Abstract
Pre-menopausal females have a comparably lower incidence of cardiovascular disease than their male counterparts. Although estrogen and activation of estrogen receptors (ER) have been found to contribute to female protection, the complex mechanisms involved are unclear. Besides altering gene transcription, estrogen could elicit its cardioprotective effect via ER-mediated nongenomic signaling pathways. In addition to the two classic nuclear ER isoforms, ERα and ERβ, a G-protein coupled ER (GPR30 or GPER), has been found to be expressed in cardiomyocytes and plays an acute cardioprotective role in ischemia reperfusion (I/R) injury. By using isoform-specific ER knockout mouse models and/or their specific modulators, the mechanisms of the different ERs involved in cardioprotection have been explored. In this review, we will focus on the signaling pathways leading to cardioprotection in I/R injury after ER activation and discuss the possibility and promise of specific ER modulators to treat ischemic heart diseases.
Keywords: estrogen receptor (ER), ischemic reperfusion, cardioprotection
Epidemiological studies have suggested that pre-menopausal females have a reduced incidence of cardiovascular disease compared to age-matched men (Vitale et al. 2009). Although clinical trials, which attempted to reintroduce estrogen to post-menopausal women such as the Women's Health Initiative (Anderson et al. 2004) and the Heart and Estrogen/progestin Replacement Study (HERS) (Hulley et al. 1998), found no beneficial cardiovascular outcomes from hormone replacement therapy, most animal studies have suggested that estrogen plays an important cardioprotective role against ischemia reperfusion (I/R) injury (Booth and Lucchesi 2008; Murphy and Steenbergen 2007). This discrepancy highlights why a better understanding of the mechanisms by which estrogen mediates protection is important to unraveling the role this hormone/receptor system plays in cardioprotection.
In accordance with the known role of the estrogen receptor (ER) as a ligand-gated transcription factor, long-term estrogen treatment has been found to cause upregulation of anti-apoptotic genes and protein expression in cardiomyocytes such as nitric oxide synthase (NOS) (Nikolic et al. 2007; Nuedling et al. 2001), which can mediate protective effects. However, recent studies suggest that ERs are also located at the plasma membrane, where they elicit rapid protective effects via activation of non-genomic signaling pathways. In this review, we will focus on some recently published studies describing the role of estrogen receptor signaling in cardioprotection. The elucidation of ER-mediated signaling might provide us a possibility and promise of a therapeutic intervention to treat ischemic heart disease.
Isoform-specific ER-mediated cardioprotection
Although epidemiological studies have suggested that females have reduced cardiovascular disease, this protection is usually ascribed to the beneficial effects of estrogen on the lipid profile or on the vasculature (Vitale et al. 2009). A number of studies have suggested that acute addition of 17β-estradiol (E2) to either ovary-intact females or ovariectomized females reduces I/R injury (Booth et al. 2003; Hale et al. 1997). However, studies comparing I/R injury between males and females have shown more discrepant results. Some (Wang et al. 2006), but not all, found reduced I/R injury in females. Studies with more severe ischemia tended to show less injury in females than in males, suggesting that the protection in females is only revealed in more severe models of ischemia (Gabel et al. 2005; Lin et al. 2009). Some of this discrepancy might result from the complexity of estrogen-mediated signaling.
The biological effects of estrogen are mainly mediated by ERs. Two classic nuclear ER isoforms, ERα and ERβ, are encoded by separate genes and have differential distribution within tissues and cells. These receptors have been shown to be expressed in both neonatal (Grohé et al. 1997) and adult (Lizotte et al. 2009) cardiac myocytes. In addition to the classic ERs, a G-protein coupled ER (GPR30 or GPER) has been found to be expressed in adult cardiomyocytes (Bopassa et al. 2010; Deschamps and Murphy 2009). Interestingly, most studies which have examined ER localization with cardiac cells do not find a difference in the distribution or abundance between males and females (Deschamps and Murphy 2009; Lizotte et al. 2009). Besides activation by E2, each of these ER isoforms has specific pharmacological agonists. 4,4′,4″-[4-propyl-(1H)-pyrazole-1,3,5-triyl]tris-phenol (PPT) is an activator of ERα, 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) activates ERβ, and the compound G-1 has been shown to be highly specific for GPR30/GPER. In addition to pharmacologic manipulation of these ERs, isoform-specific ER knockout mouse models have been used to study the complex signaling mechanisms. Using both the mouse models and the pharmacologic agents to these ER isoforms one can start to tease out the signaling pathways involved in ER activation and cardioprotection. Moreover, the ovariectomized animal model (which depletes the animal of estrogen) has been utilized to study estrogen-mediated signaling in cardioprotection. Since activation of ERα, ERβ, and GPR30 have been shown to have both non-genomic (acute) and genomic (chronic) effects, it is important to delineate, if possible, whether and to what degree each of these signaling mechanisms is responsible for the observed gender distinction in ischemic heart disease (Table 1).
Table 1.
Cardioprotective effects in I/R injury upon ER regulation
| ER | Acute agonist treatment provides protection |
Chronic agonist treatment provides protection |
Chronic genetic deletion blocks protection |
|---|---|---|---|
| ERα |
Yes (PPT, pre-ischemia) (Booth et al. 2005) Yes (PPT, post-ischemia) (Vornehm et al. 2009) |
Yes (ERA-45, pre-ischemia) (Jeanes et al. 2008) |
Yes (Increased injury in αERKO) (Wang et al. 2006) NO (E2 replacement in αERKO overiectomized female) (Babiker et al. 2007) |
| ERβ |
NO (DPN, pre-ischemia) (Booth et al. 2005) Yes (DPN, post-ischemia) (Vornehm et al. 2009) |
Yes (DPN, pre-ischemia) (Lin et al. 2009; Nikolic et al. 2007) |
Yes (Increased injury in βERKO) (Gabel et al. 2005; Wang et al. 2008; Wang et al. 2009) Yes (E2 replacement in βERKO overiectomized female) (Babiker et al. 2007) |
|
GPR30 (GPER) |
Yes (G1, pre-ischemia) (Bopassa et al. 2010; Deschamps and Murphy 2009) | No data | No data |
| WT | Yes (E2, pre-ischemia) (Booth et al. 2003; Hale et al. 1997) | Yes (E2, pre-ischemia) (Jeanes et al. 2008; Lin et al. 2009) | Not applicable |
ERα
Immunohistochemical analysis in adult murine cardiomyocytes showed that ERα was distributed in the cytosolic, nuclear and membrane compartments (Lizotte et al. 2009). The prominent anti-ERα immuno-labeling in T-tubular membranes (Ropero et al. 2006) and caveolae (Chung et al. 2009), suggest that ERα is specifically localized in these complexes to form unique signaling complex for conveying estrogen-dependent non-genomic, rapid signaling while cytosolic and nuclear location suggest a mechanism for genomic signaling. In addition to location distinction within cardiomyocytes, there appears to be a differential distribution of ERα within the heart as a whole. Lizotte et al. showed that ERα was more heavily located within the ventricular tissue compared to the atrium (Lizotte et al. 2009). In addition, Grohé et al. demonstrated that in addition to cardiac myocytes, cardiac fibroblasts also contain ERs (Grohé et al. 1997).
Acute activation
Due to the localization of ERα within membrane and caveolar compartments, it is likely that its activation results in non-genomic, acute signaling. In keeping with this idea, studies have demonstrated that acute administration of ERα agonists induces rapid changes. It has been shown that acute stimulation of ERα with E2 induces the translocation of ERα to the PI3K regulatory domain and results in endothelial NOS (eNOS) activation (Chen et al. 1999; Simoncini et al. 2000). Furthermore, in an in vivo rabbit I/R model, acute treatment with E2 or PPT, but not DPN, thirty minutes before occlusion of the left anterior descending coronary artery was found to significantly decrease infarct size in female hearts after 30 minutes of ischemia suggesting that activation of ERα is required for the acute cardioprotective effects of estrogen (Booth et al. 2005). A similar in vivo study on ovariectomized female rats found that acute estrogen-mediated cardioprotection following I/R is mimicked by pretreatment with an ERα agonist and unaffected by an ERβ antagonist (Jeanes et al. 2008). In an aging model, Novotny et al. demonstrated that in aged ovariectomized female rats, acute in vivo administration of PPT was able to reduce infarct size (Novotny et al. 2009). In summary, acute activation of ERα seems to protect the heart from ischemic injury. However, the data are conflicting regarding the role of ERα under conditions chronic estrogen exposure.
Chronic activation
A number of groups have studied whether loss of ERα (αERKO) blocks cardioprotection under conditions where cardioprotection is observed in female (or E2 treated) wild type (WT) mice. These results have been inconsistent. In an in vitro Langendorff perfused mouse heart model, Gabel et al. (2005) found that αERKO female hearts exhibited I/R injury under hypercontractile conditions similar to WT females after 20 minutes of no-flow global ischemia.(Gabel et al. 2005) Notably, in the absence of hypercontractile conditions neither the WT nor the αERKO mice exhibit gender differences in I/R injury (Gabel et al. 2005). However, a study by Meldrum's group showed a significantly more severe injury under normal condition in αERKO female hearts compared to WT females (Wang et al. 2006), implying the importance of ERα in cardioprotection.
ERβ
Compared to ERα, which is primarily found in the sarcolemma, ERβ is predominantly localized in the nucleus and cytosol of adult murine cardiomyocytes (Lizotte et al. 2009), suggesting that ERβ-mediated protective effects might depend on gene transcriptional regulation. ERβ has also been reported to be localized to the mitochondria (Yang et al. 2004), although this report has been controversial (Murphy and Steenbergen 2007). In contrast to an increased abundance of ERα within the ventricles (as compared to the atria), ERβ appears to be evenly distributed within the heart (Lizotte et al. 2009).
Acute activation
There are sparse data on the effects of acute activation of ERβ. This is an area that requires future study. As described above, an in vivo rabbit I/R study demonstrated the acute cardioprotective effect of E2 could be mimicked by PPT, the specific ERα agonist, but not by DPN, the specific ERβ agonist (Booth et al. 2005), suggesting that acute activation of ERβ might not lead to cardioprotection.
Chronic activation
In contrast to the discrepant results from αERKO studies, most studies have consistently found that ERβ knockout (βERKO) females had more I/R injury than WT females (Gabel et al. 2005; Wang et al. 2008; Wang et al. 2009). Possibilities for the increased damage include decreased gene expression involving fatty acid metabolism and nitric oxide (NO) production (Gabel et al. 2005) and reduced activation of PI3K/Akt (Wang et al. 2009). Long-term (2-week) treatment with DPN has been found to be cardioprotective in I/R injury in ovariectomized female mice (Nikolic et al. 2007). Gene profiling in this experimental model showed that long-term treatment with DPN resulted in upregulation of a number of protective genes such as those encoding NO biosynthesis and anti-apoptotic proteins (Nikolic et al. 2007). To study the impact of ER-dependent mechanisms in estrogen replacement therapy in ischemic heart disease, Babiker et al. (2007) ovariectomized WT, αERKO, and βERKO female mice and subsequently supplemented these mice with E2 or placebo for two weeks. After myocardial infarction, there was no significant difference in infarct size between E2- or placebo-treated WT mice. However, E2 treatment did result in smaller infarct sizes in ovariectomized αERKO female mice while increased the infarct size in ovariectomized βERKO female mice (Babiker et al. 2007). These data suggest that estrogen, through activation of ERβ, plays a cardioprotective role against I/R injury. The study by Babiker et al. (2007) might also suggest that chronic activation of only ERα is detrimental, while ERα and ERβ might oppose each other so that activation of ERα and ERβ together have neither beneficial nor detrimental effects (Babiker et al. 2007). Recently, Lin et al. (2009) found that 2-week E2 or DPN treatment leads to activation of protein S-nitrosylation and cardioprotection, which could be both blocked by NOS inhibition, suggesting that chronic estrogen exposure protects hearts largely via activation of ERβ and NO signaling (Lin et al. 2009).
GPR30 (GPER)
G protein-coupled estrogen receptor (GPR30 or GPER) was initially identified as a orphan G-protein coupled receptor (GPCR) until 2000, when Filardo and colleagues identified estrogen as an endogenous ligand (Filardo et al. 2000). E2 binding to GPR30 results in Gbg activation of Src and resulting matrix metalloproteinase (MMP) cleavage of heparan-bound epidermal growth factor (EGF). The latter is then able to activate the EGF receptor which subsequently results in acute PI3K and ERK activation (Filardo et al. 2000). As a transmembrane estrogen receptor, GPR30 activation may mediate rapid cell signaling (Prossnitz et al. 2008; Revankar et al. 2005).
Acute activation
Deschamps and Murphy reported that acute activation of GPR30 by the specific agonist, G-1, in Langendoff perfused rat hearts reduced infarct size when compared to control (Deschamps and Murphy 2009). Co-administration of the GPR30 agonist with an inhibitor of the PI3K pathway abolished the G-1-mediated protective effect, suggesting that one mechanism of protection by GPR30 activation is through the PI3K/AKT pathway. The activation of the PI3K pathway by GPR30, via GPR30-mediated transactivation of the EGF receptor (Filardo et al. 2000), leads not only to activation of PI3K but also to activation of ERK. Bopassa et al (2010) have shown that GPR30 activation reduces I/R injury in Langendoff perfused mouse hearts by inhibiting the mitochondria permeability transition pore opening via the activation of the ERK pathway (Bopassa et al. 2010). Furthermore, a study by Filice et al. (2009) has shown that acute administration of G-1 causes the activation of the ERK pathway (Filice et al. 2009). In addition, the authors have shown that G-1 can cause phosphorylation of eNOS, thus one can speculate that the NO system may also play a role in GPR30 mediated cardioprotection (Filice et al. 2009). Therefore, it will be interesting to study whether acute cardioprotection of GPR30 is blocked by NO/NOS inhibition.
Chronic activation
While chronic administration of G-1 has not been evaluated in the context of I/R, a study by Lindsey et al. (2009) examined the effects of a 2-week G-1 treatment in an estrogen-sensitive model of hypertension (Lindsey et al. 2009). In ovariectomized rats, G-1 was able to prevent an elevation in systolic blood pressure that occurs due to estrogen depletion, suggesting that activation of GPR30 can modulate cardiovascular function. Consistent with this, in a GPR30 deficient mouse model female mice develop a significant increase in mean arterial blood pressure by 9 months of age (Martensson et al. 2009). While there is not conclusive data to demonstrate that chronic GPR30 activation results in cardioprotection, several other lines of evidence suggest that this may be the case. This is an area which requires further study.
PI3K: a common cardioprotective signaling pathway by ER activation?
In both in vivo and in vitro studies, Patten et al. have shown that acute E2 treatment reduces cardiomyocyte apoptosis and elicits cardioprotection via activation of PI3K/Akt signaling (Patten et al. 2004). Simoncini et al. have discovered a direct protein-protein interaction between ligand-activated ERα and the regulatory subunit p85 of PI3K in endothelial cells through a nongenomic mechanism by which E2 rapidly activates eNOS via the activation of PI3K/Akt (Simoncini et al. 2000). Thus, they speculate that ERα activation of PI3K might play a role in cardioprotection (Patten et al. 2004). Interestingly, the acute cardioprotection by GPR30 is mediated through the activation of PI3K (Deschamps and Murphy 2009). Wang et al. (2009) have shown that the ablation of ERβ significantly decreased post-ischemic functional recovery in female, but not male hearts. A reduced activation of PI3K/Akt was also noted in female βERKO hearts (Wang et al. 2009). Collectively, these results suggest that activation of the PI3K/Akt signaling cascade plays an important common role in ER-mediated acute signaling in cardioprotection against I/R injury.
An important question to be answered is: Why there are discrepant results in the studies of ER activation and cardioprotection if all isoforms of ERs share a common signaling pathway? Possibly differences in the animal species, applications of ER modulators, and/or I/R models could be explanations. Alternatively, some ER isoforms might predominantly mediate acute protection and others mediate chronic or genomic effects. It is also possible that there are some opposing actions between the different ER isoforms in various tissues and species (Matthews and Gustafsson 2003).
Role of NO and protein S-nitrosylation in cardioprotection in females
NO plays an important role in the regulation of cardiovascular function. In addition to the activation of cyclic guanosine monophosphate (cGMP)-dependent pathway, NO can regulate cell function through protein S-nitrosylation, a reversible, redox-sensitive post-translational protein modification, which involves the attachment of an NO moiety to a nucleophilic protein sulfhydryl, resulting in S-nitrosothiol (SNO) formation. There are data suggesting an important role of protein S-nitrosylation in cardioprotection (Sun and Murphy 2010).
Estrogen-induced protein S-nitrosylation has been shown to be involved in a murine model of I/R resulting in a cardioprotective phenotype (Lin et al. 2009; Sun et al. 2006). In a recent study, we treated ovariectomized female mice with vehicle control, E2, or DPN for 2 weeks and evaluated cardiac function with an in vitro Langendorff model of I/R. Compared to vehicle treatment, DPN and E2 treatments significantly increased post-ischemic functional recovery. This protection was blocked by a NOS inhibitor, suggesting that increased NO signaling contributes to the cardioprotection due to chronic DPN and E2 exposure (Lin et al. 2009). With the use of a two-dimensional DyLight fluorescence differences gel electrophoresis method to identify post-translational modifications of protein S-nitrosylation, we have found that DPN- and E2-treated hearts had a significant increase in a range of S-nitrosylated proteins. A similar pattern of protein S-nitrosylation was observed between DPN and E2 treated hearts, which could also be abolished by pretreating hearts with a NOS inhibitor. These data suggest that chronic estrogen treatment and activation of ER-β would lead to increased NO/SNO signaling, which might play an essential role in cardioprotection (Lin et al. 2009).
Interestingly, many of the S-nitrosylated proteins found in DPN-treated hearts (Lin et al. 2009) have also been shown to be increased in preconditioned hearts (Sun et al. 2007) including the mitochondrial F1F0-ATPase, aconitase, malate dehydrogenase, creatine kinase, cytochrome c oxidase and heat shock proteins (HSP 27, 60, and 70). In addition to mitochondrial energetics, protein S-nitrosylation might also elicit cardioprotective effects by regulating intracellular Ca2+ handling, apoptosis, and post-infarct myocardial remodeling (Sun and Murphy 2010).
Conclusions and future perspective
Studies using isoform-specific ER modulators have shown that all ER subtypes, including ERα, ERβ, and GPR30, confer cardioprotective effects against I/R injury both in genomic and non-genomic mechanisms (Figure 1). As discussed above, the acute activation of each ER isoform causes the non-genomic activation of PI3K/Akt pathway (Deschamps and Murphy 2009; Simoncini et al. 2000; Wang et al. 2009), which would lead to downstream activation of NOS/NO/SNO signaling (Chen et al. 1999; Lin et al. 2009). Furthermore, the activation of ERβ in the heart could lead to genomic upregulation of NOS (Nikolic et al. 2007; Nuedling et al. 2001) and increased NO/SNO signaling (Lin et al. 2009). The SNO data presented by Lin et al. suggest that S-nitrosylation is an important factor in female cardioprotection (Lin et al. 2009). However, the detection and quantification of this important post-translational modification is still being developed. With more sensitive detection methods and techniques that can determine which cysteine is modified, we may be able to pinpoint which group or specific proteins are directly involved in estrogen/NO/SNO-induced protection.
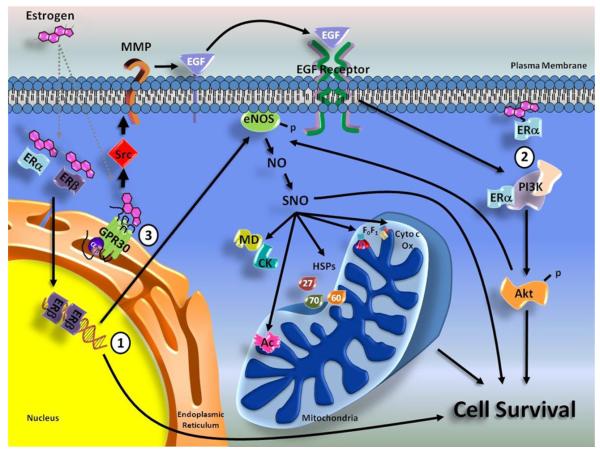
Figure 1. Cardioprotective signaling pathways via ER activation.
(1) Genomic actions of ER activation. Notable changes in gene expression, like modulators of the NO system, have been shown to be upregulated with chronic ERβ stimulation and involved in cardioprotection. (2) Activation of both PI3K/Akt and NO signaling result in cardioprotection and enhanced cell survival. The chronic activation of ERβ by DPN treatment leads to cardioprotection and significant increased S-nitrosylated proteins, including mitochondrial F1F0-ATPase (F1F0), aconitase (Ac), cytochrome c oxidase (Cyto c Ox), heat shock proteins (HSP27/60/70), creatine kinase (CK), and malate dehydrogenase (MD). (3) A G-protein coupled estrogen receptor, GPR30 or GPER, has been recently demonstrated to result in cardioprotection through a PI3K/Akt pathway. Whether through chronic or acute activation, stimulation of estrogen receptors has been shown to result in a cardioprotective phenotype.
Unlike E2, the isoform-specific ER agonists such as DPN or G1, which do not stimulate breast and ovarian proliferation, provide an attractive possibility as specific ER modulators for therapeutic intervention to treat ischemic diseases. Despite the protection afforded by administering the ER modulators before ischemia (preconditioning effect), a post-ischemic treatment approach (post-conditioning effect) with these ER-regulated pharmacological agents might be more clinically relevant. Recently, acute post-ischemic infusion with E2 has been found to improve left ventricular myocardial function after 25 min of ischemia (Terrell et al. 2008). To further distinguish which ER activation mediates this post-conditioning effect, the post-ischemic infusion was replaced with PPT or DPN. Post-ischemic treatment with PPT or DPN significantly increased myocardial functional recovery after I/R injury, suggesting that both ERα and ERβ are involved in mediating cardioprotective effect of E2-induced post-conditioning (Vornehm et al. 2009). Whether the cardioprotective effect of post-conditioning with the selective ER modulators is blocked by PI3K and NO/SNO inhibitors is worthy of further investigation. The heart is clearly a target for estrogen-mediated signaling, and a better understanding of the molecular mechanisms of estrogen-mediated cardioprotection will provide novel therapeutic strategies for protection against I/R injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson G, Limacher M, Assaf A, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Babiker FA, Lips DJ, Delvaux E, et al. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiol (Oxf) 2007;189:23–31. doi: 10.1111/j.1748-1716.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- Booth EA, Lucchesi BR. Estrogen-mediated protection in myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2008;8:101–113. doi: 10.1007/s12012-008-9022-2. [DOI] [PubMed] [Google Scholar]
- Booth EA, Marchesi M, Kilbourne E, Lucchesi BR. 17β–Estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther. 2003;307:395–401. doi: 10.1124/jpet.103.054205. [DOI] [PubMed] [Google Scholar]
- Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-α protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol. 2005;289:H2039–H2047. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, et al. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T-H, Wang S-M, Liang J-Y, et al. The interaction of estrogen receptor α and caveolin-3 regulates connexin43 phosphorylation in metabolic inhibition-treated rat cardiomyocytes. Int J Biochem Cell Biol. 2009;41:2323–2333. doi: 10.1016/j.biocel.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filice E, Recchia AG, Pellegrino D, et al. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- Gabel SA, Walker VR, London RE, et al. Estrogen receptor β mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Grohé C, Kahlert S, Löbbert K, et al. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107–112. doi: 10.1016/s0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Hale S, Birnbaum Y, Kloner R. Estradiol, administrated acutely, protects ischemic myocardium in both female and male rabbits. J Cardiovasc Pharmacol Ther. 1997;2:47–52. doi: 10.1177/107424849700200106. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Jeanes HL, Tabor C, Black D, et al. Oestrogen-mediated cardioprotection following ischaemia and reperfusion is mimicked by an oestrogen receptor (ER)α agonist and unaffected by an ERβ antagonist. J Endocrinol. 2008;197:493–501. doi: 10.1677/JOE-08-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-β activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Cohen JA, Brosnihan KB, et al. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte E, Grandy SA, Tremblay A, et al. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23:75–86. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]
- Martensson UEA, Salehi SA, Windahl S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ERα and ERβ. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Nikolic I, Liu D, Bell JA, et al. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42:769–780. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Novotny JL, Simpson AM, Tomicek NJ, et al. Rapid estrogen receptor-α activation improves ischemic tolerance in aged female rats through a novel protein kinase Cε-dependent mechanism. Endocrinology. 2009;150:889–896. doi: 10.1210/en.2008-0708. [DOI] [PubMed] [Google Scholar]
- Nuedling S, Karas RH, Mendelsohn ME, et al. Activation of estrogen receptor β is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- Patten R, Pourati I, Aronovitz M, et al. 17β-Estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, et al. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Eghbali M, Minosyan TY, et al. Heart estrogen receptor alpha: Distinct membrane and nuclear distribution patterns and regulation by estrogen. J Mol Cell Cardiol. 2006;41:496–510. doi: 10.1016/j.yjmcc.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Morgan M, Shen R-F, et al. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Picht E, Ginsburg KS, et al. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- Terrell AM, Crisostomo PR, Markel TA, et al. Postischemic infusion of 17-β-estradiol protects myocardial function and viability. J Surg Res. 2008;146:218–224. doi: 10.1016/j.jss.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C, Mendelsohn ME, Rosano GMC. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- Vornehm ND, Wang M, Abarbanell A, et al. Acute postischemic treatment with estrogen receptor-α agonist or estrogen receptor-β agonist improves myocardial recovery. Surgery. 2009;146:145–154. doi: 10.1016/j.surg.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Crisostomo PR, Markel T, et al. Estrogen receptor β mediates acute myocardial protection following ischemia. Surgery. 2008;144:233–238. doi: 10.1016/j.surg.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Wang M, Crisostomo PR, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. Am J Physiol. 2006;290:H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang Y, Weil B, et al. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol. 2009;296:R972–R978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S-H, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]