Abstract
Recent studies have linked maternal exposure to air pollution with a range of adverse pregnancy outcomes. However, the available evidence linking this exposure to congenital anomalies is still limited and controversial. The present case-control study tested the hypothesis that maternal exposure to ambient black smoke and sulfur dioxide is a risk factor for the occurrence of congenital heart disease. The authors used registry-based data on congenital heart disease for the population of the northeast of England in 1985–1996. A 2-stage spatiotemporal model was developed to predict weekly black smoke and sulfur dioxide levels at each maternal place of residence. Controls were frequency-matched to cases by year of birth (control-to-case ratio of 4:1). Two sets of analyses were performed, using predicted mean values of exposure and 1,000 simulated scenarios of exposure. The analyses were adjusted for birth year, socioeconomic status, infant sex, season of conception, and degree of urbanity. The authors found a weak association between maternal exposure to black smoke and congenital malformations of cardiac chambers and connections only when using exposure as a continuous variable. When the authors used quartiles of exposure, odds ratios did not show a dose-response relation for consecutive quartiles. For sulfur dioxide, the results were not indicative of any association.
Keywords: air pollution; congenital abnormalities; heart defects, congenital; maternal exposure; sulfur dioxide; spatiotemporal analysis
Exposure to air pollution is associated with higher mortality and morbidity rates in adults and children (1). Recent evidence suggests that air pollution also adversely affects fetal development (2–4). However, the evidence linking air pollution exposure to congenital anomalies is still limited (Table 1) (5–16).
Table 1.
Findings From Published Studies Investigating Exposure to Ambient Air Pollution and the Occurrence of Congenital Anomalies
| Study | Setting | Design | Exposure Assessment | Main Findings |
| Savel'eva, 1991 (5) | 2 districts in 1 city, Ukraine | Ecologic | Living within or outside a buffer of 600 m from a chemical factory | Increase in infant deaths due to congenital anomalies in population living within 600 m of the factory |
| Antipenko and Kogut, 1991 (6) | 3 cities, Ukraine | Ecologic | Living in 3 cities with different air pollution levels according to industrial and traffic emission rates | Increased risk of autosomal dominant and x-linked mutations and multiple congenital anomalies in proportion to increasing pollution |
| Reznik et al., 1992 (7) | 2 ecologic zones in 1 city, Ukraine | Ecologic | Living in industrial zone vs. agricultural zone | Higher rate of total congenital anomalies and multiple congenital anomalies in the industrial zone having higher levels of air pollution and radioactive radiation |
| Guminska, 1993 (8) | 1 city, Poland | Increased risk of congenital anomalies in more polluted areas | ||
| Smrcka and Leznarova, 1998 (9) | 1 district, Czech Republic | Ecologic | Living in areas with severe long-term air pollution vs. less-polluted areas | Elevated occurrence of congenital anomalies in areas of severe long-term air pollution compared with less-polluted areas |
| Ritz et al., 2002 (10) | 4 counties, United States | Case-control | Assignment of measured levels of carbon monoxide, nitrogen dioxide, ozone, and PM10 by the nearest monitor to maternal place of residence | Significant association between second-month exposure to carbon monoxide and ozone and the occurrence of specific CHDs |
| Gilboa et al., 2005 (11) | 7 counties, United States | Case-control | Assignment of measured levels of carbon monoxide, nitrogen dioxide, ozone, sulfur dioxide, and PM10 by the nearest monitor to maternal place of residence | Significant association between second-month exposure to sulfur dioxide, PM10, and carbon monoxide and the occurrence of specific CHDs |
| Kim et al., 2007 (12) | 1 city, South Korea | Cohort | Assignment of measured levels of PM10 by the monitor nearest to maternal place of residence | Significant association between maternal exposure to PM10 during the second trimester and the occurrence of congenital anomalies |
| Hwang and Jaakkola, 2008 (13) | 5 counties, Taiwan | Case-control | Interpolation (inverse distance weighting) of carbon monoxide, nitrogen dioxide, ozone, sulfur dioxide, and PM10 levels measured by monitoring site | Increased risk of oral cleft due to first- and second-month exposure to ozone |
| Rankin et al., 2009 (14) | 4 counties, England | Case-control | Assignment of the average measured black smoke and sulfur dioxide levels by the monitors within 10 km of the maternal residential postcode | Increased risk of nervous system anomalies in relation to maternal exposure to black smoke |
| Hansen et al., 2009 (15) | 1 city, Australia | Case-control | Assignment of measured levels of carbon monoxide, nitrogen dioxide, ozone, sulfur dioxide, and PM10 by the monitor nearest to maternal place of residence | Increased risk of aortic artery and valve defects and cleft lip with or without cleft palate due to exposure to sulfur dioxide and pulmonary artery and valve defects in relation to ozone |
| Strickland et al., 2009 (16) | 1 city, United States | Retrospective cohort | Assignment of measured levels of carbon monoxide, nitrogen dioxide, ozone, sulfur dioxide, and PM10 by the monitor nearest to maternal place of residence | Increased risk of patent ductus arteriosus due to exposure to PM10 |
| Dadvand et al., 2010a | 15 local authorities, England | Case-control | Assignment of measured levels of carbon monoxide, nitric oxide, nitrogen dioxide, ozone, sulfur dioxide, and PM10 by the monitor nearest to maternal place of residence | Increased risk of ventricular septal defect and congenital pulmonary valve stenosis due to maternal exposure to carbon monoxide and increased risk of ventricular septal defect and tetralogy of Fallot due to maternal exposure to nitric oxide |
Abbreviations: CHD, congenital heart disease; PM10, particulate matter with aerodynamic diameter <10 μm.
P. Dadvand, J. Rankin, S. Rushton, and T. Pless-Mulloli, unpublished data, 2010.
Congenital heart diseases (CHDs) are the most frequent group of congenital anomalies (17). CHDs are the leading cause of infant deaths due to congenital anomalies and are associated with a considerable burden on public and private resources (18–23). Although the etiology of the majority of CHDs remains unknown, it is likely to be multifactorial, with roles for both genetic and environmental causes (22–24).
A limited number of studies have recently reported some links between maternal exposure to different air pollutants and the occurrence of a range of CHD subgroups (Table 1) (10, 11, 15, 16). The findings from these recent studies are not consistent in terms of the detected associations and their direction and strength. The exposure assessment in these studies has been based on assigning the pollutant levels measured by the monitoring site nearest to the maternal place of residence. This procedure may have caused exposure misclassification in these studies (10). Gilliland et al. (25) have suggested that for the studies of the adverse effects of maternal exposure to air pollution during pregnancy that have large sample sizes, long duration, and different outcomes and exposures, the exposure assessment should rely on modeling approaches. Using a spatiotemporal modeling framework for exposure assessment, we carried out a case-control study, the Congenital Heart Disease and Air Pollution Study, to investigate whether maternal exposure to ambient black smoke (BS) and sulfur dioxide is associated with an increased risk of CHD.
MATERIALS AND METHODS
Case and control data
The Congenital Heart Disease and Air Pollution Study was carried out in the population of the northeast of England from January 1985 to December 1996. The study region encompassed 15 local authorities/unitary authorities (2,055 km2) with a population of about 2.15 million according to the 1991 United Kingdom Census (26). The northeast region has a geographically well-defined population, with 98.5% classified as white (26).
We obtained data on CHD cases from the Northern Congenital Abnormality Survey (NorCAS). NorCAS is a population-based register of all congenital anomalies occurring in pregnancies terminated after prenatal diagnosis of fetal anomaly, late miscarriages (≥20 weeks gestation), livebirths, and stillbirths in those residents in the former northern health region of England (27). For the period of study, NorCAS registered cases diagnosed with CHD up to the age of 16 years (28). All cases of CHD were confirmed by autopsy, surgery, echocardiography, or cardiac catheterization and were checked for duplication before registration onto NorCAS. NorCAS data on CHD underwent annual cross-validation with the pediatric cardiology database held at Freeman Hospital, Newcastle upon Tyne, United Kingdom. NorCAS is a member of the British Isles Network of Congenital Anomaly Registers and the European Surveillance of Congenital Anomalies (EUROCAT) and adopts the EUROCAT exclusion criteria for minor anomalies (29). The detailed descriptive epidemiology of CHD across the NorCAS region for the period of 1985–2003 has been described previously (28). The total prevalence of CHD was 85.9 per 10,000 births and terminations of pregnancy for fetal anomaly, with an increasing trend during this period (28).
Data for controls were obtained from the Office for National Statistics annual birth tapes, which include data on all births occurring annually within this population. The tapes are held by the Regional Maternity Survey Office, Newcastle upon Tyne, United Kingdom, which is a part of the North East Public Health Observatory.
Case definition and classification
Cases were classified according to the International Classification of Diseases, Tenth Revision (ICD-10) (30). ICD-10 categorizes CHD into 9 groups (codes Q20–Q28). The following groups were excluded: 1) cases of ICD-10 groups of Q24 (other congenital malformations of heart), Q27 (other congenital malformations of peripheral vascular system), and Q28 (other congenital malformations of circulatory system); 2) cases of functional or unspecified cardiac murmur, patent ductus arteriosus associated with prematurity, and peripheral pulmonary artery stenosis, in line with the EUROCAT exclusion list (29); and 3) cases with coincident chromosomal abnormalities and/or major anomalies of other organ systems, as they were more likely to have a genetic basis than to be caused by environmental insults.
Consequently, only cases with ≥1 type of CHD without any anomaly of other organ systems were included in the analysis. Because of the small number of cases of congenital malformations of great arteries (Q25) and congenital malformations of great veins (Q26), these 2 groups were grouped as congenital anomalies of great arteries and veins. The 5 most frequent individual CHDs in our data set (28), which were ventricular septal defect, atrial septal defect, congenital pulmonary valve stenosis, tetralogy of Fallot, and coarctation of aorta, were also included in the analysis. These resulted in 10 outcome groups for the analysis, including 5 ICD-10 CHD groups and 5 individual CHD subtypes (Table 2).
Table 2.
Outcome Groups and ICD-10 Codes Included in the Analysis
| Outcome Group | ICD-10 Code(s) |
| Congenital malformations of cardiac chambers and connections | Q20 |
| Congenital malformations of cardiac septa | Q21 |
| Congenital malformations of pulmonary and tricuspid valves | Q22 |
| Congenital malformations of aortic and mitral valves | Q23 |
| Congenital malformations of great arteries and veins | Q25 and Q26 |
| Ventricular septal defect | Q21.0 |
| Atrial septal defect | Q21.1 |
| Tetralogy of Fallot | Q21.3 |
| Congenital pulmonary valve stenosis | Q22.1 |
| Coarctation of aorta | Q25.1 |
Abbreviation: ICD-10, International Classification of Diseases, Tenth Revision.
Exposure assessment
The exposure assessment was performed for weeks 3–8 of pregnancy. This period is considered to be the critical exposure window for the development of CHD (31, 32). It is also in line with findings of the previous study by Ritz et al. (10).
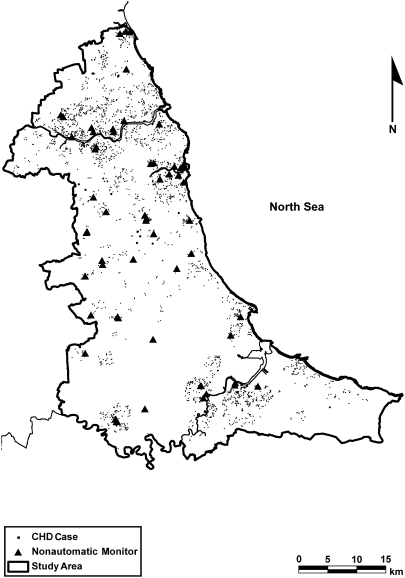
There were 56 nonautomatic monitors operating across the study region over the study period (Figure 1). Nonautomatic monitors measure daily levels of BS and sulfur dioxide. The data on these measurements were obtained from the UK National Air Quality Information Archive Web site (33).
Figure 1.
Nonautomatic monitoring stations and cases of congenital heart disease (CHD) across the northeast of England, 1985–1996.
A 2-stage spatiotemporal modeling framework was used to separately predict BS and sulfur dioxide levels at each maternal residential postcode for each individual week of weeks 3–8 of pregnancy. This modeling was developed using monitoring data on BS and sulfur dioxide together with a range of covariate data, including traffic, population density, industrial activity, meteorology, and land cover information. The data on covariates were obtained from the Countryside Information System (providing remote sensing data on land cover), the Office for National Statistics, the Digimap Web site (34), the UKBORDERS Web site (35), and the Met Office Web site (36). The first stage of the modeling was a dynamic model that separated the temporal trend in pollutant levels for the region as a whole from within-region spatial variation. The second stage was a linear model predicting BS and sulfur dioxide levels at all locations in the region for each week of the study period. For this second stage, we used spatially referenced covariate data as predictors and the predicted regional temporal trend by the first stage as an offset. A manual step-backward algorithm was used to construct the final spatiotemporal models. The covariates remaining in the final models were distance to motorway, length of local roads/streets in buffers of 250 m, distance to A- and B-type roads, easting/northing coordinates, and land cover (within a 7-km buffer zone). These models were capable of predicting 71% and 53% of the spatiotemporal variation (adjusted R2 of 0.71 and 0.53) in BS and sulfur dioxide levels, respectively, across the Congenital Heart Disease and Air Pollution Study region over the study period. We validated the modeling approach both internally and externally for each pollutant separately; results suggested that the model provided reasonable estimates of BS and sulfur dioxide levels. Further details of the exposure modeling approach have been published elsewhere (37).
Statistical analysis
During the course of the study, pollutant levels decreased and the number of diagnosed cases of CHD increased (28). Because of these changes, we determined that time could potentially act as a confounding factor, and cases and controls were therefore frequency-matched by year of birth. The control-to-case ratio was 4:1 for each outcome group.
Pollutant levels predicted by spatiotemporal models were used in the analysis. Predicted mean values and corresponding standard errors were calculated for each study participant for weeks 3–8 of pregnancy. Two separate approaches were then developed to perform the analyses. We used predicted mean values of exposure to construct logistic regression models to abstract adjusted odds ratios of exposure for each outcome group. These were adjusted for year of birth, socioeconomic status (SES), infant sex, season of conception, and degree of urbanity. The Townsend Deprivation Score, based on the 1991 census, was used to address SES. The Townsend Deprivation Score is an area-based measure of material deprivation based on 4 domains: unemployment, car ownership, owner occupation, and overcrowding (38). Two sets of logistic regression models were built, using exposure levels as continuous and categorical variables. The categorical variable was extracted as quartiles of exposure based on all study participants in each outcome group. The first quartile was considered as the reference group. The second, third, and fourth quartiles were then compared with this reference group to estimate odds ratios and to investigate dose-response relations in consecutive quartiles of exposure.
This use of predicted mean values overlooked the uncertainty of regression models in prediction of the outcome variable (exposure level in this case). Standard error was an indicator for this uncertainty. A 3-step simulation algorithm was used to address this uncertainty in the risk analysis.
As the first step, 100 possible predicted values for each week in the critical exposure window were calculated for each study participant. This was done by randomly generating a number from a normal distribution with a known mean and standard deviation, which were the predicted mean value and standard error by the exposure models, respectively. These were stored in 100 columns for each study participant. In the second step, the predicted values in each column were averaged for weeks 3–8 of pregnancy for each study participant. Finally, 1,000 simulated scenarios of exposure were generated by randomly selecting 1 exposure average from 100 exposure averages for each study participant in each simulation. These 1,000 simulated exposure scenarios were then used to construct 1,000 logistic regression models to abstract the risk by allowing for the included confounders. This process was repeated for each outcome group. Regression coefficients for the exposure covariate were abstracted. Odds ratios for the minimum, maximum, and mean of these regression coefficients, which were normally distributed, were then calculated in each outcome group. The NorCAS, as part of the British Isles Network of Congenital Anomaly Registers, has ethics approval to undertake studies involving the use of the data.
RESULTS
In total, 2,713 cases of CHD met the inclusion criteria. Table 3 summarizes the distribution of covariates in controls, pooled CHD cases, and each of the outcome groups. The sex ratio differed among the outcome groups; however, it was not different between controls and the pooled CHD cases (chi-square = 1.997, P = 0.16). The medians of the Townsend Deprivation Scores varied among outcome groups and generally were higher (i.e., participants were more deprived) than those of controls. Nevertheless, SES as measured by Townsend Deprivation Score was not different between the pooled CHD cases and controls (Wilcoxon Mann-Whitney test U = 17,326,975.5, P = 0.29). Both cases and controls were mostly from urban areas and did not differ in their degree of urbanity (chi-square = 2.222, P = 0.14).
Table 3.
Distribution of Covariates Among Controls, Pooled Congenital Heart Disease Cases, and Each Outcome Group, Northeast of England, 1985–1996
| ICD-10 Codes(s)a |
||||||||||||
| Controls (n = 9,975) | CHD (n = 2,713) | Q20 (n = 238) | Q21 (n = 1,641) | Q22 (n = 337) | Q23 (n = 330) | Q25–Q26 (n = 424) | Ventricular Septal Defect (n = 902) | Atrial Septal Defect (n = 271) | Congenital Pulmonary Valve Stenosis (n = 240) | Tetralogy of Fallot (n = 140) | Coarctation of the Aorta (n = 127) | |
| Sex, % | ||||||||||||
| Female | 48.4 | 46.9 | 37.8 | 49.1 | 49.3 | 37.3 | 44.9 | 47.6 | 57.7 | 52.9 | 42.9 | 37.0 |
| Male | 51.6 | 53.1 | 62.2 | 50.9 | 50.7 | 62.7 | 55.1 | 52.4 | 42.3 | 47.1 | 57.1 | 63.0 |
| Townsend Deprivation Score | ||||||||||||
| Minimum | −6.5 | −6.0 | −5.4 | −5.9 | −5.3 | −5.9 | −5.9 | −5.9 | −5.9 | −5.3 | −5.5 | −5.9 |
| 1st Quartile | −1.5 | −1.4 | −0.9 | −1.4 | −1.2 | −2.0 | −1.3 | −1.6 | −1.2 | −1.1 | −1.2 | −1.1 |
| Median | 2.2 | 2.4 | 2.5 | 2.4 | 2.9 | 2.1 | 2.4 | 2.4 | 2.8 | 2.9 | 2.4 | 2.2 |
| 3rd Quartile | 5.1 | 5.3 | 5.4 | 5.3 | 5.8 | 5.2 | 5.5 | 5.2 | 5.5 | 5.4 | 5.3 | 5.6 |
| Maximum | 11.0 | 11.0 | 10.0 | 10.6 | 10.6 | 10.6 | 11.0 | 10.6 | 10.6 | 10.6 | 10.6 | 11.0 |
| Urbanity, % | ||||||||||||
| Urban | 90.4 | 91.3 | 90.8 | 91.9 | 92.3 | 89.1 | 91.3 | 92.0 | 93.5 | 93.3 | 87.9 | 96.9 |
| Rural | 9.6 | 8.7 | 9.2 | 8.1 | 7.7 | 10.9 | 8.7 | 8.0 | 6.5 | 6.7 | 12.1 | 3.1 |
| Season, % | ||||||||||||
| Spring | 25.7 | 24.4 | 19.8 | 25.0 | 26.1 | 20.3 | 22.6 | 24.9 | 23.4 | 25.0 | 22.9 | 16.5 |
| Summer | 25.2 | 26.1 | 31.9 | 25.3 | 23.2 | 23.9 | 23.6 | 26.2 | 25.9 | 23.3 | 25.0 | 22.1 |
| Autumn | 24.9 | 24.7 | 24.4 | 24.6 | 25.2 | 27.3 | 24.8 | 25.4 | 23.9 | 22.9 | 25.7 | 33.1 |
| Winter | 24.2 | 24.8 | 23.9 | 25.1 | 25.5 | 28.5 | 29.0 | 23.5 | 26.8 | 28.7 | 26.4 | 28.3 |
Abbreviations: CHD, congenital heart disease; ICD-10, International Classification of Diseases, Tenth Revision.
Q20, congenital malformations of cardiac chambers and connections; Q21, congenital malformations of cardiac septa; Q22, congenital malformations of pulmonary and tricuspid valves; Q23, congenital malformations of aortic and mitral valves; Q25–Q26, congenital malformations of great arteries and veins.
Quartiles of the predicted mean values of BS and sulfur dioxide levels and the corresponding odds ratios for each outcome group are presented in Table 4. For BS, an increased risk congenital malformations of cardiac chambers and connections (ICD-10 code Q20) was detected for the fourth exposure quartile compared with the reference group. There was no clear dose-response relation for this association. For sulfur dioxide, no clear dose–response relation or significant increase in risk was detected for any of the anomaly groups or subtypes.
Table 4.
Predicted Mean Black Smoke Value and Sulfur Dioxide Levels and the Corresponding Odds Ratios for a 1–μg/m3 Increase in Black Smoke and Sulfur Dioxide Levels in Each Outcome Group, Northeast of England, 1985–1996
| Black Smoke |
Sulfur Dioxide |
|||||||||||||||||
| Second Quartile |
Third Quartile |
Fourth Quartile |
Second Quartile |
Third Quartile |
Fourth Quartile |
|||||||||||||
| Level, μgm−3 | OR | 95% CI | Level, μgm−3 | OR | 95% CI | Level, μgm−3 | OR | 95% CI | Level, μgm−3 | OR | 95% CI | Level, μgm−3 | OR | 95% CI | Level, μgm−3 | OR | 95% CI | |
| ICD-10 code(s)a | ||||||||||||||||||
| Q20 | 5.78 | 1.35 | 0.84, 2.18 | 10.09 | 1.16 | 0.71, 1.89 | 17.24 | 2.00 | 1.27, 3.17 | 17.47 | 0.89 | 0.56, 1.41 | 22.84 | 0.66 | 0.41, 1.06 | 32.64 | 1.18 | 0.76, 1.85 |
| Q21 | 5.60 | 0.96 | 0.81, 1.14 | 9.45 | 0.93 | 0.78, 1.11 | 16.00 | 0.83 | 0.68, 1.01 | 17.42 | 0.90 | 0.75, 1.06 | 22.65 | 0.84 | 0.70, 1.00 | 31.29 | 0.87 | 0.73, 1.04 |
| Q22 | 5.30 | 0.96 | 0.66, 1.40 | 9.41 | 1.05 | 0.72, 1.53 | 16.20 | 0.93 | 0.61, 1.40 | 15.91 | 1.03 | 0.71, 1.51 | 22.33 | 1.14 | 0.78, 1.65 | 31.39 | 0.96 | 0.65, 1.41 |
| Q23 | 5.76 | 0.99 | 0.68, 1.46 | 9.79 | 1.33 | 0.91, 1.95 | 16.99 | 1.08 | 0.71, 1.64 | 17.36 | 0.61 | 0.42, 0.89 | 22.92 | 0.67 | 0.46, 0.97 | 32.16 | 0.94 | 0.66, 1.35 |
| Q25–Q26 | 5.69 | 1.24 | 0.85, 1.80 | 9.46 | 1.20 | 0.82, 1.77 | 16.72 | 0.95 | 0.62, 1.44 | 17.42 | 0.56 | 0.38, 0.82 | 22.64 | 0.79 | 0.56, 1.13 | 31.46 | 0.58 | 0.40, 0.86 |
| Ventricular septal defect | 5.56 | 0.93 | 0.76, 1.14 | 9.46 | 0.83 | 0.67, 1.03 | 16.06 | 0.73 | 0.58, 0.91 | 17.31 | 0.94 | 0.76, 1.15 | 22.44 | 0.89 | 0.73, 1.10 | 31.44 | 0.90 | 0.73, 1.11 |
| Atrial septal defect | 5.82 | 0.87 | 0.56, 1.34 | 9.42 | 0.86 | 0.55, 1.34 | 16.41 | 0.94 | 0.58, 1.51 | 17.53 | 1.01 | 0.65, 1.56 | 22.25 | 0.92 | 0.59, 1.44 | 30.84 | 0.95 | 0.61, 1.48 |
| Congenital pulmonary value stenosis | 5.73 | 0.82 | 0.55, 1.23 | 9.40 | 0.82 | 0.54, 1.24 | 15.85 | 0.92 | 0.60, 1.42 | 17.44 | 0.95 | 0.64, 1.42 | 22.66 | 0.87 | 0.58, 1.30 | 31.14 | 0.90 | 0.60, 1.36 |
| Tetralogy of Fallot | 5.61 | 1.22 | 0.70, 2.13 | 9.69 | 1.68 | 0.97, 2.93 | 16.76 | 1.43 | 0.79, 2.59 | 17.43 | 0.52 | 0.30, 0.90 | 22.64 | 0.75 | 0.45, 1.25 | 31.84 | 0.75 | 0.45, 1.26 |
| Coarctation of the aorta | 5.55 | 1.18 | 0.69, 2.04 | 9.90 | 0.60 | 0.33, 1.11 | 16.62 | 0.55 | 0.29, 1.03 | 17.16 | 0.52 | 0.30, 0.91 | 22.97 | 0.74 | 0.44, 1.26 | 31.59 | 0.39 | 0.22, 0.70 |
Abbreviations: CI, confidence interval; ICD-10, International Classification of Diseases, Tenth Revision; OR, odds ratio.
Q20, congenital malformations of cardiac chambers and connections; Q21, congenital malformations of cardiac septa; Q22, congenital malformations of pulmonary and tricuspid valves; Q23, congenital malformations of aortic and mitral valves; Q25–Q26, congenital malformations of great arteries and veins.
Table 5 illustrates the pollutant-specific odds ratios for the predicted mean values of exposure (continuous variable) and odds ratios for mean, minimum, and maximum of simulated regression coefficients. Both conventional and simulated regressions showed a weak but significant association between exposure to BS and congenital malformations of cardiac chambers and connections. An inverse association between ventricular septal defect and BS was consistently detected in models with categorical and continuous exposure variables.
Table 5.
Pollutant-Specific Odds Ratios for a 1–μg/m−3 Increase in Black Smoke and Sulfur Dioxide Levels in Each Outcome Group, Northeast of England, 1985–1996
| OR for the Predicted Black Smoke Mean Values | 95% CI for the Predicted Black Smoke Mean Values | Simulated Logistic Regressions for Black Smoke Levels |
OR for the Predicted Sulfur Dioxide Mean Values | 95% CI for the Predicted Sulfur Dioxide Mean Values | Sulfur Dioxide |
|||||||||||
| OR for Mean of Regression Coefficients | OR for Minimum of Regression Coefficients | OR for Maximum of Regression Coefficients | OR for Mean of Regression Coefficients | OR for Minimum of Regression Coefficients | OR for Maximum of Regression Coefficients | |||||||||||
| ICD-10 code(s)a | ||||||||||||||||
| Q20 | 1.02 | 1.01, 1.03 | 1.02 | 1.02 | 1.02 | 1.01 | 0.99, 1.02 | 1.01 | 1.01 | 1.01 | ||||||
| Q21 | 0.99 | 0.98, 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99, 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Q22 | 1.00 | 0.98, 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99, 1.01 | 1.00 | 1.00 | 1.00 | ||||||
| Q23 | 0.99 | 0.98, 1.01 | 0.99 | 0.99 | 1.00 | 1.01 | 0.99, 1.02 | 1.00 | 0.99 | 1.01 | ||||||
| Q25–Q26 | 0.99 | 0.98, 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 0.97, 1.00 | 0.99 | 0.99 | 0.99 | ||||||
| Ventricular septal defect | 0.99 | 0.98, 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99, 1.00 | 0.99 | 0.99 | 0.99 | ||||||
| Atrial septal defect | 1.00 | 0.98, 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98, 1.01 | 1.00 | 1.00 | 1.00 | ||||||
| Congenital pulmonary value stenosis | 1.00 | 0.99, 1.02 | 0.94 | 0.60 | 1.37 | 1.00 | 0.98, 1.01 | 1.00 | 1.00 | 1.00 | ||||||
| Tetralogy of Fallot | 1.00 | 0.98, 1.02 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98, 1.02 | 1.00 | 1.00 | 1.00 | ||||||
| Coarctation of the aorta | 0.97 | 0.95, 1.00 | 0.98 | 0.97 | 0.99 | 0.97 | 0.95, 1.00 | 0.98 | 0.98 | 0.99 | ||||||
Abbreviations: CI, confidence interval; ICD-10, International Classification of Diseases, Tenth Revision; OR, odds ratio.
Q20, congenital malformations of cardiac chambers and connections; Q21, congenital malformations of cardiac septa; Q22, congenital malformations of pulmonary and tricuspid valves; Q23, congenital malformations of aortic and mitral valves; Q25–Q26, congenital malformations of great arteries and veins.
DISCUSSION
The present population-based case-control study investigated the association between maternal exposure to ambient BS and sulfur dioxide and the occurrence of CHD in offspring. The exposure assessment was based on a spatiotemporal modeling approach capable of predicting weekly BS and sulfur dioxide levels throughout the northeast of England over the period of 1985–1996. This modeling approach was used to predict ambient pollutant levels at the residential postcode of each study participant for weeks 3–8 of pregnancy. Analysis was performed as 2 subanalyses, with one using point estimates of exposure for each pregnancy location and time and the other applying a simulation approach that took into account the uncertainty of the exposure model.
Our study showed a slightly increased risk of congenital malformations of cardiac chambers and connections associated with maternal exposure to BS. However, when exposure was treated as a categorical variable, there was no dose–response relation in consecutive quartiles of exposure. The present study did not detect any increased risk for any CHD groups or subtypes after maternal exposure to sulfur dioxide.
The available evidence on the association between maternal exposure to BS and risk of congenital anomalies is still very limited. Rankin et al. (14) investigated the association between maternal exposure to BS and the occurrence of different congenital anomalies, including pooled CHD and 6 individual CHDs (ventricular septal defect, coarctation of the aorta, tetralogy of Fallot, atrioventricular septal defect, hypoplastic left heart syndrome, and patent ductus arteriosus) in northern England from 1985 to 1990. Their exposure assessment was based on assigning measured pollutant levels according to the monitoring sites within 10 km of the maternal residential postcode for the first trimester of pregnancy. They reported an increased risk of nervous system anomalies due to maternal exposure to BS, but no risk of any other anomalies. They did not perform the analysis for the ICD-10 CHD groups, and it is not possible to compare our detected significant association with their findings. However, nonsignificant associations for the individual CHDs found by our study are consistent with their reported results (14).
Gilboa et al. (11) reported an enhanced risk of ventricular septal defect in association with maternal exposure to sulfur dioxide. This finding was not replicated in our study or in other studies (14–16). Hansen et al. (15) reported an increased risk of aortic artery and valve defects due to exposure to sulfur dioxide. Our findings did not support this association, and neither did findings of other studies (11, 14, 16).
Animal and experimental studies have shown that maternal exposure to air pollutants can have teratogenic effects. Suggested mechanisms of teratogenicity of air pollutants include oxidative stress that influences the migration and differentiation of neural crest cells (39, 40), tissue hypoxia (41–44), interactions with the metabolism and detoxification of other xenobiotics (45), somatic effects on DNA interfering with basic processes such as programmed apoptosis (cell death) (46), and impact on the functionality of trophoblastic cells and early fetal growth (47–50).
Our analytical strategy resulted in a total of 60 comparisons. Instead of adjusting for multiple comparisons (51–54), we emphasized the consistency of results between the 2 subanalyses. To avoid type I errors, associations were considered to be relevant only if a direct dose–response relation was observed for consecutive quartiles of exposure (in models with categorical exposure variables) and the association was significant for the fourth quartile of exposure.
In our second subanalysis, the odds ratios based on minimum and maximum regression coefficients for the simulated exposure levels differed in the fourth or fifth decimal place. The estimated odds ratios from this subanalysis were similar to those of the first subanalysis. This was because the resulting standard errors of the spatiotemporal exposure models were very small in relation to the predicted means. Therefore, the simulated exposure measurements were very close to the predicted mean used in the first subanalysis.
The present study had the advantage of using high-quality population-based registry data on CHD from a geographically well-defined area. Our study is the first to use complex spatiotemporal modeling for exposure assessment to investigate the association between maternal exposure to ambient air pollution and the occurrence of congenital anomalies. This is important, as it has been suggested that for studies of the adverse effects of maternal exposure to air pollution during pregnancy that have large sample sizes, long duration, and different outcomes and exposures, the exposure assessment should rely on modeling approaches (25). Our modeling approach could predict 72% and 53% of spatiotemporal variation in BS and sulfur dioxide levels, respectively, at each postcode across the study region for each week of the study period. The modeling was validated internally and externally, suggesting that the model provided reasonable estimates of BS and sulfur dioxide levels. In addition to the use of point estimates of exposure, the present study used a simulation approach to address the uncertainty of the exposure model in prediction of the exposure to produce robust results.
For the period of study, there were 4 automatic monitoring sites that hourly measured particulate matter with aerodynamic diameter <10 μm, sulfur dioxide, nitrogen dioxide, nitric oxide, ozone, and carbon monoxide across the study region. Most of these sites were operational from 1992 onward. Among these monitors, 1 only measured sulfur dioxide and 1 measured nitric oxide and nitrogen dioxide. It was therefore not possible to include these pollutants in our analysis because of lack of required spatial resolution of monitoring data for the exposure modeling.
Similar to the previous studies investigating the association between maternal exposure to air pollution and the occurrence of congenital anomalies, our study relied on ambient air pollutant levels at the maternal place of residence at the time of delivery. This assignment overlooked maternal time-activity patterns leading to exposure to different pollutant levels in different microenvironments (55–57). Indoor levels of air pollutants may have differed from outdoor levels, and relying on ambient levels may have caused exposure misclassification in our study (58–60).
Hodgson et al. (61) have shown that about 9% of mothers in the NorCAS database changed their place of residence during the period between the first antenatal contact (usually about week 14 of pregnancy) and the time of delivery, with a median moving distance of 1.4 km. This change may have led to exposure misclassification, as the exposure assessment in this study was based on maternal place of residence at the time of delivery. However, 2 recently published US studies have reported that maternal relocation during pregnancy did not lead to a significant change in the assigned maternal exposure to air pollution (62, 63).
We adjusted our analyses for year of birth, SES, infant sex, degree of urbanity, and season of conception. However, we were not able to adjust our analyses for other possible confounders, such as maternal age, maternal smoking, maternal alcohol use, maternal body mass index, and maternal drug use, because of the unavailability of the data. Some evidence shows that removing cases with chromosomal abnormalities takes out the confounding effect of maternal age on the occurrence of CHD (64, 65). Therefore, exclusion of cases with chromosomal abnormalities in our analysis may have partially compensated for the lack of maternal age data. In the United Kingdom, alcohol consumption, smoking behavior, and body mass index are associated with SES (66–70). As a result, adjustment for SES is likely to at least partially address the effect of maternal body mass index, smoking, and alcohol consumption.
To date, a limited body of evidence has linked maternal exposure to ambient BS, sulfur dioxide, particulate matter with aerodynamic diameter <10 μm, carbon monoxide, ozone, and nitric oxide to the occurrence of congenital anomalies, mainly CHD; however, there is considerable inconsistency in the reported associations (5–16). Further studies are required to be able to draw a conclusion about these links. For future studies, we recommend focusing on the pollutants mentioned above and relying on modeling approaches for exposure assessment while incorporating data on maternal time-activity patterns.
Acknowledgments
Author affiliations: Institute of Health and Society, Newcastle University, Newcastle upon Tyne, United Kingdom (Payam Dadvand, Judith Rankin, Stephen Rushton, Tanja Pless-Mulloli); Institute for Research on Environment and Sustainability, Newcastle University, Newcastle upon Tyne, United Kingdom (Stephen Rushton, Tanja Pless-Mulloli); and Centre for Research in Environmental Epidemiology (CREAL), Barcelona, Spain (Payam Dadvand).
This work was carried out as a part of Payam Dadvand's doctoral research, which was partially supported by a Newcastle University International Research Scholarship.
J.R. was funded by a Personal Award Scheme Career Scientist Award from the National Institute of Health Research (UK Department of Health).
The authors thank Mary Bythell, NorCAS data manager. They are grateful to David Briggs, Nino Kunzli, and Mark Nieuwenhuijsen for their helpful suggestions on the exposure assessment method.
Conflict of interest: none declared.
Glossary
Abbreviations
- BS
black smoke
- CHD
congenital heart disease
- EUROCAT
European Surveillance of Congenital Anomalies
- ICD-10
International Classification of Diseases, Tenth Revision
- NorCAS
Northern Congenital Abnormality Survey
- SES
socioeconomic status
References
- 1.Cohen AJ, Ross Anderson H, Ostro B, et al. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A. 2005;68(13-14):1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 2.Maisonet M, Correa A, Misra D, et al. A review of the literature on the effects of ambient air pollution on fetal growth. Environ Res. 2004;95(1):106–115. doi: 10.1016/j.envres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Srám RJ, Binková B, Dejmek J, et al. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glinianaia SV, Rankin J, Bell R, et al. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 5.Savel'eva LF. Effects of air pollution on female reproductive function and congenital malformations [in Russian] Gig Sanit. 1991;4:4–5. [PubMed] [Google Scholar]
- 6.Antipenko EN, Kogut NN. Intensity of the mutagenic process in residents of cities with various levels of chemical air pollution (from the data on congenital abnormalities) [in Russian] Dokl Akad Nauk SSSR. 1991;321(1):206–209. [PubMed] [Google Scholar]
- 7.Reznik BIa, Minkov IP, Prudkii VIa, et al. Environmental pollution and congenital abnormalities according to the data of environmental health monitoring [in Russian] Gig Sanit. 1992;7-8:6–9. [PubMed] [Google Scholar]
- 8.Gumińska M. Environmental problems of Cracow and their health consequences [in Polish] Folia Med Cracov. 1993;34(1–4):19–28. [PubMed] [Google Scholar]
- 9.Smrcka V, Leznarová D. Environmental pollution and the occurrence of congenital defects in a 15-year period in a south Moravian district. Acta Chir Plast. 1998;40(4):112–114. [PubMed] [Google Scholar]
- 10.Ritz B, Yu F, Fruin S, et al. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155(1):17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Gilboa SM, Mendola P, Olshan AF, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162(3):238–252. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- 12.Kim OJ, Ha EH, Kim BM, et al. PM10 and pregnancy outcomes: a hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med. 2007;49(12):1394–1402. doi: 10.1097/JOM.0b013e3181594859. [DOI] [PubMed] [Google Scholar]
- 13.Hwang BF, Jaakkola JJ. Ozone and other air pollutants and the risk of oral clefts. Environ Health Perspect. 2008;116(10):1411–1415. doi: 10.1289/ehp.11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankin J, Chadwick T, Natarajan M, et al. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2009;109(2):181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Hansen CA, Barnett AG, Jalaludin BB, et al. Ambient air pollution and birth defects in Brisbane, Australia. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0005408. e5408. (doi:10.1371/journal.pone.0005408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strickland MJ, Klein M, Correa A, et al. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol. 2009;169(8):1004–1014. doi: 10.1093/aje/kwp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosi G. Congenital heart defects and disease: an epidemiological overview. Ital J Pediatr. 2004;30(5):261–266. [Google Scholar]
- 18.Lee K, Khoshnood B, Chen L, et al. Infant mortality from congenital malformations in the United States, 1970–1997. Obstet Gynecol. 2001;98(4):620–627. doi: 10.1016/s0029-7844(01)01507-1. [DOI] [PubMed] [Google Scholar]
- 19.Khoshnood B, De Vigan C, Vodovar V, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population-based evaluation. Pediatrics. 2005;115(1):95–101. doi: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 20.Connor JA, Gauvreau K, Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics. 2005;116(3):689–695. doi: 10.1542/peds.2004-2071. [DOI] [PubMed] [Google Scholar]
- 21.Mackie AS, Ionescu-Ittu R, Pilote L, et al. Hospital readmissions in children with congenital heart disease: a population-based study. Am Heart J. 2008;155(3):577–584. doi: 10.1016/j.ahj.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Botto LD, Correa A. Decreasing the burden of congenital heart anomalies: an epidemiologic evaluation of risk factors and survival. Prog Pediatr Cardiol. 2003;18(2):111–121. [Google Scholar]
- 23.Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young. Circulation. 2007;115(23):2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 24.Nora JJ, Nora AH. The evolution of specific genetic and environmental counseling in congenital heart diseases. Circulation. 1978;57(2):205–213. doi: 10.1161/01.cir.57.2.205. [DOI] [PubMed] [Google Scholar]
- 25.Gilliland F, Avol E, Kinney P, et al. Air pollution exposure assessment for epidemiologic studies of pregnant women and children: lessons learned from the Centers for Children's Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1447–1454. doi: 10.1289/ehp.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Office for National Statistics. Region in figures: North East: volume 2. In: Edwards G, ed. Region in Figures. London, United Kingdom: Office for National Statistics; 2000. [Google Scholar]
- 27.Richmond S, Atkins J. A population-based study of the prenatal diagnosis of congenital malformation over 16 years. BJOG. 2005;112(10):1349–1357. doi: 10.1111/j.1471-0528.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 28.Dadvand P, Rankin J, Shirley MD, et al. Descriptive epidemiology of congenital heart disease in Northern England. Paediatr Perinat Epidemiol. 2009;23(1):58–65. doi: 10.1111/j.1365-3016.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 29.European Surveillance of Congenital Anomalies. Prevalence of Congenital Anomalies. Newtownabbey, Northern Ireland: European Surveillance of Congenital Anomalies; 2004. ( http://eurocat.ulster.ac.uk/pubdata/tables.html). (Accessed January 15, 2008) [Google Scholar]
- 30.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. Geneva, Switzerland: World Health Organization; 2007. ( http://www.who.int/classifications/apps/icd/icd10online/). (Accessed January 15, 2008) [Google Scholar]
- 31.Opitz JM, Clark EB. Heart development: an introduction. Am J Med Genet. 2000;97(4):238–247. [PubMed] [Google Scholar]
- 32.Trines J, Hornberger LK. Evolution of heart disease in Utero. Pediatr Cardiol. 2004;25(3):287–298. doi: 10.1007/s00246-003-0592-2. [DOI] [PubMed] [Google Scholar]
- 33.United Kingdom Department for Environment, Food and Rural Affairs and the Devolved Administrations. UK Air Quality Archive [database]. London, United Kingdom: AEA; 2010. ( http://www.airquality.co.uk/index.php). (Accessed January 15, 2007) [Google Scholar]
- 34.EDINA. Digimap Collection [database]. Edinburgh, United Kingdom: EDINA; 2010. ( http://www.edina.ac.uk/ukborders/). (Accessed January 15, 2007) [Google Scholar]
- 35.EDINA. UKBORDERS [database]. Edinburgh, United Kingdom: EDINA; 2010. ( http://www.edina.ac.uk/ukborders/). (Accessed January 15, 2007) [Google Scholar]
- 36.UK Meteorological Office. Land Surface Stations Data (1900–2000) [database] Oxfordshire, United Kingdom: British Atmospheric Data Centre; 2010. ( http://badc.nerc.ac.uk/data/ukmo-midas/). (Accessed January 15, 2007) [Google Scholar]
- 37.Dadvand P, Rushton S, Diggle PJ, et al. Using spatio-temporal modeling to predict long-term exposure to black smoke at fine spatial and temporal scale. Atmos Environ. (doi: 10.1014/j.atmosenv.2010.10.034) [Google Scholar]
- 38.Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Bristol, United Kingdom: Croom Helm Ltd; 1987. [Google Scholar]
- 39.Hassler JA, Moran DJ. Effects of ethanol on the cytoskeleton of migrating and differentiating neural crest cells: possible role in teratogenesis. J Craniofac Genet Dev Biol Suppl. 1986;2:129–136. [PubMed] [Google Scholar]
- 40.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrielli A, Layon AJ. Carbon monoxide intoxication during pregnancy: a case presentation and pathophysiologic discussion, with emphasis on molecular mechanisms. J Clin Anesth. 1995;7(1):82–87. doi: 10.1016/0952-8180(94)00017-x. [DOI] [PubMed] [Google Scholar]
- 42.Aubard Y, Magne I. Carbon monoxide poisoning in pregnancy. BJOG. 2000;107(7):833–838. doi: 10.1111/j.1471-0528.2000.tb11078.x. [DOI] [PubMed] [Google Scholar]
- 43.Singh J. Mechanism of developmental toxicity of carbon monoxide. Reprod Toxicol. 2007;24(1):66. [Google Scholar]
- 44.Longo LD. Carbon monoxide in the pregnant mother and fetus and its exchange across the placenta. Ann N Y Acad Sci. 1970;174(1):313–341. doi: 10.1111/j.1749-6632.1970.tb49798.x. [DOI] [PubMed] [Google Scholar]
- 45.Garvey DJ, Longo LD. Chronic low level maternal carbon monoxide exposure and fetal growth and development. Biol Reprod. 1978;19(1):8–14. doi: 10.1095/biolreprod19.1.8. [DOI] [PubMed] [Google Scholar]
- 46.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75(6):1163–1171. doi: 10.1016/s0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]
- 47.Walker SK, Hartwich KM, Robinson JS. Long-term effects on offspring of exposure of oocytes and embryos to chemical and physical agents. Hum Reprod Update. 2000;6(6):564–577. doi: 10.1093/humupd/6.6.564. [DOI] [PubMed] [Google Scholar]
- 48.Barroso RP, Osuamkpe C, Nagamani M, et al. Nitric oxide inhibits development of embryos and implantation in mice. Mol Hum Reprod. 1998;4(5):503–507. doi: 10.1093/molehr/4.5.503. [DOI] [PubMed] [Google Scholar]
- 49.Wu TP, Huang BM, Tsai HC, et al. Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch Androl. 2004;50(3):173–179. doi: 10.1080/01485010490425494. [DOI] [PubMed] [Google Scholar]
- 50.Inoue T, Kaibara M, Sakurai-Yamashita Y, et al. Increases in serum nitrite and nitrate of a few-fold adversely affect the outcome of pregnancy in rats. J Pharmacol Sci. 2004;95(2):228–233. doi: 10.1254/jphs.fp0040125. [DOI] [PubMed] [Google Scholar]
- 51.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 52.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. (doi:10.1186/1471-2288-2-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas DC, Siemiatycki J, Dewar R, et al. The problem of multiple inference in studies designed to generate hypotheses. Am J Epidemiol. 1985;122(6):1080–1095. doi: 10.1093/oxfordjournals.aje.a114189. [DOI] [PubMed] [Google Scholar]
- 55.Jerrett M, Finkelstein M. Geographies of risk in studies linking chronic air pollution exposure to health outcomes. J Toxicol Environ Health A. 2005;68(13-14):1207–1242. doi: 10.1080/15287390590936085. [DOI] [PubMed] [Google Scholar]
- 56.Briggs D. The role of GIS: coping with space (and time) in air pollution exposure assessment. J Toxicol Environ Health A. 2005;68(13-14):1243–1261. doi: 10.1080/15287390590936094. [DOI] [PubMed] [Google Scholar]
- 57.Nethery E, Leckie SE, Teschke K, et al. From measures to models: an evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup Environ Med. 2008;65(9):579–586. doi: 10.1136/oem.2007.035337. [DOI] [PubMed] [Google Scholar]
- 58.Raw GJ, Coward SK, Brown VM, et al. Exposure to air pollutants in English homes. J Expo Anal Environ Epidemiol. 2004;14(suppl 1):85S–94S. doi: 10.1038/sj.jea.7500363. [DOI] [PubMed] [Google Scholar]
- 59.Weisel CP, Zhang J, Turpin BJ, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA): part I. Collection methods and descriptive analyses. Res Rep Health Eff Inst. 2005;130(1):1–107. [PubMed] [Google Scholar]
- 60.Turpin BJ, Weisel CP, Morandi M, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA): part II. Analyses of concentrations of particulate matter species. Res Rep Health Eff Inst. 2007;130(2):1–77. [PubMed] [Google Scholar]
- 61.Hodgson S, Shirley M, Bythell M, et al. Residential mobility during pregnancy in the north of England. BMC Pregnancy Childbirth. 2009;9:52. doi: 10.1186/1471-2393-9-52. (doi:10.1186/1471-293-9-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lupo PJ, Symanski E, Chan W, et al. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol. 2010;24(2):200–208. doi: 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, Bell EM, Caton AR, et al. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110(2):162–168. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Bassili A, Mokhtar SA, Dabous NI, et al. Risk factors for congenital heart diseases in Alexandria, Egypt. Eur J Epidemiol. 2000;16(9):805–814. doi: 10.1023/a:1007601919164. [DOI] [PubMed] [Google Scholar]
- 65.Yauck JS, Malloy ME, Blair K, et al. Proximity of residence to trichloroethylene-emitting sites and increased risk of offspring congenital heart defects among older women. Birth Defects Res A Clin Mol Teratol. 2004;70(10):808–814. doi: 10.1002/bdra.20060. [DOI] [PubMed] [Google Scholar]
- 66.Evans JM, Newton RW, Ruta DA, et al. Socio-economic status, obesity and prevalence of type 1 and type 2 diabetes mellitus. Diabet Med. 2000;17(6):478–480. [PubMed] [Google Scholar]
- 67.Graham H, Hunt S. Women's smoking and measures of women's socio-economic status in the United Kingdom. Health Promot Int. 1994;9(2):81–88. [Google Scholar]
- 68.Shohaimi S, Luben R, Wareham N, et al. Residential area deprivation predicts smoking habit independently of individual educational level and occupational social class. A cross sectional study in the Norfolk cohort of the European Investigation Into Cancer (EPIC-Norfolk) J Epidemiol Community Health. 2003;57(4):270–276. doi: 10.1136/jech.57.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiles NJ, Lingford-Hughes A, Daniel J, et al. Socio-economic status in childhood and later alcohol use: a systematic review. Addiction. 2007;102(10):1546–1563. doi: 10.1111/j.1360-0443.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- 70.Wardle J, Waller J, Jarvis MJ. Sex differences in the association of socioeconomic status with obesity. Am J Public Health. 2002;92(8):1299–1304. doi: 10.2105/ajph.92.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]