Eur Addict Res. 2008;14(1):47–52. doi: 10.1159/000110410. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt LG, Dufeu P, Heinz A, et al. Serotonergic dysfunction in addiction: effects of alcohol, cigarette smoking and heroin on platelet 5-HT content. Psychiatry Res. 1997;72(3):177–185. doi: 10.1016/s0165-1781(97)00102-9. [DOI] [PubMed] [Google Scholar]
- 16.Seibyl JP, Laruelle M, van Dyck CH, et al. Reproducibility of iodine-123-beta-CIT SPECT brain measurement of dopamine transporters. J Nucl Med. 1996;37(2):222–228. [PubMed] [Google Scholar]
- 17.Seibyl JP, Marek K, Sheff K, et al. Test/retest reproducibility of iodine-123-betaCIT SPECT brain measurement of dopamine transporters in Parkinson's patients. J Nucl Med. 1997;38(9):1453–1459. [PubMed] [Google Scholar]
- 18.Shi J, Zhao LY, Copersino ML, et al. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008;579(1–3):160–166. doi: 10.1016/j.ejphar.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Simantov R. Chronic morphine alters dopamine transporter density in the rat brain: possible role in the mechanism of drug addiction. Neurosci Lett. 1993;163:121–124. doi: 10.1016/0304-3940(93)90360-w. [DOI] [PubMed] [Google Scholar]
- 20.Spampinato U, Esposito E, Romandini S, et al. Changes of serotonin and dopamine metabolism in various forebrain areas of rats injected with morphine either systemically or in the raphe nuclei dorsalis and medianis. Brain Res. 1985;328:89–95. doi: 10.1016/0006-8993(85)91326-5. [DOI] [PubMed] [Google Scholar]
- 21.Spampinato U, Gozlan H, Daval G, et al. Dopamine receptor subsensitivity in the substantia nigra after chronic morphine treatment in rats. Eur J Pharmacol. 1988;150(1–2):113–122. doi: 10.1016/0014-2999(88)90756-x. [DOI] [PubMed] [Google Scholar]
- 22.Staley J, Krishnan-Sarin S, Zoghbi S, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Liu Y, Lei Y, et al. Extremely low-frequency electromagnetic field exposure during chronic morphine treatment strengthens downregulation of dopamine D2 receptors in rat dorsal hippocampus after morphine withdrawal. Neurosci Lett. 2008;433(3):178–182. doi: 10.1016/j.neulet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Wise R, Leone P, Rivest R, et al. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]