Synopsis
Salivary diagnostics is a dynamic and emerging field utilizing nanotechnology and molecular diagnostics to aid in the diagnosis of oral and systemic diseases. Here, we critically review the latest advances using oral biomarkers for disease detection. The use of oral fluids is broadening perspectives in clinical diagnosis, disease monitoring and decision making for patient care. Important elements determining the future possibilities and challenges in this field are also discussed.
Keywords: Point-of Care diagnostics, saliva, systematic disease, oral disease
Introduction
Saliva is a clinically informative, biological fluid (biofluid) that is useful for novel approaches to prognosis, laboratory or clinical diagnosis, and monitoring and management of patients with both oral and systemic diseases. It is easily collected and stored and ideal for early detection of disease as it contains specific soluble biological markers (biomarkers). Saliva contains multiple biomarkers which make it useful for multiplexed assays that are being developed as point-of-care (POC) devices, rapid tests, or in more standardized formats for centralized clinical laboratory operations. Salivary diagnostics is a dynamic field that is being incorporated as part of disease diagnosis, clinical monitoring and for making important clinical decisions for patient care.
Salivary diagnostics has been the subject of recent meetings and reviews [1, 2] and an overview of the principles of salivary gland secretion, methods of saliva collection, and discussion of general uses can be found in a report of a meeting published in the Annals of the New York Academy of Sciences [3]. These topics were updated in a subsequent meeting [4]and also in a recent textbook, Salivary Diagnostics, 2008 [5].
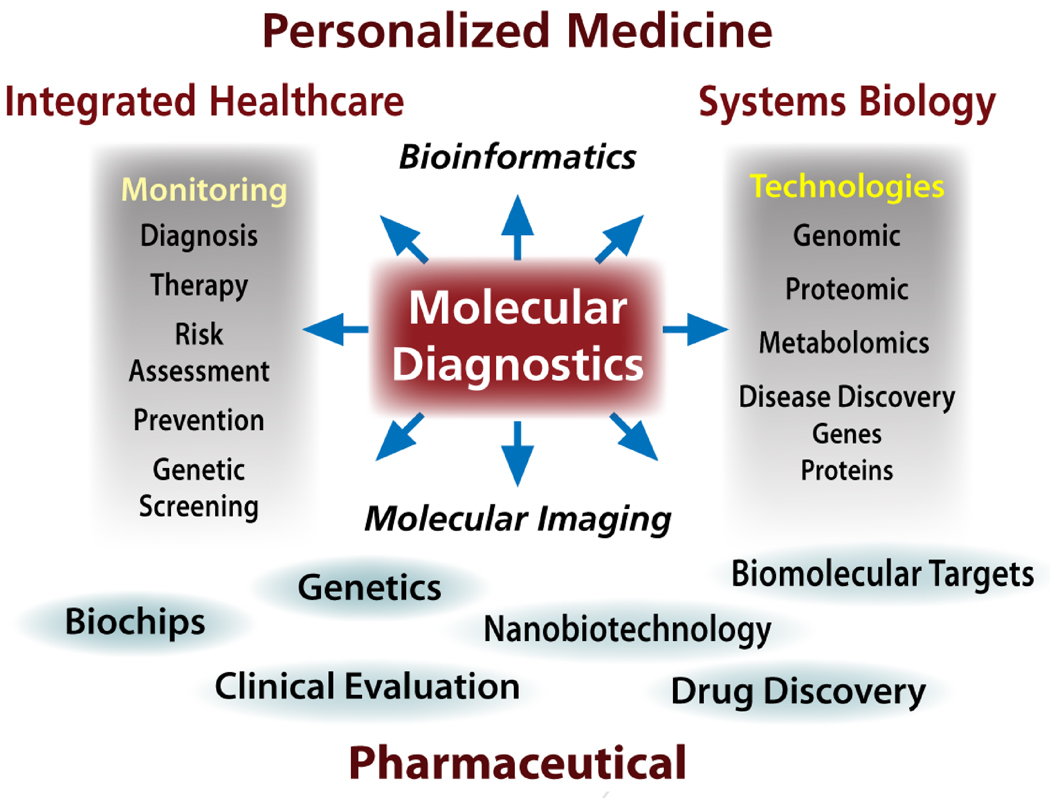
Salivary diagnostics has evolved into a sophisticated science, and serves as a subset of the larger field of molecular diagnostics, now recognized as a central player in a wide variety of biomedical basic and clinical areas (see Figure 1). Molecular diagnostics feeds into a wide range of disciplines including drug development, personalized medicine (pharmacogenomics) and plays a major role in discovery of biomarkers for the diagnosis of oral and systemic diseases. This is especially true since most of the biomarkers present in blood and urine can also be detected in a sample of saliva. In the present review we focus on the use of saliva and other oral samples for the diagnosis of systemic and oral diseases.
Figure 1.
Overview of the centrality of molecular diagnostics to biomedical activities.
Salivary Diagnostics for Systemic Diseases
Historically, systemic diseases are diagnosed via (1) patient reported symptoms, (2) examination and a medical history obtained by a physician or other medical professional, (3) chemical analysis of blood and/or urine samples. The patient’s samples are typically sent to a remote, clinical diagnostic laboratory for determination of the levels of a broad series of markers including ions, antibodies, hormone levels, and a variety of disease-specific biomarkers. After some time (from minutes to days depending upon the assay) the laboratory report is returned to the physician, and the results are then communicated to the patient. Generally, oral samples are only taken if there is a suspicion of an oral infection, for example a throat swab for Streptococcus pyogenes to diagnose “strep throat”, or a mucosal biopsy for suspected oral cancer. However, there has been increasing interest in the use of saliva, and other oral samples, for the diagnosis of oral and systemic diseases. In a sense, the rationale is obvious. If it is possible to obtain similar or identical information with an oral sample that is easy to collect and that does not require invasive procedures, the need for a blood draw would become unnecessary. This is particularly important in a number of populations and situations, which include handling pediatric and geriatric patients, or when access to health care is limited in remote geographic areas where phlebotomists are unavailable. A recent survey reported that dentists believe that screening for medical conditions is important and they are willing to participate when the sample is saliva, as opposed to a finger stick [6].
To substantiate the concept that an oral diagnostic test would be preferred as compared to a more invasive alternative, one only needs to look at the success of the oral thermometer to detect fever which has totally replaced its predecessor, the rectal thermometer. More recently, the confirmation that an oral test for detection of antibodies to the Human Immunodeficiency Virus (HIV) is as sensitive and specific as a blood test [7, 8] has led to a large increase in HIV testing at a variety of locations including emergency rooms, sexually transmitted diseases (STD) clinics, community health centers, bath houses, and most recently in dental settings. The ability to accurately detect antibodies to HIV strongly suggests the potential to detect antibodies to many other pathogens. Indeed recent literature documents this for a large number of viral and bacterial pathogens [1].
Oral samples that are useful for the diagnosis of systemic diseases include saliva, gingival crevicular fluid (GCF), oral swabs, dental plaque, and volatiles. Indeed, published data indicates the successful use of all of these types of oral samples to detect or predict susceptibility to systemic diseases.
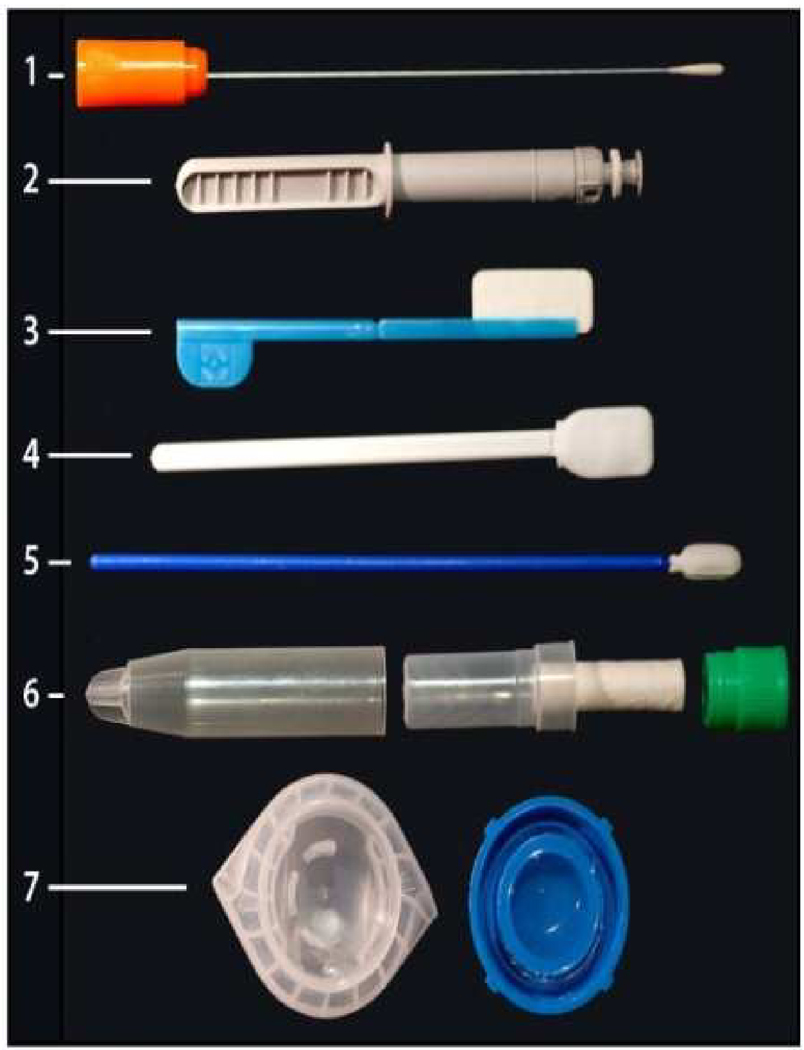
The ability to accurately assess biomarkers in samples obtained from the oral cavity depends on the biochemical nature of the marker, the source and type of sample being taken, and the mechanism by which the marker enters the oral cavity. The most widely used type of oral sample is a swab that collects a deoxyribonucleic acid (DNA) sample. This has been employed for many years in forensic studies [9, 10] and more recently for single nucleotide polymorphisms (SNP) analyses for mutations associated with specific diseases [11]. While a DNA sample can be collected from a wide range of sites on/in the human body, oral sampling has been used most often because of the ease of the sampling procedure, i.e. a buccal brushing that is placed in a stabilizing transport medium and sent off to a laboratory for evaluation. The commercial success of genotyping individuals for disease-related DNA sequences, while still somewhat controversial for its medical value, is not questioned for its scientific accuracy. Several companies have developed kits for collection of oral swabs for this purpose. Genomic profiles are returned within weeks that can predict ancestral origins and susceptibility to a number of diseases. Figure 2 presents examples of a selection of commercially available devices for collection of oral samples. Note that some of these devices (#3, 4, and 5) are shipped with a stabilizing solution for transport of oral samples to a testing laboratory. The salivette (#6) contains a cotton pad that is placed in the mouth, chewed; the pad is returned to its carrier and closed. The saliva sample is then recovered from the pad by centrifugation. Culture swab collection and transport system from BBL, (2) UpLink saliva collector from OraSure Technologies Inc., (3) Intercept Oral Specimen Collection Device from OraSure Technologies, Inc., (4) Aware Messenger Device for the Collection, Stabilization and Transport of Oral Fluid Specimens from Calypte Biomedical Corporartion, (5) Saliva Collection device for collection of DNA samples from children, DNA genotek, (6) Salivette from Sarstedt AG & Corporation, (7) Oragene saliva kit for collection of saliva samples for DNA analysis, DNA genotek.
Figure 2.
Examples of commercial collectors for saliva.
In terms of the number of publications, the second major use of oral samples is for the quantitation of steroid hormone levels. Assays are commercially available for cortisol, estriol, estrogen, testosterone, and consistently provide accurate detection of these hormones [12, 13, 14, 15, 16, 17]. However, salivary levels do not correlate well with serum levels in the case of conjugated steroid hormones. Thus, while dehydroepiandrosterone (DHEA) can be reliably monitored in saliva and the measurements reflect blood levels of the hormone, the sulfated derivative of the steroid, DHEA-S, can be measured in saliva, but the levels are not correlated with serum levels. The reason for this discrepancy appears to be the route of entry of the hormone into the oral cavity. DHEA as a steroid can readily cross the phospholipid membrane of epithelial cells lining the blood vessels, so that elevated serum levels translate as elevated saliva levels by simple diffusion of the hormone. The addition of the charged sulfate group, however, impedes membrane transport and the substance detected in saliva likely represents leakage from the blood rather than diffusion. These observations raise the general issue of a qualitative versus quantitative assay for biomarkers. When qualitative (i.e., yes/no) results are needed, as in the case of pregnancy or bacterial and viral infections, saliva sampling will generally be useful. When a quantitative result is needed, for example when analyzing glucose or DHEA-S levels, one must determine the saliva/plasma ratio. If this ratio is close to 1, as it is for ethyl alcohol and unconjugated steroid hormones, then quantitative salivary testing is feasible, if not, then a quantitative salivary-based assay will not be suitable for that biomarker.
The application of salivary diagnostics for systemic diseases received a major boost in 2002 as a result of funding a program by the National Institute of Dental and Craniofacial Research (NIDCR), (“Development and Validation Technologies for Saliva Based Diagnostics”), designed to establish collaborative research teams between engineers with skills in nanotechnology and microfluidic techniques, with scientists from the oral biology community to develop portable POC diagnostic platforms for rapid detection and analysis of oral biomarkers. Initially 7 research teams were funded by this program, and 4 of these projects were refunded in 2006. The currently funded teams are led by Dr. David Walt (Tufts University, Medford, MA) who is monitoring chronic obstructive pulmonary disease (COPD) and cystic fibrosis, Dr. John McDevitt (Rice University, Houston, TX) who is developing biomarkers for acute myocardial infarction, Dr. David Wong (University of California, Los Angeles, CA) who is focusing on detection of oral cancer, and Dr. Daniel Malamud (New York University, New York, NY) who is developing a multiplexed test for HIV, TB and Malaria. All of these projects utilize advanced techniques for rapid POC diagnostics for detection of the relevant biomarkers [18, 19, 20, 21].
A summary of selected molecules that have been accurately detected in saliva is presented in table 1 below:
Table 1.
Analytes detected in saliva
| Analyte | Examples | References |
|---|---|---|
| Hormones | [17, 19] | |
| Steroids | cortisol, androgens, (testosterone), estriol, estrogen, progesterone, aldosterone, DHEAS |
[7, 12, 13, 14, 15, 16, 17, 22, 23, 24, 25, 26, 27, 28, 29] |
| Antibodies | IgG, IgA, sIgA, IgM | [7, 22, 29] |
| Growth Factors | EGF, NGF, VEGF, IGF | [30, 31, 32, 33, 34] |
| Cytokines and Chemokines | IL-1 beta,IL-8, IL-6, MCP-1, CX3CL1, GRO-1 alpha, troponin I, TNF alpha |
[35, 36, 37, 38, 39] |
| Nucleic Acids | human DNA, microbial DNA, mRNA, siRNA, micro RNA (miR-125a and miR-200a) |
[9, 40, 41, 42, 43] |
| Proteins | 100’s-1,000s | [44, 45, 46] |
| Drugs | drugs of abuse (NIDA 5), ethanol, therapeutic drugs, anticonvulsants, antipyretic/analgesics, anti- neoplastic agents, anti- bacterial agents, bronchodilators, cotinine |
[47, 48, 49, 50] |
Salivary Biomarkers in cardiovascular disease
There are numerous published reports demonstrating that C-reactive protein (CRP) can be monitored in salivary samples, however CRP remains a non-specific inflammatory response factor that increases in many conditions including periodontal diseases [51]. Similarly salivary immunoglobulins levels are known to increase in association with coronary artery disease [52] but once again immunoglobulins, particularly salivary IgA is elevated in response to many local and systemic conditions. Recently, Floriano et al [51] reported that a group of salivary biomarkers can complement findings of an electrocardiogram (ECG) following an acute myocardial infarction. These markers include CRP, myoglobin and myeloperoxidase, in combination with an ECG, showed a highly significant correlation with myocardial infarct patients as compared to healthy controls. Salivary biomarkers have also been incorporated into POC devices for the rapid assessment of cardiovascular disease (CVD) with potential association with distinct disease stages, demonstrating promising results to identify CVD [53]. Elevated salivary lysozyme levels, a biomarker for oral infection and hyperglycemia, has also shown a significant association with hypertension, an early stage of CVD [44]. In spite of the progress made in biomarker discovery, robust clinical studies are required to validate salivary biomarkers for CVD and its different clinical stages.
Salivary biomarkers for renal disease
Walt et al [21] and Arregger et al [54] reported on a series of salivary markers that were associated with end stage renal disease. The list of markers included cortisol, nitrite, uric acid, sodium, chloride, pH, amylase and lactoferrin. In a subsequent study [35] by these same investigators , colormetric test strips were used to monitor salivary nitrate and uric acid before and after hemodialysis. It was suggested that a salivary test could be used by patients to decide when dialysis is required, thereby eliminating unnecessary visits to a dialysis clinic [55]. Salivary phosphate has been successfully used as a clinical biomarker for hyperphosphatemia, which is an important contributor to cardiovascular calcification in chronic renal failure (CRF) [55, 56, 57]. In this clinical study, sixty eight patients undergoing hemodialysis (HD) and one hundred ten patients with various degrees of CRF were evaluated. It was demonstrated that both HD and CRF patients had significantly higher salivary phosphate levels compared with healthy control subjects. Furthermore, evaluation of phosphate levels in saliva correlated positively with serum creatinine and the glomerular filtration rate. Thus, salivary phosphate may provide a better marker than serum phosphate for the initiation of treatment of hyperphosphatemia in CRF and HD. These results may also offer new approaches in hyperphosphatemia therapy by establishing measures to bind salivary phosphate in the oral cavity before saliva is swallowed [56].
Salivary biomarkers in psychological research
Stress and pain are often interrelated events. Investigators have attempted to distinguish them using a variety of model systems that induce either stress or pain, and subjects are monitored for changes in salivary biomarkers. Typical markers that have been identified include salivary amylase, cortisol, substance P, lysozyme and secretory IgA. Pain responses in dental pulp have been specifically associated with neuropeptides including calcitonin gene-related peptide (CGRP), substance P, neurokinin A and neurokinin P. Salivary testosterone levels have been associated with increased aggressive behavior and also with athletic activities [58]. Several reports relate cognitive behavior to levels of tryptophan and serotonin, the latter being monitored in saliva. It should be pointed out that for studies in psychological and behavior fields, POC collection of saliva samples can play a key role, as a blood draw may induce both stress and pain in some individuals.
Salivary markers for systemic malignancies
The search for biomarkers for a variety of malignancies has been ongoing for decades. Once such biomarkers are detected in serum, it is a natural progression to look for these same markers in saliva. The ability to detect specific markers, particularly for malignancies that have few early symptoms such as ovarian and pancreatic cancer would have tremendous impact on survival rates. Mutations of the tumor suppressor p53 were first reported for salivary gland adenomas in 1992 [59] and subsequently described in a pilot study of saliva from breast cancer subjects [60]. Subsequently, there were reports of elevated levels of the cancer antigen, CA15-3 and the oncogene c-erB2, in woman with breast cancer as compared to controls [1, 2, 60, 61]. Chen et al [62] identified the tumor marker C125 in saliva of subjects with malignant ovarian tumors. Other studies have reported down-regulation of the tumor suppressor DMBT1 in mammary tumors in mice [63] and humans [64]. Zhang et al [65] have identified four mRNA biomarkers that could distinguish pancreatic cancer subjects from pancreatitis and control subjects. It is likely that there will be an increased effort to substantiate and extend these findings in a variety of solid tumors in order to develop an early diagnostic profile.
Diabetes biomarkers
Because of the large diabetic population, combined with the current epidemic of Type 2 diabetes, an oral test to monitor blood glucose would be highly desirable. Unfortunately, while it is relatively easy to measure salivary glucose, due to the multiple sources of this material in the oral cavity, salivary glucose levels do not correlate with blood glucose levels. However, several other approaches are under investigation. A recent report by Rao et al [66] demonstrated a unique proteomic signature in saliva obtained from Type-2 diabetics as compared to control saliva, with 65 proteins showing greater than a 2-fold change. Many of these proteins were associated with metabolic and immune regulatory pathways. While further studies are clearly needed, these findings suggest that there may indeed be a unique salivary biomarker profile associated with diabetes. Another interesting approach to detect Type 1 diabetic hyperglycemia involves measuring exhaled methyl nitrate [67]. These investigators demonstrated a correlation between blood glucose levels and exhaled methyl nitrate, presumably due to interaction of superoxide dismutase with nitric oxide as a byproduct of elevated oxidative reactions.
Finally, Strauss et al [68] have proposed using gingival crevicular blood as a measure of blood glucose. In a study of fifty four subjects, blood obtained during a routine periodontal exam was collected and compared to blood obtained with a finger-stick; the study showed good correlation between samples collected from the two sites.
Saliva tests for forensics
Salivary test have been used for a wide variety of forensic studies. Samples can be obtained from drinking glasses, cigarette butts, envelopes, and other sources and then used to detect blood-group substances or salivary genetic proteins (primarily proline-rich protein polymorphisms). Approximately 85% of individuals secrete blood-group antigens in their saliva including A, B, H, and Lewis antigens that have been used for identification of individuals in both criminal cases and paternity law suits. With the widespread use of DNA testing, samples of DNA taken from the buccal surface with an oral swab can be easily obtained by untrained individuals without the need for a phlebotomist. Saliva is often present at crime scenes, along with other body fluids, and since DNA is relatively stable in the dry state, these samples can be used to place an individual at the scene of a crime.
Salivary diagnostics for autoimmune diseases
Major rheumatoid factor diseases include Lupus Erythematosis, Scleroderma, and Sjogren's syndrome. These autoimmune diseases are characterized by the production of auto-antibodies that attack healthy tissue. Sjogren's syndrome is a disease characterized by dryness of the eyes and mouth [69] and it may occur as a primary or a secondary disease. The clinical symptoms in the primary form are more restricted and are associated with lacrimal and salivary gland dryness. In secondary Sjogren's syndrome, patients undergo one of the autoimmune diseases mentioned above before Sjogren's symptoms develop. In contrast, the primary Sjogren's Syndrome (pSS) occurs by itself and it is the third most common autoimmune disease with a reported prevalence between 0.05 and 4.8% [70], mostly (90%) occurring in women.
For decades, the pSS diagnosis has been based on oral examination, detection of blood biomarkers (autoantibodies to self-antigens (SS-A and SS-B), Rheumatoid factor and antinuclear antibodies, and by obtaining a confirmatory salivary gland biopsy [71, 72]. Patients with pSS have forty times higher risk of developing lymphoma, a fatal lymphocytic cancer. In contrast, patients with secondary Sjogren's syndrome tend to have more health problems because they suffer from a primary condition as well as SS. They are also less likely to have the antibodies associated with the pSS.
More recently, a panel of salivary biomarkers that can distinguish pSS patients from healthy subjects has been reported by Hu et al [73]. Using cutting-edge proteomics and genomics technologies, investigators searched globally for markers in saliva from pSS patients and healthy controls, and found that whole saliva (i.e., the combination of saliva in the mouth plus saliva from the individual salivary glands), contained a series of biomarkers that could detect pSS. In addition, the proteomic and genomic profile of these salivary markers reflected the damage to glandular cells, activated anti-viral immune response, or programmed cell death known to be involved in pSS pathogenesis. The value of these candidate salivary biomarkers for pSS diagnosis has been confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) and immunoblotting techniques by the same investigators. Similar to pSS, the progress made in cataloguing oral biomarkers derived from the salivary proteome has provided a unique opportunity and a novel approach for the future use of salivary diagnostics in many other conditions [45, 74, 75, 76].
Salivary biomarkers for infectious diseases
This topic was recently reviewed [1, 3] and will only be briefly summarized here. The previous review identified twenty three viruses that could be identified in salivary samples by specific antibody reactivity, antigen detection, or nucleic acid via PCR. These include a large range of Herpes viruses, Hepatitis viruses, HIV, Human Papillomavirus (HPV), Influenza virus, and Poliovirus. Fourteen bacterial pathogens were detected (by antibody, antigen or nucleic acid) including Escherichia coli, Mycobacterium tuberculosis, Helicobacter pylori, Treponema pallidum and a wide range of streptococcal species. Non-viral and non-bacterial infectious agents including Candida albicans, Toxoplama gondii, and Schistosoma mansoni were detectable, typically by antibodies to these infectious agents. These pathogens are responsible for both systemic and oral diseases. Tests for these, and many other pathogens, are currently under development by a large number of commercial and academic entities, so that it is likely that additional salivary-based tests for infectious diseases will continue to emerge. Some of the common pathogen- and non-pathogen-induced oral diseases and the role of saliva in their diagnosis are described below.
Salivary diagnostics of common oral diseases
The physicochemical and biochemical properties of saliva along with its complex composition endowes this fluid with multiple functions, including: anti-bacterial, anti-viral and anti-fungal properties; buffering capacity for plaque acids; digestive activity (amylase, protease, nuclease enzymes) needed for food mastication; mineralizing agents for protection and repair of hard tissues; lubricant and viscoelastic properties essential for the maintenance of oral health; and protective and repairing fluid for mucosal surfaces. Saliva is a hypotonic biofluid composed of 99.5% water and 0.5% ions (e.g., potassium, calcium, chloride, sodium and phosphates), and organic micro- and macro-molecules (e.g., amino acids, histatins, cystatins, defensins, statherins, lysozyme, proline-rich proteins, carbonic anhydrases, peroxidases, lactoferrin, mucins, secretory immunoglobulins, and lipids among others). The origin of these salivary components is diverse and complex and they are not reviewed here. Whole saliva can be easily collected with stimulating agents (using paraffin for mastication, or using citric acid or sour candy drops on the tongue) or without stimulation. The unstimulated whole saliva is often used in diagnostics as stimulated whole saliva contains a diluted concentration of biomarkers that may be difficult to detect.
Another oral fluid of interest for clinical diagnostics is the GCF, which is an interstitial biofluid or inflammatory transudate that flows out via the gingival crevice and contains cells (desquamated epithelial cells, neutrophils, lymphocytes and monocytes, and pathogens such as bacteria); electrolytes similar to plasma (e.g., potassium and calcium); and organic components also similar to plasma (e.g., albumin, globulins, complement s, protease inhibitors, lactate, urea, and multiple enzymes). The GCF has a protective role in the oral cavity by removing potentially harmful cells, molecules and pathogens, and also has an antibacterial role by virtue of its pathogen neutralizing antibodies.
Many of the salivary or GCF derived molecules are used as diagnostic biomarkers for oral diseases including oral cancer, and conditions caused by fungi (Candida species), viruses (HPV, Epstein-Barr Virus [EBV], Cytomegalovirus [CMV]) and bacteria (multiple species involved in periodontal diseases and caries). In many instances, pathogen-induced oral diseases have been reported as opportunistic or secondary infections and are referred to as early manifestations of the Acquired Immunodeficiency Syndrome (AIDS) in HIV infected subjects. The frequency of many AIDS-related oral manifestations varies, but increases in the absence of highly active antiretroviral therapy (HAART), and may indicate inadequate HAART treatment, development of drug resistance, or therapeutic failure.
Salivary diagnostics in oral squamous cell carcinoma
The oral squamous cell carcinoma (OSCC) is the most common malignancy of the oral cavity among oral cancers (e.g. adenocarcinomas, lymphomas, sarcomas, verrucous or mucoepidermoid carcinomas, malignant melanoma, and Kaposi’s sarcoma), accounting for more than 90% of clinical cases and ranking among the top ten types of cancers worldwide [77]. However, oral cancers have also been reported with less frequency in the oral mucosa, tongue, pharynx, lips, gums, palate, salivary glands, tonsils and sinuses. Epidemiological data have shown an increased incidence and mortality of oral cavity cancers in many countries [78]. The latter is associated with risk factors, including tobacco, alcohol, oral pathogen infections, environmental factors, and poor oral hygiene [52]. Classically, clinical diagnosis of oral cancer has been based on visual and palpation assessment, followed by biopsy and histopathological evaluation. However, this clinical assessment has been broadened by use of magnetic resonance imaging and computerized tomography [79]; toluidine blue staining [80] and light-based detection techniques [81]. More recently, detection of biomarkers in saliva has emerged as a novel approach for the diagnosis of OSCC and its developmental stages including, initial process, invasion, recurrence and treatment. A comprehensive description of these oral cancer biomarkers has been previously described including, oncogenes (e.g. C-myc, c-Fos, C-Jun), anti-oncogenes (e.g. p53, p16), cytokines (e.g. TGF-β1, IL-8, and IL-1 β), growth factors (e.g. VEGF, EGF and IGF), extracellular matrix-degrading proteinases (MMP1, MMP2, MMP9), hypoxia markers (HIF-α, CA-9), epithelial-mesenchymal transition markers (e.g. E-cadherin, N-cadherin and β-catenin), epithelial tumor factors (CYFRA 21-1), cytokeratins (CK13, 14 and 16), micro RNA molecules and hypermethylation of cancer-related genes (p16 and DAP-K) [43, 82, 83, 84, 85, 86, 87, 88]. These biomarkers have been defined using molecular, transcriptomic, genomic, proteomic, metabolomic and phenotypic techniques. However, further development and validation of these biomarkers is needed for routine implementation in clinical diagnostics to assist with early cancer detection, risk assessment and response to therapies. Refinement of a panel of soluble salivary biomarkers will depend on their stability and accuracy of detection, incorporation into sensitive and reproducible assays easy to perform, high sensitivity and specificity to indicate specific diseases and their stages of development, easy quantification in the clinical laboratory, and cost effectiveness integration into clinical diagnostic algorithms.
Salivary diagnostics in oral fungal diseases
The oral cavity of immunocompetent individuals contains resident microbiota co-existing under a delicate immunophysiological balance and including an important fungal component known as the oral mycobiome. The latter includes culturable and non-culturable fungi, some of which may be pathogenic, causing common oral diseases such as oropharyngeal candidiasis (OPC), frequently observed in immunocompromised individuals. A recent study characterized the oral mycobiome of twenty healthy individuals showing that Candida species were the most frequently isolated fungi (present in 75% of participants), followed by Cladosporium (65%), Aureobasidium, Saccharomycetales (50% for both), Aspergillus (35%), Fusarium (30%), and Cryptococcus (20%) [89]. There are numerous factors that can disturb the balance of microorganisms in the oral microbiome and mycobiome, predisposing individuals to fungal diseases, including: physiological changes that occur in the geriatric and pediatric populations and during pregnancy; disturbances of soft and hard tissues caused by lesions or poor oral hygiene; prolonged use of antibiotics with a broad antimicrobial spectrum; extended use of steroids that impair the immune system; nutritional deficiencies in micro- or macro-nutrients; endocrinological malfunction associated with diseases such as hypothyroidism; chemotherapy and radiotherapy-induced immunosuppression due to cancer; immunodeficiencies caused by pathogens such as the HIV or congenital defects such as thymic aplasia; Xerostomia; autoimmune diseases (Sjogren's syndrome); use of prosthodontic appliances; and diabetes. Mycotic infections of the oral cavity show different etiologies, pathogenesis, and clinical forms. Primary OPC has been described as pseudomembranous, erythematous and hyperplastic; while secondary OPC has been described as a chronic mucocutaneous presentation [90]. OPC being one of the most common oral diseases occurs as a mixed yeast-bacterial biofilm infection and it is most commonly caused by Candida albicans. However, other Candida species are also seen in medically compromised patients with a history of liberal use of azoles [91]. Classically, the diagnosis of oral mycoses, including OPC, is based on an oral clinical examination along with the collection of oral specimens (swab, sputum or saliva) for clinical laboratory analysis. The latter involves in vitro culture to isolate and identify the etiological agent, direct microscopic analysis for pathogen visualization, and histopathological staining to confirm the etiological agent and assess the severity of tissue damage [92, 93]. To date, saliva samples for clinical diagnosis of fungal infections are only used for pathogen isolation and not for direct clinical assay applications. Yet, the performance of a commercial enzyme-linked immunosorbent assay (ELISA) kit to detect Candida’s mannan antigen in oral rinse solutions has been reported, but further assay optimization is needed for oral specimens [94]. Experimental attempts have also been made to detect salivary IgA or IgG antibodies to Candida [95, 96, 97], but immunodiagnosis remains elusive because of differences observed in sensitivity and specificity of different assays when detecting various Candida antigen preparations.
Salivary diagnostics in oral diseases caused by viruses
Oral diseases caused by viruses are prevalent including papillomaviruses (HPV associated with oral cancer –OSSC- and oral warts) and herpesviruses (EBV causing Hairy Leukoplakia and is also associated with various types of lymphoid and epithelial malignancies; Cytomegalovirus [CMV] causing opportunistic infections after solid organ transplantation, retinitis, gastrointestinal and neurological disorders, and oral ulcerations; Herpes Simplex Viruses 1 and 2 [HSV-1 and HSV-2] and Varicella Zoster Virus [VZV] also causing oral ulcerations of the aphthous type; and Human Herpesvirus 8 [HHV-8] causing oral and systemic Kaposi’s sarcoma). These oral diseases have been more frequently reported in immunocompromised patients due to impairment of the immune system, especially those with HIV/AIDS not receiving HAART, which represents more than 70% of people residing in countries where the AIDS epidemic is most devastating [98, 99, 100, 101, 102, 103, 104, 105, 106]. Upon initiation of highly active antiretroviral therapy (HAART), HIV/AIDS patients show lower prevalence of some of these oral diseases (Hairy Leukoplakia and Kaposi’s sarcoma), but other conditions continue or may be even more prevalent under HAART (oral warts, oral cancers, salivary gland disease and oral lesions associated with immune reconstitution inflammatory syndrome -IRIS) [107, 108]. The clinical diagnosis of the oral diseases and viral agents described above is based on clinical examination supported by confirmatory clinical laboratory testing, including histopathological staining of tissue specimens, microscopic visualization of lesions and pathogens, virus isolation from clinical specimens, and nucleic- and immunoassays for detection of viral and host biomarkers. For instance, the standard approach for HIV clinical laboratory diagnosis has been testing serum or plasma samples in a sensitive ELISA followed by a Western blot if the ELISA is positive. However, oral fluids have also been successfully used in lab diagnostics to detect HIV antigen and antibodies in different nucleic- and immunoassay formats such as qRT-PCR, ELISA, rapid test, POC and microfluidic diagnostic devices [18, 109, 110, 111, 112, 113, 114, 115, 116]. Additionally, HIV neutralizing innate immune factors such as defensins have also been successfully detected in saliva using sophisticated experimental methodologies such as liquid chromatography-tandem mass spectrometry that involves limited sample manipulation and that can be easily automated [117].
Experimental detection of HPV in saliva samples has utilized nucleic acid assays such as HPV DNA amplification by PCR [118, 119] and this methodology has also been used to detect different HPV types [99]. Antibodies to HPV have been simultaneously tested in serum, saliva and oral mucosal transudate specimens yielding promising results when using oral fluids, but further optimization has also been recommended as a reliable alternative to serum HPV testing [120].
The EBV DNA load in blood and saliva detected by PCR has shown similar results in cohorts of HIV infected patients [121, 122, 123]. The nested PCR technique has been used to consistently detect CMV in subgingival plaque, unstimulated saliva and peripheral blood of patients with chronic periodontitis [124] and it has been reported that saliva is as reliable as urine for CMV detection in large screening programs [125]. More recently, saliva specimens have been successfully used for direct genotyping of CMV strains in a new PCR-restriction fragment length polymorphism (RFLP) method, coupled with capillary electrophoresis fragment detection for genotyping [126]. Comparative nested PCR analyses of saliva and peripheral blood specimens have consistently demonstrated HSV-1 detection with similar frequencies in both types of samples [101]. Similarly, reliable detection and quantification of nucleic acids for HSV-1, HSV-2 and VZV in oral fluids have been reported. There is a new standardized liquid phase-based saliva collection system, followed by a fully automated viral nucleic acid extraction and RT-PCR, using commercially available in vitro diagnostics (IVD)/Conformité Européene (CE) labeled molecular assays [127]. Lastly, shedding of HHV-8 in saliva has also been demonstrated using PCR and immunohistochemistry [128, 129], and HHV-8 load in blood, serum and saliva have shown comparable titers by qRT-PCR [130].
The simultaneous detection of viruses in oral fluids using different assays with multiple applications is an emerging field. Many of these assays are changing to incorporate multiplexing capabilities and to take advantage of nanotechnology approaches, yielding automated, reliable and sensitive diagnostic devices. Yet, these novel detection systems require further optimization and validation prior to implementation in routine clinical diagnostics.
Salivary diagnostics in oral diseases caused by bacteria
Caries and periodontitis are the most commonly known polymicrobial-driven diseases of the oral cavity. Periodontal disease is a chronic inflammatory process of the periodontium in response to bacterial plaque deposited on the adjacent teeth. Bacterial infections forming biofilms, destroy the alveolar bone and periodontal ligament, induce gingivitis, cause apical migration of the epithelial attachment resulting in the formation of periodontal pockets, and induce irreversible loss and exfoliation of the teeth. If left untreated, gingivitis may progress into periodontitis, leading to tooth loss and severe lesions of soft and hard tissues. Periodontitis is also linked to systemic illness, such as CVD and diabetes. Caries is also caused by bacterial plaque that in combination with fermentable carbohydrates produces acids (e.g., lactic acid) that lower the pH at the surface of the tooth compromising the enamel, dentin and cementum, and ultimately affect the structural integrity of the tooth.
Clinical diagnosis of periodontal disease is based on an oral examination, consisting of inspection of the gingival tissue on the buccal and lingual side of every tooth, conducting a periodontal screening and recording pocket depths for each tooth, checking attachment level, measuring plaque index, testing bleeding on probing, testing tooth mobility, and taking radiographs to assess bone loss. Caries are also clinically diagnosed upon visual examination and by taking radiographs. In both oral diseases, identification of the etiological agents may be carried out. For this, oral specimens including plaque, GCF and saliva are sent and analyzed in diagnostic laboratories. Isolation of bacterial species from oral specimens using classical in vitro methods is only possible for cultivable species such as Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia present as a complex biofilm in destructive periodontitis [131], and Streptococcus mutans and Lactobacillus sp. frequently found in caries [132, 133]. However, the majority of the oral bacterial species are uncultivable. For instance, the oral microbiota contains over 700 individual taxa with approximately 200 characterized bacterial species and only about 100 of them representing cultivable strains in vitro. To bridge this gap, novel approaches in salivary diagnostics have been developed to characterize the role of the uncultivable microbiome in disease initiation and progression. Other studies are conducting a comparative analysis of salivary proteomic profiles in patients with periodontitis and healthy subjects, showing distinctive profiles with alterations of salivary proteins in presence of periodontal inflammation, which may contribute to the improvement of periodontal diagnosis [134, 135, 136]. Recently, a clinical study was conducted in which one hundred individuals were enrolled either into a group of healthy/gingivitis subjects or into a group of subjects with periodontitis to identify pathogen and host-response salivary biomarkers correlated with periodontitis [137]. For this purpose, a rapid POC chairside diagnostics was utilized, which had the capacity to characterize early stages of periodontal infection and its progression into disease. Whole saliva was collected and analyzed using antibody arrays to measure the levels of multiple proinflammatory cytokines and bone resorptive/turnover markers. Salivary biomarker data were correlated to comprehensive clinical, radiographic, and microbial plaque biofilm level (measured by quantitative PCR) to generate periodontal disease identification models. As previously described, biomarkers such as MMP-8 and −9 (matrix metalloproteinases) were elevated in subjects with advanced periodontitis, which was predicted when assessing multiple combinations of salivary biomarkers (e.g., MMP-8 and −9 and osteoprotegerin) along with red-complex anaerobic periodontal pathogens (e.g., Porphyromonas gingivalis or Treponema denticola). In addition, disease severity was also predicted when obtaining elevated salivary MMP-8 and T. denticola biofilm levels. This approach proved the usefulness of monitoring salivary and host-response biomarkers for an oral disease. Studies are ongoing to apply this approach to the longitudinal predictions of disease activity [137]. A similar POC diagnostic approach was previously developed as a portable microfluidic device consisting of a chip-based immunoassay to detect biomarkers of periodontal disease in saliva [138].
The clinical value of salivary proteomic biomarkers in periodontal disease diagnosis is under experimental development and is based on profile changes in molecules involved in inflammation, collagen degradation and bone loss [65, 137, 139]. In spite of this progress, some of the biomarkers identified are not disease specific. As with salivary proteomics, salivary transcriptomics and genomics in high-throughput platforms have also been developed using oral diseases as models [65], but face similar challenges as described above.
In addition to using plaque specimens for conventional bacterial isolation methods and saliva samples for proteomic biomarker profiling, GCF specimens have also been tested by ELISA to assess differential expression of specific host biomarkers (e.g., RANKL and cathepsin-K indicators of osteoclast activity) for the experimental diagnosis of periodontal disease [140, 141], demonstrating the use of GCF specimens in oral diagnostics. In fact, detection of GCF constituents (mostly inflammatory mediators) in saliva is the current focus of most saliva-based tests for periodontal disease.
The value of salivary diagnostics in caries has also been reported. An experimental assay was developed using biomarkers (genetically determined oligosaccharides profiles present on salivary glycoproteins) for caries risk assessment with prognostic value for caries susceptibility [142, 143]. Evaluation of this unique assay is underway for future diagnostic applications.
Summary
Early studies attempting to utilize saliva as a diagnostic fluid were hampered by a lack of understanding on how these biomarkers enter saliva, the difficulty in detecting some markers due to low levels in saliva as compared to serum, and lack of attention to the method of collection and storage of samples prior to analysis. These challenges have largely been met as a result of careful studies of salivary gland physiology, development of sensitive amplification methods (e.g. ELISA, qRT-PCR), and education of the scientific community in the methodology for obtaining and dealing with salivary samples. The recent advances in oral fluid biomarker diagnostics has been fueled by novel molecular approaches (e.g. proteomics, transcriptomics and genomics) and metagenomic analyses that have broadened the discovery of microbial pathogens associated with systemic and oral diseases. Similarly, these experimental approaches have been successfully used in the diagnosis of non-infectious systemic and oral conditions (e.g., cancers, autoimmune diseases, renal disease and diabetes). The future of this field will depend on further validation of disease (and stage) specific biomarkers and their incorporation into state-of-the-art, multiplex assays that are versatile, quantitative, reliable, sensitive, specific, rapid, robust, and cost effective for broad implementation in diagnostic programs.
Acknowledgments
This work acknowledges NIH grants UO1 DE14964 and U19 DE18385, the New York State office of Science Technology and Academic Research (NYSTAR), and National Institute of Dental and Craniofacial Research for continued support to the fields of Salivary Diagnostics and AIDS-related oral manifestations, malignancies and Immunosuppression, the contribution of Dr. William Abrams in preparing the figures and reviewing the manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corstjens PLAMMD. Point-of-care Diagnostics for infectious diseases. In: Wong DT, editor. Saliva Diagnostics. Ames: Wiley-Blackwell; 2008. pp. 243–254. [Google Scholar]
- 2.Farnaud SJ, Kosti O, Getting SJ, et al. Saliva: physiology and diagnostic potential in health and disease. ScientificWorldJournal. 2010;10:434–456. doi: 10.1100/tsw.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malamud D, Tabak LA, editors. Saliva as a Diagnostic Fluid: Ann NY Acad Sci. 1992. Malamud D, Tabak LA, eds. Saliva as a Diagnostic Fluid; No. 694. [Google Scholar]
- 4.Malamud D, Niedbala RS. Oral-based diagnostics. Boston: Mass; 2007. New York Academy of Sciences. Published by Blackwell Pub. on behalf of the New York Academy of Sciences. [Google Scholar]
- 5.Wong D. Salivary diagnostics. Ames, Iowa: Wiley-Blackwell; 2008. [Google Scholar]
- 6.Greenberg BL, Glick M, Frantsve-Hawley J, et al. Dentists' attitudes toward chairside screening for medical conditions. J Am Dent Assoc. 2010;141(1):52–62. doi: 10.14219/jada.archive.2010.0021. [DOI] [PubMed] [Google Scholar]
- 7.Parisi MR, Soldini L, Di Perri G, et al. Offer of rapid testing and alternative biological samples as practical tools to implement HIV screening programs. New Microbiol. 2009;32(4):391–396. [PubMed] [Google Scholar]
- 8.White DA, Scribner AN, Huang JV. A comparison of patient acceptance of fingerstick whole blood and oral fluid rapid HIV screening in an emergency department. J Acquir Immune Defic Syndr. 2009;52(1):75–78. doi: 10.1097/QAI.0b013e3181afd33d. [DOI] [PubMed] [Google Scholar]
- 9.Lijnen I, Willems G. DNA research in forensic dentistry. Methods Find Exp Clin Pharmacol. 2001;23(9):511–517. doi: 10.1358/mf.2001.23.9.662139. [DOI] [PubMed] [Google Scholar]
- 10.Virkler K, Lednev IK. Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int. 2009;188(1–3):1–17. doi: 10.1016/j.forsciint.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Kohnemann S, Pfeiffer H. Application of mtDNA SNP analysis in forensic casework. Forensic Sci Int Genet. 2010 doi: 10.1016/j.fsigen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Gerritsen L, Geerlings MI, Beekman AT, et al. Early and late life events and salivary cortisol in older persons. Psychol Med. 2009:1–10. doi: 10.1017/S0033291709991863. [DOI] [PubMed] [Google Scholar]
- 13.Hedriana HL, Munro CJ, Eby-Wilkens EM, et al. Changes in rates of salivary estriol increases before parturition at term. Am J Obstet Gynecol. 2001;184(2):123–130. doi: 10.1067/mob.2001.108338. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Lee YJ, Ahn RS. Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Med J. 2010;51(2):212–218. doi: 10.3349/ymj.2010.51.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klebanoff MA, Meis PJ, Dombrowski MP, et al. Salivary progesterone and estriol among pregnant women treated with 17-alpha-hydroxyprogesterone caproate or placebo. Am J Obstet Gynecol. 2008;199(5):506, e501–e507. doi: 10.1016/j.ajog.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007;24(5):969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- 17.Touitou Y, Auzeby A, Camus F, et al. Daily profiles of salivary and urinary melatonin and steroids in healthy prepubertal boys. J Pediatr Endocrinol Metab. 2009;22(11):1009–1015. doi: 10.1515/jpem.2009.22.11.1009. [DOI] [PubMed] [Google Scholar]
- 18.Abrams WR, Barber CA, McCann K, et al. Development of a microfluidic device for detection of pathogens in oral samples using upconverting phosphor technology (UPT) Ann N Y Acad Sci. 2007;1098:375–388. doi: 10.1196/annals.1384.020. [DOI] [PubMed] [Google Scholar]
- 19.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 20.Gau V, Wong D. Oral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platform. Ann N Y Acad Sci. 2007;1098:401–410. doi: 10.1196/annals.1384.005. [DOI] [PubMed] [Google Scholar]
- 21.Walt DR, Blicharz TM, Hayman RB, et al. Microsensor arrays for saliva diagnostics. Ann N Y Acad Sci. 2007;1098:389–400. doi: 10.1196/annals.1384.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 23.Dias Fernandes CS, Salum FG, Bandeira D, et al. Salivary dehydroepiandrosterone (DHEA) levels in patients with the complaint of burning mouth: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(4):537–543. doi: 10.1016/j.tripleo.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Gavrilova N, Lindau ST. Salivary sex hormone measurement in a national, population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64 Suppl 1:i94–i105. doi: 10.1093/geronb/gbn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray SH, Ebe LK, Feldman HA, et al. Salivary Progesterone Levels Before Menarche: A Prospective Study of Adolescent Girls. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton LD, van Anders SM, Cox DN, et al. The effect of competition on salivary testosterone in elite female athletes. Int J Sports Physiol Perform. 2009;4(4):538–542. doi: 10.1123/ijspp.4.4.538. [DOI] [PubMed] [Google Scholar]
- 27.Manolopoulou J, Gerum S, Mulatero P, et al. Salivary Aldosterone as a Diagnostic Aid in Primary Aldosteronism. Horm Metab Res. 2010 doi: 10.1055/s-0030-1248287. [DOI] [PubMed] [Google Scholar]
- 28.Vitzthum VJ, Worthman CM, Beall CM, et al. Seasonal and circadian variation in salivary testosterone in rural Bolivian men. Am J Hum Biol. 2009;21(6):762–768. doi: 10.1002/ajhb.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warrener L, Slibinskas R, Brown D, et al. Development and evaluation of a rapid immunochromatographic test for mumps-specific IgM in oral fluid specimens and use as a matrix for preserving viral nucleic acid for RT-PCR. J Med Virol. 2010;82(3):485–493. doi: 10.1002/jmv.21693. [DOI] [PubMed] [Google Scholar]
- 30.Adisen E, Aral A, Aybay C, et al. Salivary epidermal growth factor levels in Behcet's disease and recurrent aphthous stomatitis. Dermatology. 2008;217(3):235–240. doi: 10.1159/000148250. [DOI] [PubMed] [Google Scholar]
- 31.Eckley CA, Rios Lda S, Rizzo LV. Salivary egf concentration in adults with reflux chronic laryngitis before and after treatment: preliminary results. Braz J Otorhinolaryngol. 2007;73(2):156–160. doi: 10.1016/S1808-8694(15)31060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam JW, Chung JW, Kho HS, et al. Nerve growth factor concentration in human saliva. Oral Dis. 2007;13(2):187–192. doi: 10.1111/j.1601-0825.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 33.Taichman NS, Cruchley AT, Fletcher LM, et al. Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest. 1998;78(7):869–875. [PubMed] [Google Scholar]
- 34.Upile T, Jerjes W, Kafas P, et al. Salivary VEGF: a non-invasive angiogenic and lymphangiogenic proxy in head and neck cancer prognostication. Int Arch Med. 2009;2(1):12. doi: 10.1186/1755-7682-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blicharz TM, Rissin DM, Bowden M, et al. Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: a proof of principle. Clin Chem. 2008;54(9):1473–1480. doi: 10.1373/clinchem.2008.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh KI, Kim YK, Kho HS. Salivary levels of IL-1beta, IL-6, IL-8, and TNF-alpha in patients with burning mouth syndrome. Arch Oral Biol. 2009;54(9):797–802. doi: 10.1016/j.archoralbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Teles RP, Likhari V, Socransky SS, et al. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44(3):411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas MV, Branscum A, Miller CS, et al. Within-subject variability in repeated measures of salivary analytes in healthy adults. J Periodontol. 2009;80(7):1146–1153. doi: 10.1902/jop.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome. Oral Dis. 2009;15(8):519–526. doi: 10.1111/j.1601-0825.2009.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malamud D, Abrams WR, Bau H, et al. Oral-based techniques for the diagnosis of infectious diseases. J Calif Dent Assoc. 2006;34(4):297–301. [PubMed] [Google Scholar]
- 41.Palanisamy V, Sharma S, Deshpande A, et al. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5(1):e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starke EM, Smoot JC, Wu JH, et al. Saliva-based diagnostics using 16S rRNA microarrays and microfluidics. Ann N Y Acad Sci. 2007;1098:345–361. doi: 10.1196/annals.1384.007. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann BG, Park NJ, Wong DT. Genomic targets in saliva. Ann N Y Acad Sci. 2007;1098:184–191. doi: 10.1196/annals.1384.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qvarnstrom M, Janket S, Jones JA, et al. Salivary lysozyme and prevalent hypertension. J Dent Res. 2008;87(5):480–484. doi: 10.1177/154405910808700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan W, Apweiler R, Balgley BM, et al. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl. 2009;3(1):116–134. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zehetbauer S, Wojahn T, Hiller KA, et al. Resemblance of salivary protein profiles between children with early childhood caries and caries-free controls. Eur J Oral Sci. 2009;117(4):369–373. doi: 10.1111/j.1600-0722.2009.00641.x. [DOI] [PubMed] [Google Scholar]
- 47.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27(3):147–159. [PMC free article] [PubMed] [Google Scholar]
- 49.Langel K, Engblom C, Pehrsson A, et al. Drug testing in oral fluid-evaluation of sample collection devices. J Anal Toxicol. 2008;32(6):393–401. doi: 10.1093/jat/32.6.393. [DOI] [PubMed] [Google Scholar]
- 50.Langman LJ. The use of oral fluid for therapeutic drug management: clinical and forensic toxicology. Ann N Y Acad Sci. 2007;1098:145–166. doi: 10.1196/annals.1384.001. [DOI] [PubMed] [Google Scholar]
- 51.Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55(8):1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010 doi: 10.1016/j.oraloncology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Miller CS, Foley JD, Bailey AL, et al. Current developments in salivary diagnostics. Biomark Med. 2010;4(1):171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arregger AL, Cardoso EM, Tumilasci O, et al. Diagnostic value of salivary cortisol in end stage renal disease. Steroids. 2008;73(1):77–82. doi: 10.1016/j.steroids.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Nagler RM. Saliva analysis for monitoring dialysis and renal function. Clin Chem. 2008;54(9):1415–1417. doi: 10.1373/clinchem.2008.112136. [DOI] [PubMed] [Google Scholar]
- 56.Savica V, Calo L, Santoro D, et al. Salivary phosphate secretion in chronic kidney disease. J Ren Nutr. 2008;18(1):87–90. doi: 10.1053/j.jrn.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Savica V, Calo LA, Granata A, et al. A new approach to the evaluation of hyperphosphatemia in chronic kidney disease. Clin Nephrol. 2007;68(4):216–221. [PubMed] [Google Scholar]
- 58.Dabbs JM., Jr Salivary testosterone measurements in behavioral studies. Ann N Y Acad Sci. 1993;694:177–183. doi: 10.1111/j.1749-6632.1993.tb18351.x. [DOI] [PubMed] [Google Scholar]
- 59.Azuma M, Kasai Y, Tamatani T, et al. Involvement of p53 mutation in the development of human salivary gland pleomorphic adenomas. Cancer Lett. 1992;65(1):61–71. doi: 10.1016/0304-3835(92)90214-g. [DOI] [PubMed] [Google Scholar]
- 60.Streckfus C, Bigler L, Tucci M, et al. A preliminary study of CA15-3, c-erbB-2, epidermal growth factor receptor, cathepsin-D, and p53 in saliva among women with breast carcinoma. Cancer Invest. 2000;18(2):101–109. doi: 10.3109/07357900009038240. [DOI] [PubMed] [Google Scholar]
- 61.Streckfus C, Bigler L. The use of soluble, salivary c-erbB-2 for the detection and post-operative follow-up of breast cancer in women: the results of a five-year translational research study. Adv Dent Res. 2005;18(1):17–24. doi: 10.1177/154407370501800105. [DOI] [PubMed] [Google Scholar]
- 62.Chen DX, Schwartz PE, Li FQ. Saliva and serum CA 125 assays for detecting malignant ovarian tumors. Obstet Gynecol. 1990;75(4):701–704. [PubMed] [Google Scholar]
- 63.Blackburn AC, Hill LZ, Roberts AL, et al. Genetic mapping in mice identifies DMBT1 as a candidate modifier of mammary tumors and breast cancer risk. Am J Pathol. 2007;170(6):2030–2041. doi: 10.2353/ajpath.2007.060512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braidotti P, Nuciforo PG, Mollenhauer J, et al. DMBT1 expression is down-regulated in breast cancer. BMC Cancer. 2004;4:46. doi: 10.1186/1471-2407-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Henson BS, Camargo PM, et al. The clinical value of salivary biomarkers for periodontal disease. Periodontol 2000. 2009;51:25–37. doi: 10.1111/j.1600-0757.2009.00315.x. [DOI] [PubMed] [Google Scholar]
- 66.Rao PV, Reddy AP, Lu X, et al. Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res. 2009;8(1):239–245. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 67.Novak BJ, Blake DR, Meinardi S, et al. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(40):15613–15618. doi: 10.1073/pnas.0706533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strauss SM, Wheeler AJ, Russell SL, et al. The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: an examination based on characteristics of the blood collection site. J Periodontol. 2009;80(6):907–914. doi: 10.1902/jop.2009.080542. [DOI] [PubMed] [Google Scholar]
- 69.Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren's Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pillemer SR, Matteson EL, Jacobsson LT, et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76(6):593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 71.Cummins MJ, Papas A, Kammer GM, et al. Treatment of primary Sjogren's syndrome with low-dose human interferon alfa administered by the oromucosal route: combined phase III results. Arthritis Rheum. 2003;49(4):585–593. doi: 10.1002/art.11199. [DOI] [PubMed] [Google Scholar]
- 72.Kruszka P, O'Brian RJ. Diagnosis and management of Sjogren syndrome. Am Fam Physician. 2009;79(6):465–470. [PubMed] [Google Scholar]
- 73.Hu S, Wang J, Meijer J, et al. Salivary proteomic and genomic biomarkers for primary Sjogren's syndrome. Arthritis Rheum. 2007;56(11):3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7(5):1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu S, Xie Y, Ramachandran P, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5(6):1714–1728. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- 76.Sondej M, Denny PA, Xie Y, et al. Glycoprofiling of the Human Salivary Proteome. Clin Proteomics. 2009;5(1):52–68. doi: 10.1007/s12014-008-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M, Li L, Tang YL, et al. Biomarkers in tongue cancer: understanding the molecular basis and their clinical implications. Postgrad Med J. 2010;86(1015):292–298. doi: 10.1136/pgmj.2009.086504. [DOI] [PubMed] [Google Scholar]
- 78.Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009;45(4–5):454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 79.Tshering Vogel DW, Zbaeren P, Thoeny HC. Cancer of the oral cavity and oropharynx. Cancer Imaging. 2010;10:62–72. doi: 10.1102/1470-7330.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guneri P, Epstein JB, Ergun S, et al. Toluidine blue color perception in identification of oral mucosal lesions. Clin Oral Investig. 2010 doi: 10.1007/s00784-010-0398-6. [DOI] [PubMed] [Google Scholar]
- 81.Seoane Leston J, Diz Dios P. Diagnostic clinical aids in oral cancer. Oral Oncol. 2010 doi: 10.1016/j.oraloncology.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 82.Xie H, Onsongo G, Popko J, et al. Proteomics analysis of cells in whole saliva from oral cancer patients via value-added three-dimensional peptide fractionation and tandem mass spectrometry. Mol Cell Proteomics. 2008;7(3):486–498. doi: 10.1074/mcp.M700146-MCP200. [DOI] [PubMed] [Google Scholar]
- 83.Williams MD. Integration of biomarkers including molecular targeted therapies in head and neck cancer. Head Neck Pathol. 2010;4(1):62–69. doi: 10.1007/s12105-010-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sugimoto M, Wong DT, Hirayama A, et al. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6(1):78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JM, Garon E, Wong DT. Salivary diagnostics. Orthod Craniofac Res. 2009;12(3):206–211. doi: 10.1111/j.1601-6343.2009.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu S, Arellano M, Boontheung P, et al. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14(19):6246–6252. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bigler LR, Streckfus CF, Dubinsky WP. Salivary biomarkers for the detection of malignant tumors that are remote from the oral cavity. Clin Lab Med. 2009;29(1):71–85. doi: 10.1016/j.cll.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Bilodeau E, Alawi F, Costello BJ, et al. Molecular diagnostics for head and neck pathology. Oral Maxillofac Surg Clin North Am. 2010;22(1):183–194. doi: 10.1016/j.coms.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Ghannoum MA, Jurevic RJ, Mukherjee PK, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6(1):e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 91.Richardson R, Antilla VJ. [Diagnosis and treatment of oral candidosis] Duodecim. 2010;126(2):174–180. [PubMed] [Google Scholar]
- 92.Iatta R, Napoli C, Borghi E, et al. Rare mycoses of the oral cavity: a literature epidemiologic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):647–655. doi: 10.1016/j.tripleo.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Terai H, Shimahara M. Usefulness of culture test and direct examination for the diagnosis of oral atrophic candidiasis. Int J Dermatol. 2009;48(4):371–373. doi: 10.1111/j.1365-4632.2009.03925.x. [DOI] [PubMed] [Google Scholar]
- 94.Kurita H, Kamata T, Zhao C, et al. Usefulness of a commercial enzyme-linked immunosorbent assay kit for Candida mannan antigen for detecting Candida in oral rinse solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(4):531–534. doi: 10.1016/j.tripleo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 95.Naglik JR, Scott J, Rahman D, et al. Serum and saliva antibodies do not inhibit Candida albicans Sap2 proteinase activity using a BSA hydrolysis assay. Med Mycol. 2005;43(1):73–77. doi: 10.1080/13693780410001712070. [DOI] [PubMed] [Google Scholar]
- 96.Pomarico L, Cerqueira DF, de Araujo Soares RM, et al. Associations among the use of highly active antiretroviral therapy, oral candidiasis, oral Candida species and salivary immunoglobulin A in HIV-infected children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(2):203–210. doi: 10.1016/j.tripleo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pomarico L, de Souza IP, Castro GF, et al. Levels of salivary IgA antibodies to Candida spp. in HIV-infected adult patients: a systematic review. J Dent. 2010;38(1):10–15. doi: 10.1016/j.jdent.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Andrews E, Seaman WT, Webster-Cyriaque J. Oropharyngeal carcinoma in non-smokers and non-drinkers: a role for HPV. Oral Oncol. 2009;45(6):486–491. doi: 10.1016/j.oraloncology.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Andrews E, Shores C, Hayes DN, et al. Concurrent human papillomavirus-associated tonsillar carcinoma in 2 couples. J Infect Dis. 2009;200(6):882–887. doi: 10.1086/605442. [DOI] [PubMed] [Google Scholar]
- 100.Gennaro S, Naidoo S, Berthold P. Oral health & HIV/AIDS. MCN Am J Matern Child Nurs. 2008;33(1):50–57. doi: 10.1097/01.NMC.0000305658.32237.7d. [DOI] [PubMed] [Google Scholar]
- 101.Grande SR, Imbronito AV, Okuda OS, et al. Herpes viruses in periodontal compromised sites: comparison between HIV-positive and -negative patients. J Clin Periodontol. 2008;35(10):838–845. doi: 10.1111/j.1600-051X.2008.01307.x. [DOI] [PubMed] [Google Scholar]
- 102.Heinic GS, Greenspan D, Greenspan JS. Oral CMV lesions and the HIV infected. Early recognition can help prevent morbidity. J Am Dent Assoc. 1993;124(2):99–105. doi: 10.14219/jada.archive.1993.0051. [DOI] [PubMed] [Google Scholar]
- 103.Hille JJ, Webster-Cyriaque J, Palefski JM, et al. Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. Oral Dis. 2002;8 Suppl 2:161–168. doi: 10.1034/j.1601-0825.2002.00028.x. [DOI] [PubMed] [Google Scholar]
- 104.Itin PH, Lautenschlager S. Viral lesions of the mouth in HIV-infected patients. Dermatology. 1997;194(1):1–7. doi: 10.1159/000246047. [DOI] [PubMed] [Google Scholar]
- 105.Merchant VA. An update on the herpesviruses. J Calif Dent Assoc. 1996;24(1):38–46. [PubMed] [Google Scholar]
- 106.Reichart PA. Oral ulcerations in HIV infection. Oral Dis. 1997;3 Suppl 1:S180–S182. doi: 10.1111/j.1601-0825.1997.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 107.Ramirez-Amador VA, Espinosa E, Gonzalez-Ramirez I, et al. Identification of oral candidosis, hairy leukoplakia and recurrent oral ulcers as distinct cases of immune reconstitution inflammatory syndrome. Int J STD AIDS. 2009;20(4):259–261. doi: 10.1258/ijsa.2008.008351. [DOI] [PubMed] [Google Scholar]
- 108.Shiboski CH, Patton LL, Webster-Cyriaque JY, et al. The Oral HIV/AIDS Research Alliance: updated case definitions of oral disease endpoints. J Oral Pathol Med. 2009;38(6):481–488. doi: 10.1111/j.1600-0714.2009.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen D, Mauk M, Qiu X, et al. An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed Microdevices. 2010 doi: 10.1007/s10544-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chohan BH, Lavreys L, Mandaliya KN, et al. Validation of a modified commercial enzyme-linked immunoassay for detection of human immunodeficiency virus type 1 immunoglobulin G antibodies in saliva. Clin Diagn Lab Immunol. 2001;8(2):346–348. doi: 10.1128/CDLI.8.2.346-348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu C, Qiu X, Ongagna S, et al. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip. 2009;9(6):768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiu X, Thompson JA, Chen Z, et al. Finger-actuated, self-contained immunoassay cassettes. Biomed Microdevices. 2009;11(6):1175–1186. doi: 10.1007/s10544-009-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherman GG, Lilian RR, Coovadia AH. Oral fluid tests for screening of human immunodeficiency virus-exposed infants. Pediatr Infect Dis J. 2010;29(2):169–172. doi: 10.1097/INF.0b013e3181b9a19c. [DOI] [PubMed] [Google Scholar]
- 114.Whitney JB, Luedemann C, Bao S, et al. Monitoring HIV vaccine trial participants for primary infection: studies in the SIV/macaque model. AIDS. 2009;23(12):1453–1460. doi: 10.1097/QAD.0b013e32832b43d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu X, Jackson S. Plasma and salivary IgA subclasses and IgM in HIV-1-infected individuals. J Clin Immunol. 2002;22(2):106–115. doi: 10.1023/a:1014435920321. [DOI] [PubMed] [Google Scholar]
- 116.Yapijakis C, Panis V, Koufaliotis N, et al. Immunological and molecular detection of human immunodeficiency virus in saliva, and comparison with blood testing. Eur J Oral Sci. 2006;114(3):175–179. doi: 10.1111/j.1600-0722.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 117.Gardner MS, Rowland MD, Siu AY, et al. Comprehensive defensin assay for saliva. Anal Chem. 2009;81(2):557–566. doi: 10.1021/ac801609r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adamopoulou M, Vairaktaris E, Panis V, et al. HPV detection rate in saliva may depend on the immune system efficiency. In Vivo. 2008;22(5):599–602. [PubMed] [Google Scholar]
- 119.Chuang AY, Chuang TC, Chang S, et al. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44(10):915–919. doi: 10.1016/j.oraloncology.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cameron JE, Snowhite IV, Chaturvedi AK, et al. Human papillomavirus-specific antibody status in oral fluids modestly reflects serum status in human immunodeficiency virus-positive individuals. Clin Diagn Lab Immunol. 2003;10(3):431–438. doi: 10.1128/CDLI.10.3.431-438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Idesawa M, Sugano N, Ikeda K, et al. Detection of Epstein-Barr virus in saliva by real-time PCR. Oral Microbiol Immunol. 2004;19(4):230–232. doi: 10.1111/j.1399-302X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 122.Ling PD, Vilchez RA, Keitel WA, et al. Epstein-Barr virus DNA loads in adult human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2003;37(9):1244–1249. doi: 10.1086/378808. [DOI] [PubMed] [Google Scholar]
- 123.Mbulaiteye SM, Walters M, Engels EA, et al. High levels of Epstein-Barr virus DNA in saliva and peripheral blood from Ugandan mother-child pairs. J Infect Dis. 2006;193(3):422–426. doi: 10.1086/499277. [DOI] [PubMed] [Google Scholar]
- 124.Imbronito AV, Grande SR, Freitas NM, et al. Detection of Epstein-Barr virus and human cytomegalovirus in blood and oral samples: comparison of three sampling methods. J Oral Sci. 2008;50(1):25–31. doi: 10.2334/josnusd.50.25. [DOI] [PubMed] [Google Scholar]
- 125.Yamamoto AY, Mussi-Pinhata MM, Marin LJ, et al. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36(3):228–230. doi: 10.1016/j.jcv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 126.Grosjean J, Hantz S, Cotin S, et al. Direct genotyping of cytomegalovirus envelope glycoproteins from toddler's saliva samples. J Clin Virol. 2009;46 Suppl 4:S43–S48. doi: 10.1016/j.jcv.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 127.Raggam RB, Wagner J, Michelin BD, et al. Reliable detection and quantitation of viral nucleic acids in oral fluid: Liquid phase-based sample collection in conjunction with automated and standardized molecular assays. J Med Virol. 2008;80(9):1684–1688. doi: 10.1002/jmv.21245. [DOI] [PubMed] [Google Scholar]
- 128.Gandhi M, Koelle DM, Ameli N, et al. Prevalence of human herpesvirus-8 salivary shedding in HIV increases with CD4 count. J Dent Res. 2004;83(8):639–643. doi: 10.1177/154405910408300811. [DOI] [PubMed] [Google Scholar]
- 129.Widmer IC, Erb P, Grob H, et al. Human herpesvirus 8 oral shedding in HIV-infected men with and without Kaposi sarcoma. J Acquir Immune Defic Syndr. 2006;42(4):420–425. doi: 10.1097/01.qai.0000226790.31463.e6. [DOI] [PubMed] [Google Scholar]
- 130.Mancuso R, Biffi R, Valli M, et al. HHV8 a subtype is associated with rapidly evolving classic Kaposi's sarcoma. J Med Virol. 2008;80(12):2153–2160. doi: 10.1002/jmv.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 132.Kanasi E, Johansson I, Lu SC, et al. Microbial risk markers for childhood caries in pediatricians' offices. J Dent Res. 2010;89(4):378–383. doi: 10.1177/0022034509360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wen ZT, Yates D, Ahn SJ, et al. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goncalves Lda R, Soares MR, Nogueira FC, et al. Comparative proteomic analysis of whole saliva from chronic periodontitis patients. J Proteomics. 2010;73(7):1334–1341. doi: 10.1016/j.jprot.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 135.Haigh BJ, Stewart KW, Whelan JR, et al. Alterations in the salivary proteome associated with periodontitis. J Clin Periodontol. 2010;37(3):241–247. doi: 10.1111/j.1600-051X.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- 136.Wu Y, Shu R, Luo LJ, et al. Initial comparison of proteomic profiles of whole unstimulated saliva obtained from generalized aggressive periodontitis patients and healthy control subjects. J Periodontal Res. 2009;44(5):636–644. doi: 10.1111/j.1600-0765.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- 137.Ramseier CA, Kinney JS, Herr AE, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80(3):436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Herr AE, Hatch AV, Giannobile WV, et al. Integrated microfluidic platform for oral diagnostics. Ann N Y Acad Sci. 2007;1098:362–374. doi: 10.1196/annals.1384.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koss MA, Castro CE, Salum KM, et al. Changes in saliva protein composition in patients with periodontal disease. Acta Odontol Latinoam. 2009;22(2):105–112. [PubMed] [Google Scholar]
- 140.Mogi M, Otogoto J. Expression of cathepsin-K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol. 2007;52(9):894–898. doi: 10.1016/j.archoralbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 141.Mogi M, Otogoto J, Ota N, et al. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J Dent Res. 2004;83(2):166–169. doi: 10.1177/154405910408300216. [DOI] [PubMed] [Google Scholar]
- 142.Denny PC. A saliva-based prognostic test for dental caries susceptibility. J Dent Hyg. 2009;83(4):175–176. [PubMed] [Google Scholar]
- 143.Denny PC, Denny PA, Takashima J, et al. A novel saliva test for caries risk assessment. J Calif Dent Assoc. 2006;34(4):287–290. 292–284. [PubMed] [Google Scholar]