Abstract
Background.
This descriptive cross-sectional study investigated the relationships between cerebral oxygen reserve and cognitive function in community-dwelling older adults.
Methods.
Participants (72 women and 40 men) underwent standard polysomnography, including regional measures of percent oxyhemoglobin saturation (rcSO2) determined by cerebral oximetry. Two variables were used to calculate cerebral oxygen reserve: (a) awake rcSO2 (mean presleep rcSO2) and (b) the change in rcSO2 from before sleep to the end of the first non-rapid-eye movement cycle. General linear models, adjusted for the effects of education and occupation, tested differences in performance on standard tests of memory, attention, and speed of mental processing.
Results.
Awake rcSO2 values were normal (60%–79.9%) in 64 participants, marginal (50%–59.9%) in 41, and low (43%–49.9%) in 7. Participants with normal awake levels had higher cognitive function than those with low levels (p < .05). Changes in rcSO2 were greatest in participants with marginal awake rcSO2 values; among whom, those who increased rcSO2 during sleep (n = 17) had better memory function than the 24 who did not (p < .05).
Conclusions.
Low awake rcSO2 values mark individuals with low cerebral oxygen reserves and generally lower cognitive function; marginal awake rcSO2 values that fall during sleep may indicate loss of cerebral oxygen reserve and an increased risk for cognitive decline. Further studies may clarify the significance of and mechanisms underlying individual differences in awake rcSO2 and the changes that occur in rcSO2 while asleep.
Keywords: Cerebral oxygenation, Sleep, Cognition
THE brain comprises 2% of total body weight but uses 20% of the body's energy(1). A small fraction (<5%) of that energy is used for task-evoked brain activations; more than 90% is devoted to intrinsic activity, which remains markedly stable during states of quiet wakefulness (2) and declines by 10%–15% during light sedation (3) or early NREM sleep (4). Typically measured at rest with eyes closed, intrinsic activity refers to the ongoing neural and metabolic activity that is not directly associated with participants’ performance of a task but is important for synaptic homeostasis and the maintenance of functional connectivity between brain areas (2).
The brain's energy is derived from nearly complete (>90%) oxidation of glucose (5): Autoregulation of cerebral blood flow ensures a plentiful supply of oxygen (cerebral oxygen reserve) (6). Because awake resting cerebral blood flow declines by 5%–10% between ages 40 and 75 years (7), resting brain metabolism dictates that oxygen extraction from capillary blood must increase (6) and cerebral oxygen reserve decline.
Prior studies suggest that age-associated loss of cerebral oxygen reserve may first be manifested during sleep, when cerebral blood flow (8) and arterial oxygenation (9) fall. Using cerebral oximetry, we evaluated cerebral oxyhemoglobin saturation (rcSO2) in 9 old (65–84 years) and 10 young (20–39 years) adults without sleep apnea (10). The two groups had mean awake rcSO2 values greater than 60%, but rcSO2 levels declined (median = −3.1%, range: −11.1% to 2.1%) during sleep in 8 of 9 old and increased (median = 1.8%, range: −1.4% to 7.9%) in 9 of 10 young participants.
At present, we do not know whether low rcSO2 levels during sleep mark individuals at risk for cognitive impairment, but rcSO2 levels less than 50% during surgery are linked to postoperative cognitive impairment (11,12), so it is plausible that low rcSO2 levels (awake or sleeping) might impair cognition in otherwise healthy participants and that falling rcSO2 during sleep may indicate impaired cerebral oxygen reserve. Therefore, we examined the relationship between awake resting rcSO2 levels, the change in rcSO2 levels during sleep, and cognitive function in community-dwelling older adults.
METHODS
Participants
Procedures for participant recruitment have been described elsewhere (13). Of 167 volunteers, 38 were excluded because they were less than 70 years old or had sleep apnea, depression, or dementia. Another 14 were eligible, but 2 lost interest, and 12 had acute changes in health or scheduling conflicts. The remaining 115 participants underwent sleep study. Five had mild sleep apnea (5–10 apneas or hypopneas per hour); data from three were excluded because of missing data, but those from two were included after auxiliary analyses showed that inclusion did not alter study findings.
The final sample (112 participants) was 64% female, 86% Caucasian, and aged 70–92 years (mean age = 78.3 years). Fifty-four percent of participants had at least 4 years of college, 37% had completed technical college or high school, and 9% had less formal education. Half had held professional jobs; 30%, small businesses, office, or clerical jobs; and 15% had been employed as skilled and 5% as unskilled laborers. All were retired; 75% did volunteer work.
No participant had a neurological condition that would affect sleep or cognitive function. All had Mini-Mental State Examination (14) scores greater than 27 (mean ± SD: 28.8 ± 1.1) and Older Adults Resource Services Independent Activity of Daily Living Scale (15) scores greater than 12 (13.5 ± 0.1). Scores on the 15-item Geriatric Depression Scale (16) were all less than 5 (1.2 ± 1.5). Sleep problems were minimal; all had Pittsburgh Sleep Quality Index scores (17) less than 8 (4.9 ± 3.5) and Epworth Sleepiness Scale (18) scores less than 10 (5.0 ± 2.1).
Number and severity of health problems were assessed with the Cumulative Illness Rating Scale (19). Subscale scores range from 0 (no history) to 4 (extremely severe); no participant scored greater than 3 and only 10% greater than 2. Body mass indices were between 18.6 and 32 kg/m2; 95% were less than 28 kg/m2. Serum hemoglobin concentrations were greater than 12 gm/dL. The institutional committee for the protection of human participants approved the study. All participants gave informed consent.
Cognitive Assessments
Cognitive tests were administered prior to the sleep study and scored by trained assistants, supervised by the project psychologist (M.H.). Ten percent of sessions were videotaped and reviewed monthly to ensure consistent test administration. Interrater reliability was assessed bimonthly; correlations exceeded .90 for all instruments.
Memory was assessed using 30-minute delayed recall scores from the logical memory and visual reproduction subscales of the Wechsler Memory-III test (20). Attention and processing speed were assessed using the Trail-Making Test Part B (21), Symbol Digit Modalities Test (22), color–word subscale of the Stroop Color–Word Test (23), and C-F-L version of the Controlled Word Association Test (24). Because all participants had near perfect scores (110–112 points) on the Stroop Color–Word test, we used time to complete the task to assess processing speed. The Controlled Word Association Test measures naming abilities within a 60-second interval, so it, too, is a reasonable measure of processing speed.
We used Petersen and colleagues criteria (25) to identify mild cognitive impairment (MCI). MCI was defined as a score greater than or equal to 25 on the MAC-Q (26) and an education-adjusted score greater than 1.5 SD below the mean on any of the above-mentioned tests. All participants had normal scores on the Mini-Mental State Examination and Older Adults Resource Services Independent Activity of Daily Living Scale and so were considered to have normal functional abilities and no dementia.
Sleep Monitoring
Sleep studies were conducted on two consecutive weekday nights in the Biobehavioral Laboratory at the University of North Carolina-Chapel Hill. Night 1 allowed participants to adjust to sleeping in the laboratory; data for analysis were collected on Night 2. Beginning at 11:00 PM, participants were asked to lie awake for 10 minutes with their eyes closed; thereafter, lights were extinguished and participants told to try to fall asleep; they were awakened at 6 AM. Participants maintained their typical daytime routines between study nights.
The collection of physiological signals has been described elsewhere (13). Briefly, standard polysomnography (27) was sampled at 250 Hz using a Grass Model 15 digital recorder (Astromed-Grass, Warwick, RI). Standard measures and criteria set by the American Academy of Sleep Medicine (28) were used to define arterial oxygen desaturations as well as apneas and hypopneas. Arterial oxyhemoglobin saturation (SaO2) was measured every 3 seconds with a Nellcor pulse oximeter (Mallinckrodt Inc., St. Louis, MO). Respiratory effort was recorded using a calibrated respiratory inductance plethysmograph (Ambulatory Monitoring, Ardsdale, NJ). Airflow at the nose and mouth was monitored using a single-channel oro-nasal thermocouple (Pro Tech, Woodville, WA).
Cerebral oximetry data, collected every 4 seconds using an INVOS 4100 cerebral oximeter (Somanetics, Troy, MI), were stored separately and merged with the polysomnograms using computer time stamps. The sensors were applied to the forehead, 2 cm above the eyebrows and 2 cm to the right and left of midline. We collected data for the entire night but restricted analysis to the first NREM sleep cycle because preliminary data showed that the greatest changes in rcSO2 occurred during the first cycle and did not differ significantly in subsequent cycles.
Signal Processing
Standard scoring rules (27) verified that participants were awake for 10 minutes and were used to score each 30-second epoch during sleep. A valid awake observation was defined as a continuous 10-minute segment of wakefulness before lights out. A valid epoch of sleep was defined as one consisting of NREM sleep that occurred between the onset of sleep and last epoch of continuous REM sleep. Interrater agreement for scoring sleep was excellent (κ = .91).
The difference between right- and left-sided measures was negligible, F(1,110) = 0.02, p = .98, so we averaged right and left to estimate rcSO2 . The average rcSO2 during the 10 minutes before lights out was used to classify participants as follows: normal awake rcSO2 (29) (presleep rcSO2 ≥60%), low awake rcSO2 (as suggested from studies correlating rcSO2 levels with cognitive function (11,12), a presleep rcSO2 <50%), or marginal awake rcSO2 (presleep rcSO2 50%–59.9%). The rcSO2 groups were subdivided according to whether rcSO2 increased, decreased, or did not change during sleep. The change in rcSO2 during sleep (ΔrcSO2, calculated as the difference between the average awake rcSO2 and the average of rcSO2 values during sleep) ranged from −6.9% to +5.4%. Because the repeatability of rcSO2 measurements is reportedly ±1% (http://www.somanetics.com), we defined an increase in rcSO2 as a ΔrcSO2 greater than +1.0% and a decrease as a ΔrcSO2 greater than −1.0%.
Data Analysis
General linear models were used to test the effect of awake rcSO2 on cognitive function. Because more education or a history of engagement in mentally challenging occupations improves test performance, tests for the effect of rcSO2 on cognition were adjusted for the combined effects of education and occupation using the two-factor Hollingshead Index of Social Status (30). The interaction of Hollingshead Index with awake rcSO2 level was not significant, so we present only the tests of adjusted main effects of awake rcSO2 levels on cognitive function.
Within each awake rcSO2 level, we used t tests to examine how cognitive function differed between those who increased and those who did not increase rcSO2 during sleep. We used Satterthwaite's correction to adjust for unequal variances among groups. All statistical analyses were performed using SAS, v9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Awake Cerebral Oxygenation
Of the 112 participants studied, 7 had low awake rcSO2 levels (43%–49.9%), 41 had marginal levels (50%–59.9%), and 64 had normal levels (60%–79.9%). Demographic and health data (Table 1) show that the groups with marginal and low awake rcSO2 levels had higher percentages of females ( = 27.6, p < .001) and minorities ( = 14.7, p < .001) but were similar in age to the normal group, F(2,112) = 0.87, p = .42. Those with low and marginal awake rcSO2 levels had higher Hollingshead Index Scores, F(2,112) = 6.06, p<.01, indicating less education or employment in less mentally challenging occupations compared with the group with normal levels.
Table 1.
Demographic and Health-Related Measures by Awake rcSO2 Level
| Awake rcSO2 |
All | |||
| Low: <50% | Marginal: 50–59.9% | Normal: ≥60% | ||
| M (SD) | M (SD) | M (SD) | ||
| N | 7 | 41 | 64 | 112 |
| Awake rcSO2 | 44.8 (3.7) | 56.0 (3.7) | 65.6 (5.7) | 61.6 (7.4) |
| Demographics | ||||
| % Female† | 100 | 90.2 | 44.9 | 62.3 |
| % Minority† | 14.3 | 29.3 | 3.1 | 13.4 |
| Age (years) | 80.6 (7.1) | 78.5 (6.0) | 77.7 (5.3) | 78.2 (5.7) |
| Hollingshead Index*,‡ | 39.1 (21.0) | 31.7 (15.4) | 23.8 (12.8) | 27.7 (15.0) |
| Illness burden | ||||
| CIRS-G total | 6.7 (4.5) | 5.2 (3.8) | 4.4 (3.1) | 4.9 (3.5) |
| Subscale scores >1 pt | ||||
| Vascular (%) | 57.1 | 61.0 | 51.5 | 55.3 |
| Arthritis† (%) | 71.4 | 46.3 | 28.1 | 37.5 |
| Endocrine† (%) | 42.9 | 26.8 | 18.8 | 23.2 |
| Heart (%) | 57.1 | 26.8 | 25.0 | 27.8 |
| Body mass index | 26.1(5.4) | 24.9 (4.6) | 25.1 (4.4) | 25.1 (4.5) |
| Type 2 diabetes (%)† | 19.1 | 3.0 | 10.0 | 7.6 |
| Obesity (BMI >30 kg/m2; %) | 14.3 | 22.01 | 28.0 | 25.0 |
| Hypertension (%) | 56.1 | 60.0 | 50.2 | 54.3 |
| None of these conditions (%) | 0.0 | 29.3 | 39.1 | 33.0 |
Notes: BMI = body mass index; CIRS = Cumulative Illness Rating Scale.
Lower scores indicate more education and more mentally challenging occupation.
Fisher’s Exact Test, p < .017.
F(2,109) = 6.06. Contrasts (Tukey's HSD), p < .05, low and marginal > normal.
Compared with the normal and marginal groups, participants with low awake rcSO2 had higher Cumulative Illness Rating Scale for Geriatrics scores (ie, greater illness burden; F(2,112) = 3.6, p = .01. Because we specifically excluded persons with debilitating illness, these higher scores reflect a greater number of organ systems with mild to moderate dysfunction. Average body mass index did not differ across the three groups, F(2,112) = 0.22, p = .81. Hypertension, cardiac disease, or endocrine disorder (primarily hypothyroidism) were equally prevalent in the three groups, but the low awake rcSO2 group had significantly more diabetes ( = 7.5, p = .02). Only 29% of the marginal and 30% of the normal awake oxygen groups had two or more risk factors for cardiovascular disease, but all participants with low awake rcSO2 did ( = 9.5, p < .004).
Awake Cerebral Oxygenation and Cognitive Function
Table 2 shows how cognitive function, adjusted for combined effects of education and occupation, differed across the three groups. The group with low awake rcSO2 levels performed significantly worse than the group with normal awake levels on tests of delayed memory (Logical Memory and Visual Reproduction tests) and two tests of attention and processing speed (Stroop Color–Word and the Controlled Oral Word Association Test). The marginal group differed from the others on the Symbol Digit Symbol Modalities Test; a similar group trend was observed with the Trail-Making Test Part B.
Table 2.
Measures of Cognitive Function by Awake Cerebral Oxygen Levels
| Awake rcSO2 Levels |
All | Partial F(2,108)* | p | |||
| Low: <50% | Marginal: 50–59.9% | Normal: ≥60% | ||||
| M (SD) | M (SD) | M (SD) | ||||
| N | 7 | 41 | 64 | 112 | ||
| Delayed memory | ||||||
| Logical memory | 15.9 (7.8) | 21.4 (8.4) | 23.7 (7.6) | 22.4 (8.1) | 3.08 | .05† |
| Visual reproduction | 23.0 (11.7) | 37.3 (19.7) | 47.9 (21.6) | 42.4 (21.5) | 5.69 | <.01† |
| Speed of processing and attention | ||||||
| Trail Making‡ | 130.9 (45.5) | 120.7 (68.9) | 97.9 (45.9) | 108.3 (56.3) | 2.96 | .06 |
| DSMT | 27.7 (5.9) | 37.1 (10.1) | 42.3 (8.9) | 39.5 (10.0) | 8.79 | <.01§ |
| Stroop Color–Word‡ | 210.7 (50.8) | 174.4 (47.5) | 163.0 (52.6) | 170.4 (51.6) | 3.14 | .05‖ |
| COWA | 29.4 (10.9) | 35.4 (11.1) | 43.3 (12.3) | 39.6 (12.6) | 6.90 | <.01¶ |
Notes: COWA = Controlled Oral Word Association; DSMT = Digit Symbol Modalities Test.
Significant contrasts of mean adjusted for education and occupation.
Low < normal.
A higher score indicates poorer performance. These variables were also extremely skewed, and thus, the data were log transformed prior to analysis.
Low < marginal < normal.
Low > normal.
Low and marginal < normal.
Ten participants met criteria for MCI; nine showed impaired attention and speed of processing (primarily the Symbol Digit Modalities Test) and one impaired memory (Visual Reproduction Test). At least one participant in each group met criteria for MCI, but a higher percentage was found in the group with low awake rcSO2 (43% vs 12% in the marginal and 3% in the normal groups; = 13.1, p = .001).
Sleep Characteristics
Polysomnographic measures did not differ between the three groups (see Supplementary Table 1). On average, the participants’ first NREM sleep cycle lasted approximately 118 minutes, with 103 minutes spent sleeping (mean sleep efficiency = 87.3%); more time was spent in slow-wave sleep than in Stages 1–2 NREM and Stage REM sleep.
Electroencephalographically detected arousals ranged from 2.0–14.8 per hour (median = 6.7). The participants’ awake SaO2 values ranged from 93% to 100%; mean SaO2 values decreased from 96.2% at rest to 95.9% during sleep. In all participants, the average SaO2 was above 90%; they spent less than 10% of time asleep with SaO2 levels less than 90%; end-tidal carbon dioxide levels were normal (30–45 mm Hg).
Changes in Cerebral Oxygenation During Sleep
Of the 64 participants with normal (>60%) awake oxygen levels, 11 (17%) increased, 29 (45%) decreased, and 24 (36%) showed no change in rcSO2 during sleep (see Supplementary Figure 1). We saw little change in rcSO2 levels during sleep in participants whose awake levels were greater than 70% (range: −1.8% to 2.8%). In only eight (15%), all of whom had low normal awake values (60%–65%), did rcSO2 values fall below 60% during sleep (range: 55.5%–59.8%).
The greatest changes in rcSO2 (range: −6.9% to +5.3%) occurred in participants with marginal awake oxygen levels. Of the 41 participants in this group, 16 (39%) decreased, 8 (20%) increased, and 17 (41%) showed no change during sleep. Of the 16 participants whose rcSO2 decreased during sleep, 11 (69%) had awake values below 55%, and in 2, the asleep values fell below 50%. All eight subjects who increased rcSO2 levels during sleep had awake rcSO2 values above 55% (55.1%–59.3%); six reached rcSO2 levels above 60% (60.1%–64.3%) during sleep.
Unlike the marginal and normal groups, five of seven participants with low rcSO2 declined further during sleep. We observed a strong association between the magnitude of decline in rcSO2 and the participant's awake rcSO2 level (Spearman ρ, r = .99). Ranging from−1.2% to −6.3%, declines in rcSO2 were greatest in participants with the lowest awake values and least in those with the highest.
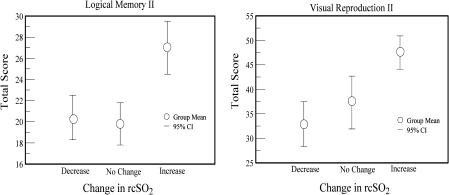
Change in Cerebral Oxygen During Sleep and Cognitive Function
Because changes in rcSO2 may influence cognition (11,12), we examined the relationship between ΔrcSO2 and cognitive function in the group that showed the largest change in rcSO2 during sleep—those with marginal awake rcSO2 levels (Figure 1). Performance on the six tests did not differ between those whose rcSO2 declined or did not change during sleep. But, compared with those whose rcSO2 declined or did not change, those whose rcSO2 rose during sleep performed significantly better on the Logical Memory (20.3 ± 8.0 vs 27.0 ± 7.1, t = −2.2, p < .05) and Visual Reproduction (32.6 ± 18.2 vs 47.5 ± 15.2, t = −2.3, p < .05) tests. There were no group differences on tests of attention or processing speed
Figure 1.
Differences in memory function in participants with marginal awake rcSO2 levels who either increased (n = 17), decreased (n = 16), or had no change (n = 8) in rcSO2 during sleep. Each point indicates the group mean. The bars indicate the 95% confidence intervals.
DISCUSSION
This study of community-dwelling older adults found significant correlations between rcSO2 and cognitive function. Specifically, older adults with low awake cerebral oxygen levels (rcSO2 <50%) had more cognitive dysfunction than those with normal levels (rcSO2 >60%). Compared to those with normal and marginal rcSO2 levels, a greater percentage of the low group met criteria for MCI. The association of awake rcSO2 and cognition was quite striking because the differences in function spanned a wide range of cognitive abilities and remained significant even after controlling for the effects of education and occupational status.
Cognitive function in the marginal group (awake rcSO2 between 50% and 60%) fell between that of the normal and low awake groups but did not differ significantly from either. Those with marginal awake rcSO2 who increased their levels during sleep had better memory function than those who did not. Together, these findings suggest preservation of cognitive function in older adults who can maintain (the normal awake group) or restore cerebral oxygen during sleep (as in the marginal group) to levels that better support the brain's intrinsic activity.
Mechanisms of Hypoxic Injury
We cannot draw conclusions from this cross-sectional study about causal effects of rcSO2 on cognitive function, but our findings open the door for further study. The brain uses a great deal of oxygen, largely to support intrinsic activity (5). Cerebral blood flow is coupled to energy demand, but age-related decline in regional blood flow can lead to a state of chronic hypoperfusion (7,31). Because the brain oxidizes nearly all its available glucose (5), chronic hypoperfusion is likely to result in mitochondrial dysfunction and release of reactive oxygen species (31). In blood vessels, reactive oxygen species decrease the supply of endothelium-derived nitric oxide, leading to endothelial dysfunction, further fall in regional blood flow, even lower rcSO2 levels (32), and further increased oxidative stress and damage.
Systemic diseases may also aggravate pathological processes within the brain, possibly by impairing cerebral blood flow (33), inducing the release of proinflammatory mediators (34), or by depleting circulating stores of antioxidants (35). Only 30% of participants with normal awake rcSO2 levels, but all participants with low awake levels, had two or more risk factors for cardiovascular disease. In addition, the low awake group had a larger percentage of individuals with diabetes and arthritis, conditions associated with inflammation and depletion of antioxidants (36). The fact that the low awake group had a higher percentage of persons with MCI suggests that systemic diseases may aggravate the effects of cerebral hypoperfusion, possibly placing individuals at greater risk for cognitive loss.
Sleep and Cerebral Oxygen Reserves
We do not know the factors that contribute to changes in rcSO2 during sleep in the old. Studies of young adults have shown that cerebral blood flow and metabolism decline during sleep (8) so that oxygen extraction in the young remains stable during sleep. However, we have previously seen increases in rcSO2 in young adults, (10) suggesting that regional flow can increase during sleep above the level needed to maintain intrinsic activity.
In those with marginal awake levels, rises in rcSO2 during sleep often restore oxygen to normal levels (>60%). Of importance for potential interventions, such rises may protect cognition because those in the marginal group who increased their rcSO2 performed better on tests of memory than those with similar awake levels who did not. Clearly, more study is needed to better delineate the mechanisms that raise rcSO2 during sleep.
Declines in rcSO2 during sleep occurred in all three groups but were most prevalent in participants with low awake rcSO2 levels. In those whose rcSO2 declined during sleep, the magnitude of decline increased as awake rcSO2 levels decreased (r = .99). A number of these individuals met the criteria for MCI, suggesting that low cerebral oxygen reserves may place these individuals at risk of hypoxia, oxidative stress, and mitochondrial dysfunction. If so, we might expect changes in brain intrinsic activity and an impaired ability to repair damaged neurons, thus jeopardizing the brain's functional connectivity (2). Clearly, investigations of the relationship between cerebral oxygen reserve, intrinsic activity, and cognitive function are important areas for future research.
Limitations and Directions for Future Research
Despite opening new avenues for future research, our study has at least two limitations. The sample, although one of the largest to measure rcSO2 in persons older than 70 years of age, was predominantly Caucasian, and the mean education level was fairly high. Our findings need corroboration in an ethnically and educationally diverse population. Second, our assessment of cerebral oxygenation is obtained from a small portion of the frontal area of the brain. Although not representative of the entire brain, disruption of cerebral white matter in the frontal lobe has been associated with lower cognitive function in healthy older adults (37). It is also an area that shows great age-associated reductions in regional blood flow (7).
Clearly, investigating how oxidative stress and inflammation relate to blood vessel reactivity and cerebral oxygen levels and to changes in cognitive function are important areas for future study. If, as our research suggests, cerebral oxygen reserve is diminished in those with low and marginal awake oxygen levels, then these individuals are likely to benefit from improved oxygen delivery (eg, nocturnal oxygen supplementation), enhanced cerebral blood flow (adjustments to antihypertensive medications), counteracting the activation of inflammatory or oxidative pathways (antioxidants), or improving vascular function (eg, exercise). Thus, the assessment of change in cerebral oxygen levels during sleep, particularly in participants who have marginal or low awake rcSO2 levels, may provide a simple minimally intrusive indicator of early decline in cerebral oxygen reserves. If so, this method opens new opportunities to initiate interventions at a stage when interventions may prove most effective for forestalling cognitive loss.
FUNDING
This work is supported by grants from the National Institutes of Health (RO1 NR08032, M01RR00046, and UL1RR025747).
SUPPLEMENTARY MATERIAL
Supplementary Table 1 and Figure 1 can be found at: http://biomed.gerontologyjournals.org/
References
- 1.Clark DD, Sokoloff L. Circulation and energy metabolism of the brain. In: GL Siegel, BW Aranoff, RW Albers, et al., editors. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadelphia, PA: Lippincot-Raven; 1999. p. 100. [Google Scholar]
- 2.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–12734. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 6.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125:595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 7.Larsson A, Skoog I, Aevarsson AA, et al. Regional cerebral blood flow in normal individuals aged 40, 75 and 88 years studied by 99Tc(m)-d, l-HMPAO SPET. Nucl Med Commun. 2001;22:741–746. doi: 10.1097/00006231-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Madsen PL. Blood flow and oxygen uptake in the human brain during various states of sleep and wakefulness. Acta Neurol Scand Suppl. 1993;148:3–27. [PubMed] [Google Scholar]
- 9.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. 1996;110:1489–1492. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 10.Carlson BW, Neelon VJ, Carlson JR, Hartman M, Dogra S. Exploratory analysis of cerebral oxygen reserves during sleep onset in older and younger adults. J Am Geriatr Soc. 2008;56:914–919. doi: 10.1111/j.1532-5415.2008.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casati A, Fanelli G, Pietropaoli P, et al. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery: a prospective cohort study. Eur J Anaesthesiol. 2007;24:59–65. doi: 10.1017/S0265021506001025. [DOI] [PubMed] [Google Scholar]
- 12.Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 13.Carlson BW, Neelon V, Carlson J, Hartman M, Dogra S. Cerebrovascular disease and patterns of cerebral oxygen reserves during sleep in elders. Biol Res Nurs. 2009;10:307–317. doi: 10.1177/1099800408330396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. ”Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Fillenbaum GG. Center for the Study of Aging and Human Development. Multifactorial Functional Assessment Questionnaire. Durham, NC: Duke University Press; 1978. [Google Scholar]
- 16.Sheikh JI, Yesavage JS. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 17.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. WMS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 21.Partington J, Leiter R. Partington's pathway test. Psychol Serv Cent Bull. 1949;1:9–20. [Google Scholar]
- 22.Smith A. The Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Service Center; 1991. [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;28:643–662. [Google Scholar]
- 24.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 25.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Crook TH, III, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr. 1992;4:165–176. doi: 10.1017/s1041610292000991. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: National Institute of Neurobiological Diseases and Blindness: Neurological Information Network; 1968. [Google Scholar]
- 28.The report of an American Academy of Sleep Medicine Task Force: sleep-related breathing disorders in adults: recommendation for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 29.Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58:541–560. doi: 10.1016/s0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 30.Hollingshead A. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1957. [Google Scholar]
- 31.de la Torre JC. Cerebral hypoperfusion, capillary degeneration, and development of Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14:S72–S81. doi: 10.1097/00002093-200000001-00012. [DOI] [PubMed] [Google Scholar]
- 32.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 33.Abbatecola AM, Barbieri M, Rizzo MR, et al. Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. J Gerontol A Biol Sci Med Sci. 2008;63:991–996. doi: 10.1093/gerona/63.9.991. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RO, Geda YE, Knopman DS, et al. Association of C-reactive protein with mild cognitive impairment. Alzheimers Dement. 2009;5:398–405. doi: 10.1016/j.jalz.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.