Abstract
Background.
The adverse effects of smoking on individual medical conditions are well known; however, the cumulative effect of smoking on physical performance is not well characterized, particularly in midlife.
Methods.
In the British 1946 Birth Cohort Study, cigarette pack-years were examined with standing balance, chair rising, grip strength, and an overall composite index. Pack-years were calculated from data collected at ages 20, 25, 31, 36, 43, and 53 years, whereas physical performance, cognitive function, anthropometry, and spirometry were assessed at age 53 years in 2,394 men and women. Regression and cubic splines were used to assess the relationship between pack-years and physical performance.
Results.
Greater pack-years smoked were associated with lower overall physical performance and lower performance in standing balance and chair rising; however, there was no association with grip strength. For every 10 pack-years smoked, the overall physical performance index decreased by 0.11 SD (95% confidence interval: 0.07–0.15, p < .001), standing balance time decreased by 0.09 SD (0.05–0.13), and the reciprocal of chair rise time decreased by 0.11 SD (0.07–0.16). Adjustment for education, social class, lung function, cognitive function, and medical conditions attenuated the effect, but pack-years remained significantly associated with standing balance and chair rising time.
Conclusions.
Lifetime cigarette pack-years are strongly related to physical performance in the fifth decade of life, suggesting that smokers will enter older adulthood with decreased physiological reserve. As smoking prevalence remains high in many developed countries and is rapidly growing in developing countries, these findings underscore the need for effective smoking cessation and prevention programs.
Keywords: Physical performance, Aging, Smoking, Cigarette pack-years
SMOKING is one of the most studied risk factors for disease and premature mortality, but it is not well understood how smoking relates to physical performance in middle age. Decline in physical performance with aging increases risk of disability and premature death (1); thus, understanding factors acting through life that influence physical performance in midlife may help us improve the future health of elderly populations. Previous results from the Medical Research Council's National Survey of Health and Development (also known as the 1946 British birth cohort) showed midlife physical performance to be related to a range of factors acting all the way from childhood, such as birth weight, childhood socioeconomic conditions, and adolescent cognitive function (2–4), through numerous factors in adulthood, such as physical activity, health status, weight, adult cognitive function, and socioeconomic conditions (5,6). Although other studies have found a link between smoking and physical performance, they have not investigated the life-long impact of smoking. In the Honolulu Heart study and the Alameda County Study, smoking status was found to be inversely related to healthy aging and physical performance in older adulthood (7,8). Among older adults, smoking status is also found to be an important risk factor for mobility problems (9), functional status (10,11), and disability (12). It is not known how smoking through life affects physical performance in midlife as most of the evidence is based on cross-sectional studies (13) or has been performed on older people (7–9,12).
We will build on previous work from the British 1946 birth cohort (14) and use smoking histories constructed from six interview waves from age 20 to 53 years to investigate if smoking and cigarette pack-years are related to different aspects of physical performance at age 53 years. This new work will allow smoking to be modeled more flexibly than in our earlier reports, and we are able to adjust for numerous confounders and mediators, such as education, social class, lung function, cognitive function, and health conditions. Three objective measures of physical performance (grip strength, balance, and chair rise) will be used in concert with a summary index combining the three (4).
METHODS
Study Population
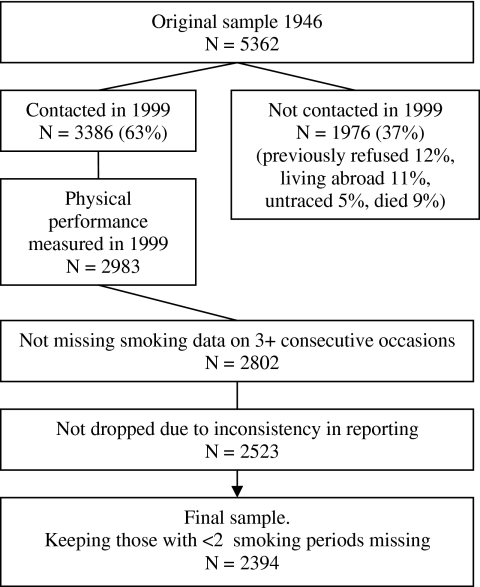
The Medical Research Council's National Survey of Health and Development is a prospective cohort of 2,547 women and 2,815 men, a socially stratified sample of all births that took place in England, Scotland, and Wales during a week in 1946 (15). In 1999 at age 53 years, 3,386 study members were contacted, and of these, 3,035 provided information. Of the remaining 1,976 (37%), no attempt for contact was made as these had previously refused (12%), were living abroad (11%), were untraced (5%), or had died (9%; Figure 1).
Figure 1.
Smoking data from the British 1946 birth cohort.
Physical Performance at Age 53 Years
During home visits at age 53 years, trained nurses measured study participants’ (N = 2,983, 98% of the interviewed) physical performance in three different tests, following a written protocol (5): standing balance time on one foot with eyes closed and folded arms across the chest for a maximum of 30 seconds, time used to perform 10 chair rises from sitting to standing position and then back to sitting position again, and handgrip strength in kilograms. Standing balance time in seconds was logged to get a more normal distribution. Reciprocal chair rise time ([1/chair rise time] × 100) was used so that highest score equaled better performance (as on the other tests), and grip strength was measured isometrically using an electronic handgrip dynamometer (16), and the measure was analyzed without transformation. Study members who were unable to perform the tests for health reasons were assigned values corresponding to the first percentile for balance time (1 second for 107 study members) and grip strength (11.2 kg for 64 study members) and a value corresponding to the 99th percentile for chair rises (30 seconds for 146 study members). In addition to using the three measures separately, they were combined to get an index of overall physical performance as used previously on this cohort (4). This was done by rescaling, separately for men and women, the outcomes to a 0–1 scale, where 0 indicates poor performance and 1 indicates good performance. Balance time was divided by highest possible time (30 seconds), and persons unable to perform the test were assigned score value 0. Chair rise time was rescaled using the equation 1 − (chair rise time/48 seconds), where 48 was the 99th percentile of time. People unable to do the test were assigned score 0. Grip strength adjusted for height was divided by the sex-specific 99th percentile value of adjusted grip strength (0.4346 kg/cm for men and 0.2838 kg/cm for women). Those with grip strength above the 99th percentile were assigned score 1 and those unable to perform the test were assigned score 0. These three rescaled scores were summed to create a normally distributed aggregate physical performance score ranging from 0 to 3, where a higher score indicates better overall physical performance. Finally, all four measures (overall, balance, chair rises, and grip strength) were standardized to have mean 0 and SD 1.
Cigarette Pack-Years
Cigarette pack-years are a measure of smoking intensity and duration. It was derived by multiplying the number of packs of cigarettes (assuming 20 cigarettes a pack) smoked per day (intensity) by the number of years the person has smoked (duration), and thus, one cigarette pack-year is equal to smoking one pack of cigarettes a day for 1 year (or half a pack a day for 2 years). Smoking histories were constructed from six interview waves. On each of the interview waves at age 20, 25, 31, 36, 43, and 53 years, the study members were asked about their smoking status (current, former, and never) and the number of cigarettes they smoked per day. They were also asked about age at smoking initiation (asked at 20 and 25 years only) and cessation (asked at ages 25, 31, 36, and 43 years). Cigarette pack-years were derived as the number of cigarettes smoked per day divided by 20, and this quantity was multiplied by the length in years of the period. This was done separately for the following six periods: 20 years and younger, 20–25 years, 25–31 years, 31–36 years, 36–43 years, and 43–53 years. For each period, the amount smoked at the start of the period was multiplied by half the length of the period and then summed with the amount smoked at the end of the period, which was also multiplied by half the length of the period. To construct overall pack-years up to age 53 years, some selection and imputations were made. First, the median age at smoking initiation (16 years) was imputed for current smokers at age 20 years with missing age at initiation (n = 20). Second, participants with missing smoking data on three or more consecutive occasions were dropped from analyses (n = 181). Furthermore, if the respondent was a current smoker at the beginning of the period and an ex-smoker at the end, then pack-years were calculated for half the length of the period, and the other half was set to zero. A similar procedure was applied if the respondent was a nonsmoker at the start and then became a smoker at the end of the period. Third, if smoking status for a period was known but not the amount smoked, the amount was imputed from adjacent time periods (n = 198). Participants who never smoked were assigned a pack-years value of zero. Finally, to calculate lifetime pack-years, the pack-years in the six age periods were summed up, leaving out those with more than one period missing, and for those with one period missing, the pack-years in this period were imputed based on the mean of the other periods. Analyses were also run using only those with nonmissing periods, and the results were similar. Of the 2,983 with physical performance assessments, the analyses included 2,394 (80%) participants with data on smoking history through age 53 years (Figure 1). We also ran analyses on a sample with nonmissing smoking information on all waves (N = 1,845).
Mediators and Confounders
Height in centimeters and weight in kilograms were measured using standard protocols at age 53 years (5). Education at age 26 years was categorized into four groups (advanced degree level [n = 212, 9%], advanced secondary qualifications [n = 588, 25%], ordinary secondary level [n = 553, 24%], and no formal qualifications [n = 956, 41%]). Household social class at age 53 years was categorized in four groups (professional and intermediate [n = 1,001, 42%], skilled nonmanual [n = 531, 22%], skilled manual [n = 429, 18%], and partly skilled and unskilled [n = 415, 17%]). If household social class at age 53 years was missing, household social class at age 43 (n = 41) or 36 years was used (n = 16). Lung function at age 53 years was denoted by forced expiratory volume in 1 second (FEV1) and measured using the Micromedical turbine electronic spirometer. Cognitive function at age 53 years was assessed by four different tests: Verbal memory was assessed with a 15-item word-learning task, search speed and concentration were assessed by a timed letter search, verbal fluency was assessed by counting the number of animals the study members were able to name in 1 minute, and general cognitive ability was assessed using the National Adult Reading Test (6). The presence of potentially disabling medical conditions was identified at 53 years, such as diabetes, cancer, epilepsy in the last 10 years, or cardiovascular disease (one or more of the following: heart attack or stroke, aortic stenosis or valvular disease in the last 10 years, physician-diagnosed angina or Rose angina Grade I or II elicited by standardized questions, or intermittent claudication). As only 13% reported any of these conditions at 53 years, it was included in the analysis as a simple indicator variable (yes/no) (5).
Those seen at 53 years with complete data on smoking behavior, mediators, and confounders (n = 2,116) did not differ for gender, current height and weight, education, adult socioeconomic position (SEP), lung function (FEV1), cognitive function (except verbal fluency), chair rise time, or balance performance from others seen at 53 years (n = 867), but those with complete data had better grip strength (p = .04), better overall physical performance (p = .02), and were less likely to have disabling medical conditions (p = .04). Most of those excluded due to incomplete data (N = 867) were excluded due to missing pack-years (N = 589), but the remainder (N = 278) did not differ regarding smoking behavior from those with complete data.
Statistical Methods
First, we assessed the effect of lifetime cigarette pack-years on overall performance, balance, chair rises, and grip strength at age 53 years. Second, we examined whether any of these effects are confounded by socioeconomic position and mediated by lung function, cognitive function, and age-associated medical conditions.
All analyses were weighted and adjusted for the initial sampling design using the svy and pweighs functions in Stata 10.0. Men and women were combined in all the analyses as there were no significant gender by pack-year interactions in predicting physical performance outcomes. Ordinary least squares regression was used to assess the relationship between cigarette pack-years and each of the four physical performance outcomes. Three different analyses were performed to assess the shape of the relationship between cigarette pack-years and physical performance. First, pack-years were categorized into eight groups (pack-years: 0, 0.15–4.99, 5.00–9.99, 10.00–14.99, 15.00–19.99, 20.00–29.99, 30.00–39.99, and 40.00+) and regressed with the physical performance outcomes, adjusting for gender, height and weight, and gender by height. Second, pack-years were treated as a continuous variable and modeled linearly. To check for deviation from linearity, a squared pack-years term was included, but this was nonsignificant for all outcomes and therefore not included in the presented analyses. The relationship of cigarette pack-years (on a continuous scale) with physical performance outcomes was investigated, including never-smokers in the analysis using an established method (17). This was done by centering pack-years to zero by subtracting the mean value among smokers and keeping the value 0 for never-smokers. Both the pack-years variable and a smoking status at age 53 years indicator variable (current/former smokers = 1 and never-smokers = 0) were included simultaneously in the regression model. The linear transformation of pack-years does not change the effect of pack-years on the outcomes, but the interpretation of the smoking status indicator variable becomes more meaningful as it compares the average smoker's physical performance with the never-smokers (If the pack-years variable is not centered before assigning never-smokers a 0 value, then the smoking status variable would compare smokers with 0 pack-years with never-smokers) (17). Third, the relationship between pack-years and physical performance was modeled using Restricted Cubic Splines with three knots. This is more flexible because it is not restricted to be linear. All analyses were performed using STATA software, version 10.0.
RESULTS
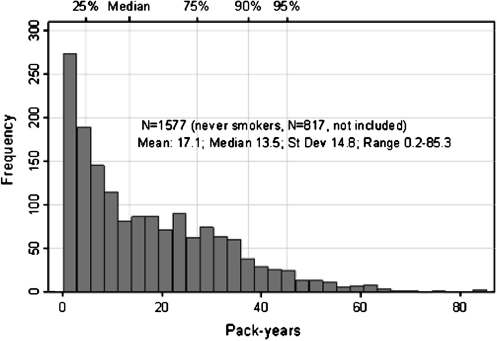
The characteristics of the study sample are shown in Table 1. Men had on average higher number of cigarette pack-years (14.4 vs 10.0), and they had a lower percentage of never-smokers (29% vs 39%) compared with women (Table 1). Among the 1,577 ever-smokers, the gender-combined median pack-years were 13.5, and pack-years range was 0.2–85.3 (Figure 2).
Table 1.
Participant Characteristics of Study Sample, N = 2,394
| Total |
Men |
Women |
||||
| M (SD) | N | M (SD) | N | M (SD) | N | |
| Never-smokers | 34% | 817 | 29% | 344 | 39% | 473 |
| Ever-smokers | 66% | 1577 | 71% | 842 | 61% | 735 |
| Cigarette pack-years | 12.2 (14.9) | 2,394 | 14.4 (16.2) | 1,186 | 10.0 (13.0) | 1,208 |
| Overall physical performance | 1.34 (0.39) | 2,216 | 1.40 (0.40) | 1,095 | 1.28 (0.37) | 1,121 |
| Balance time, s | 6.4 (6.2) | 2,326 | 7.3 (6.9) | 1,151 | 5.5 (5.2) | 1,175 |
| Chair rise time | 23.9 (17.9) | 2,335 | 22.8 (15.7) | 1,156 | 25.0 (19.8) | 1,179 |
| Grip strength (kg) | 36.9 (14.5) | 2,328 | 46.8 (12.9) | 1,156 | 27.1 (7.9) | 1,172 |
| Lung function FEV1 | 2.71 (0.77) | 2,329 | 3.16 (0.70) | 1,151 | 2.27 (0.53) | 1,178 |
| Height (cm) 53 years | 167.5 (8.9) | 2,366 | 174.0 (6.3) | 1,171 | 161.0 (5.7) | 1,195 |
| Weight (kg) 53 years | 77.6 (15.2) | 2,362 | 83.7 (13.5) | 1,170 | 71.7 (14.3) | 1,192 |
| Cognitive test scores at 53 years | ||||||
| Search speed | 276 (75) | 2,351 | 268 (73) | 1,168 | 284 (77) | 1,183 |
| Verbal memory | 23.2 (6.3) | 2,319 | 22.4 (6.1) | 1,149 | 24.0 (6.3) | 1,170 |
| Verbal fluency | 23.0 (6.7) | 2,362 | 23.4 (6.5) | 1,169 | 22.6 (6.9) | 1,193 |
| Reading test (National Adult Reading Test) | 32.8 (9.7) | 2,266 | 33.3 (9.7) | 1,117 | 32.3 (9.7) | 1,149 |
| Presence of potentially disabling health condition at 53 years (eg, diabetes, cancer, epilepsy, or cardiovascular disease) | 13% | 291 | 12% | 138 | 13% | 153 |
Figure 2.
Cigarette pack-years among smokers (upper x-axis shows percentiles) and frequency.
Light smokers (less than five pack-years) did not differ in physical performance (all three components and the aggregate measures) from never-smokers (Table 2). There was an indication of a threshold effect; up to 15 pack-years, the overall performance, balance, and chair rises did not differ significantly from the never-smokers, but above 15 pack-years, physical performance decreased significantly with increased pack-years for all three outcomes. This threshold effect was not evident for grip strength, and there was no overall association between pack-years and grip strength. The effect of pack-years with physical performance did not differ between the genders; none of the interactions of pack-years by gender were significant (results not shown).
Table 2.
Mean Difference (95% confidence interval) in Physical Performance (standard deviations unit) at Age 53 Years by Lifetime Cigarette Pack-Years, Adjusting for Gender, Height, and Weight, and Gender by Height
| Pack-Years | Physical Performance Measure |
|||
| Overall Performance (N = 2,203) | Balance (N = 2,301) | Chair Rises (N = 2,311) | Grip Strength (N = 2,318) | |
| 0 | Ref | Ref | Ref | Ref |
| 0.2–4.9 | 0.10 (−0.03–0.22) | 0.06 (−0.07–0.19) | 0.03 (−0.12–0.17) | 0.10 (−0.00–0.20) |
| 5.0–9.9 | −0.05 (−0.20–0.10) | 0.00 (−0.16–0.16) | −0.15 (−0.31–0.01) | 0.06 (−0.05–0.18) |
| 10.0–14.9 | 0.01 (−0.17–0.19) | −0.02 (−0.21–0.17) | 0.03 (−0.18–0.24) | 0.07 (−0.07–0.21) |
| 15.0–19.9 | −0.18 (−0.35,−0.01) | −0.28 (−0.47,−0.09) | −0.33 (−0.51,−0.14) | 0.12 (−0.01–0.24) |
| 20.0–29.9 | −0.29 (−0.46,−0.12) | −0.22 (−0.38,−0.07) | −0.30 (−0.45,−0.14) | −0.01 (−0.13–0.11) |
| 30.0–39.9 | −0.31 (−0.51,−0.12) | −0.22 (−0.41,−0.04) | −0.42 (−0.60,−0.24) | 0.02 (−0.12–0.15) |
| 40.0+ | −0.45 (−0.69,−0.22) | −0.42 (−0.64,−0.21) | −0.53 (−0.77,−0.30) | 0.02 (−0.16–0.19) |
| Overall p value | <.001 | <.001 | <.001 | .416 |
Note: Overall p value calculated using the Wald test. Analyses are weighted for study design.
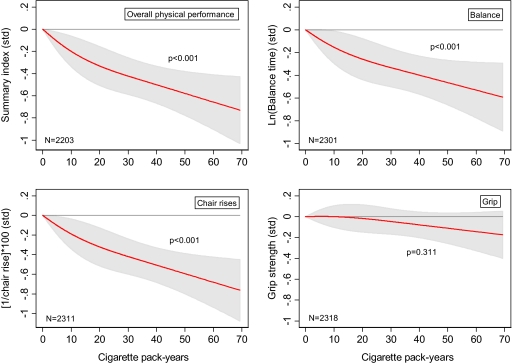
Compared with never-smokers, ever-smokers (with mean pack-year) had poorer overall physical performance (p = .009), balance (p = .03), and chair rises (p < .001) but not poorer grip strength (Table 3, Model 1). Pack-years were inversely related to physical performance; for every 10 pack-years smoked, the physical performance index decreased by 0.10 SDs (p < .001), standing balance decreased by 0.09 SD (p < .001; 1 SD = 6.3 seconds), and chair rises decreased by 0.11 SD (p < .001; 1 SD = 17.4 seconds). The more flexible Spline analysis also indicated a linear decrease in physical performance with increased pack-years for balance, chair rises, and overall performance but not for grip strength, where no relationship was observed (Figure 3).
Table 3.
Mean Difference in Four Measures of Physical Performance (standard deviations unit) at Age 53 Years by Lifetime Cigarette Pack-Years and Lifetime Smoking Status
| Smoking Status and Pack-Years | Overall Physical Performance N = 1,967 | p Value | Standing Balance N = 2,049 | p Value | Chair Rises N = 2,059 | p Value | Grip Strength N = 2,056 | p Value |
| Model 1 | ||||||||
| 10 pack-years* | −0.10 (−0.13–−0.06) | <.001 | −0.09 (−0.13–−0.05) | <.001 | −0.11 (−0.15–−0.07) | <.001 | −0.01 (−0.04–0.02) | .557 |
| Ever-smoker | −0.13 (−0.23–−0.03) | .009 | −0.11 (−0.21–−0.01) | .030 | −0.19 (−0.30–−0.08) | <.001 | 0.05 (−0.03–0.13) | .197 |
| Model 2 | ||||||||
| 10 pack-years* | −0.07 (−0.11–−0.03) | <.001 | −0.06 (−0.10–−0.02) | .003 | −0.09 (−0.14–−0.05) | <.001 | −0.01 (−0.04–0.02) | .731 |
| Ever-smoker | −0.07 (−0.17–0.03) | .169 | −0.04 (−0.15–0.05) | .386 | −0.15 (−0.26–−0.04) | .005 | 0.06 (−0.02–0.13) | .137 |
| Model 3 | ||||||||
| 10 pack-years* | −0.06 (−0.10–−0.03) | .001 | −0.07 (−0.11–−0.03) | <.001 | −0.09 (−0.13–−0.04) | <.001 | 0.01 (−0.02–0.04) | .548 |
| Ever-smoker | −0.09 (−0.19–0.01) | .079 | −0.09 (−0.19–0.01) | .082 | −0.16 (−0.27–−0.05) | .004 | 0.08 (0.00–0.15) | .047 |
| Model 4 | ||||||||
| 10 pack-years* | −0.09 (−0.13–−0.05) | <.001 | −0.08 (−0.12–−0.04) | <.001 | −0.10 (−0.15–−0.06) | <.001 | −0.01 (−0.04–0.02) | .589 |
| Ever-smoker | −0.12 (−0.22–−0.02) | .015 | −0.11 (−0.21–−0.01) | .037 | −0.18 (−0.29–−0.07) | .001 | 0.05 (−0.02–0.13) | .181 |
| Model 5 | ||||||||
| 10 pack-years* | −0.07 (−0.11,−0.04) | <.001 | −0.06 (−0.10,−0.02) | .002 | −0.09 (−0.13,−0.04) | <.001 | −0.01 (−0.04–0.02) | .500 |
| Ever-smoker | −0.10 (−0.20,−0.01) | .039 | −0.08 (−0.18–0.03) | .147 | −0.16 (−0.27,−0.06) | .003 | 0.05 (−0.03–0.12) | .222 |
| Model 6 | ||||||||
| 10 pack-years* | −0.04 (−0.08–0.00) | .061 | −0.04 (−0.08–−0.00) | .034 | −0.06 (−0.11–−0.02) | .005 | 0.01 (−0.02–0.04) | .563 |
| Ever-smoker | −0.03 (−0.13–0.06) | .475 | −0.03 (−0.13–0.07) | .590 | −0.14 (−0.24–−0.03) | .020 | 0.08 (0.01–0.16) | .036 |
Notes: All models included ever- and never-smokers and were adjusted for gender, height, and weight and then in Models 2–5 additionally for education, adult social class, lung function (FEV1), cognitive function, and health condition. All models are weighted for study design. Model 1 adjusted for gender, height, and weight at 53 years (in none of the models were gender by pack-years significant; thus, genders were collapsed). Model 2, adjusted additionally for education and adult SEP. Model 3, adjusted for the factors in Model 1 and additionally for lung function (FEV1). Model 4, adjusted for the factors in Model 1 and additionally for disabling health conditions. Model 5, adjusted for the factors in Model 1 and additionally for cognitive function at age 53 years (reading test, search speed, verbal memory, and fluency). Model 6, fully adjusted for all variables in Models 1–5.
Pack-years were centered. For never-smokers, centered pack-years = 0; for ever-smokers, centered pack-years = pack-years minus mean pack-years. Pack-years squared did not improve the fit of the model and were not included.
Figure 3.
Mean difference in four measures of physical performance (standard deviations unit) at age 53 years by lifetime cigarette pack-years among smokers. Adjusted for gender, height, and weight at 53 years (modeled using restricted cubic Splines with three knots). p Values are calculated using the Wald test. Shaded area is 95% confidence interval.
Table 3 shows the results of smoking history with physical performance adjusting for potential confounders and mediators. Education and social class did not attenuate the relationship between pack-years and overall physical performance to a notable extent, and the relationship was still significant after adjustment (p < .001; Model 2). The same applied when lung function, cognitive function, and medical conditions were adjusted for successively one by one (Models 3, 4, and 5). However, when all the factors were included in Model 6, less than half of the initial relationship between pack-years and overall physical performance remained, and the relationship was no longer significant using conventional levels (p = .06). The relationship of pack-years with standing balance and chair rises was also attenuated in the fully adjusted Model 6, but the effect of pack-years was still significant for both measures (p = .03 for balance and p = .005 for chair rises). Pack-years were not related to grip strength neither before nor after adjustment.
When adjusted in turn by education, social class, and lung function, the relationship of poorer overall physical performance among ever-smokers (with average pack-years) compared with never-smokers was modified and no longer significant, whereas adjustment for cognitive function and medical conditions did not modify the relationship (Table 3). For balance, adjustment by education, social class, and cognitive function attenuated the relationship with smoking status, whereas the other factors did not modify this relationship much. For chair rises, none of the factors modified the poorer performance among ever-smokers compared with never-smokers and the relationship remained significant after inclusion of all mediators and confounders (p = .02; Model 6). Ever-smokers had a nonsignificant tendency for better grip strength compared with never-smokers, which was amplified and significant when adjusted for lung function (p = .05; Models 3 and 6).
DISCUSSION
Findings from this postwar British birth cohort showed an inverse relationship between pack-years smoked and overall physical performance, standing balance, and chair rises. Education, social class, lung function, cognitive function, and medical conditions attenuated these relationships, but pack-years were still significantly related to chair rises and to a lesser extent standing balance at age 53 years. Pack-years were not related to grip strength neither before nor after adjustment for mediators and confounders.
Numerous reports show a relationship between smoking status and measures of physical performance among older adults (7–9,11–13), but prospective studies investigating the relationship of lifetime smoking with physical performance in midlife are scarce. A Norwegian study reported poorer physical fitness (assessed on a bicycle ergometer test) among 40- to 59-year-old smokers during 7 years of follow-up compared with nonsmokers (18), and in the Alameda County study, California, USA, nonsmokers aged 65–89 years in 1984 had higher overall physical performance (assessed by a combined self-reported physical performance and exercise scale) than smokers (8).
In the Women's Health and Aging Study, lung function measured by FEV1 was associated with objective physical function in older women (19). Furthermore, it was previously reported from the 1946 cohort that life-long smokers had a more rapid decline in FEV1 than never-smokers (14). Although FEV1 was associated with standing balance and chair rising performance, FEV1 mediated only a small amount of the effect of pack-years on balance and chair rises. This indicates that the effect of smoking on physical performance does not primarily work through lung function.
In line with our results, an effect of cigarette pack-years on chair rises and a lack of such effect on grip strength was reported in a cross-sectional study of American women aged 65 years and older (13). Grip strength has differed from chair rises and balance also in other aspects in previous reports from the 1946 birth cohort; for example, grip strength was equal across socioeconomic groups in childhood as opposed to balance and chair rises where sharp childhood socioeconomic differences were observed (5). The mechanisms causing pack-years to affect both balance and chair rises but not grip strength are not straightforward and as far as we are aware of not previously investigated. One possible explanation for the missing link between pack-years and grip strength could be due to confounding by social class (stronger grip strength and more smoking among manual workers), but the effect of pack-years was not strongly attenuated by adjustment for social class. Furthermore, earlier reports have shown better grip strength with higher adult social class (2). Another explanation for the lack of effect of smoking on grip strength is that chair rising and standing balance are more complex measures involving several body systems, whereas grip is a more simple measure of strength (5,6).
Balance and chair rises were strongly related to cigarette pack-years, and the data suggested a threshold effect at low levels of consumption (up to 15 pack-years) and a dose–response relationship thereafter, with poorer performance with higher number of pack-years. The explanation for this relationship is probably partly due to decreased lung function, poorer overall health and cognitive function, lower education, and social class among the heavy smokers. However, some effect of pack-years remained also after adjustment for these factors, and there are likely additional pathways linking smoking with balance and chair rises.
In a recent report from the 1946 cohort, cognitive function in adulthood was strongly related to standing balance and chair rises but not to grip strength (6). Furthermore, previous results from our cohort (20) and numerous other prospective studies indicate that heavy smoking is related to cognitive function and cognitive decline (21–23), so therefore cognitive function might be on the pathway linking cigarette pack-years with balance and chair rises. However, our results do not indicate cognitive function at 53 years to be a main factor for this link.
There is some evidence for smoking to be related to poor peripheral nerve system (PNS) (24,25), and it might be that the oxidative stress of smoking adversely affects the PNS, which can reduce balance and chair rise performance. Indeed, a large epidemiological study of male veterans ages 31–46 years showed that smoking was significantly associated with decreased nerve conduction velocity and amplitude of the peroneal motor nerve (24,25). Furthermore, there is evidence that skeletal muscle is more fatigable in smokers than in nonsmokers, possibly due to reduced oxygen delivery to muscle (26,27).
An advantage of this study is that smoking status was prospectively collected throughout adulthood, unlike the retrospective recall made in most studies in aging, and we were able to relate smoking through life with objectively assessed tests of physical performance in middle age and to adjust for a range of objectively measured confounders and mediators also collected prospectively through life.
A limitation is the attrition that is part of all cohort studies followed up for many years. Although there has been some dropout, comparisons with census data of the sample at 36 years show that the 1946 cohort is still representative for the national population of a similar age in terms of childhood social class, whereas at age 53 years, men with higher education were underrepresented (28). The complete study sample generally did not differ from those excluded from analyses due to missing values on mediators and confounders, with one exception with possible impact on our results. Those in the complete sample had better grip strength, better overall physical performance, and were less likely to have a disabling health condition than those excluded. It is difficult to assess the effect these differences might have, but they may lead to a slight underestimation of the true effect of smoking on physical performance.
Study members not able to perform the tests because of a health problem were included in the analyses and assigned values as described in the methods. Excluding these respondents can lead to bias (29). Analyses performed without these study members gave similar conclusions, but the relation for the outcomes with pack-years was a little weaker, especially for chair rises. A likely explanation for this is that those not able to perform the chair rise test smoked more (on average 10.1 pack-years more than those who were able to perform the test), and the odds of being unable to perform the test increased with pack-years.
Some imputations were made in the construction of lifetime cigarette pack-years, which might introduce bias. As a check of validity, all analyses were run on the subsample with complete smoking histories (N = 1,845), and the results were similar.
This is one of the first studies to show that lifetime cigarette pack-years are related to physical performance in the fifth decade of life, suggesting that smokers will enter older adulthood with decreased physiological reserve. As smoking prevalence remains high in many developed countries and is rapidly growing in developing countries, these findings underscore the need for effective smoking cessation and prevention programs. Further work is required to understand the pathways linking smoking intensity with physical performance.
FUNDING
The manuscript is funded by Medical Research Council Unit for Lifelong Health and Ageing in UK and supported in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health in USA, and the Research Council of Norway.
Acknowledgments
We acknowledge the valuable comments made by Dr. Bram Van den Borst on an earlier version of this paper.
References
- 1.Harris T, Kovar MG, Suzman R, Kleinman JC, Feldman JJ. Longitudinal study of physical ability in the oldest-old. Am J Public Health. 1989;79:698–702. doi: 10.2105/ajph.79.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife physical performance: evidence from a British birth cohort. Am J Epidemiol. 2006;164:110–121. doi: 10.1093/aje/kwj193. [DOI] [PubMed] [Google Scholar]
- 3.Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife grip strength: findings from a birth cohort study. J Gerontol A Biol Sci Med Sci. 2006;61:702–706. doi: 10.1093/gerona/61.7.702. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Butterworth S, Wadsworth ME, Kuh D. Childhood socioeconomic status predicts physical functioning a half century later. J Gerontol A Biol Sci Med Sci. 2006;61:694–701. doi: 10.1093/gerona/61.7.694. [DOI] [PubMed] [Google Scholar]
- 5.Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60:224–231. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 6.Kuh D, Cooper R, Hardy R, Guralnik J, Richards M. Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosom Med. 2009;71:38–48. doi: 10.1097/PSY.0b013e31818a1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benfante R, Reed D, Brody J. Biological and social predictors of health in an aging cohort. J Chronic Dis. 1985;38:385–395. doi: 10.1016/0021-9681(85)90134-1. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989;79:703–708. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 10.Rapuri PB, Gallagher JC, Smith LM. Smoking is a risk factor for decreased physical performance in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:93–100. doi: 10.1093/gerona/62.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Pinsky JL, Leaverton PE, Stokes J., III Predictors of good function: the Framingham Study. J Chronic Dis. 1987;40(suppl 1):159S–167S. doi: 10.1016/s0021-9681(87)80045-0. 181S–182S. [DOI] [PubMed] [Google Scholar]
- 12.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338:1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 13.Nelson HD, Nevitt MC, Scott JC, Stone KL, Cummings SR. Smoking, alcohol, and neuromuscular and physical function of older women. Study of osteoporotic fractures research group. JAMA. 1994;272:1825–1831. doi: 10.1001/jama.1994.03520230035035. [DOI] [PubMed] [Google Scholar]
- 14.Clennell S, Kuh D, Guralnik JM, Patel KV, Mishra GD. Characterisation of smoking behaviour across the life course and its impact on decline in lung function and all-cause mortality: evidence from a British birth cohort. J Epidemiol Community Health. 2008;62:1051–1056. doi: 10.1136/jech.2007.068312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 national birth cohort (MRC National Survey of Health and Development) Int J Epidemiol. 2006;35:49–54. doi: 10.1093/ije/dyi201. [DOI] [PubMed] [Google Scholar]
- 16.Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond) 1993;84:331–337. doi: 10.1042/cs0840331. [DOI] [PubMed] [Google Scholar]
- 17.Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–823. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 18.Sandvik L, Erikssen G, Thaulow E. Long term effects of smoking on physical fitness and lung function: a longitudinal study of 1393 middle aged Norwegian men for seven years. BMJ. 1995;311:715–718. doi: 10.1136/bmj.311.7007.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson CF, Punjabi NM, Wolfenden L, Shardell M, Shade DM, Fried LP. Relationship between lung function and physical performance in disabled older women. J Gerontol A Biol Sci Med Sci. 2005;60:350–354. doi: 10.1093/gerona/60.3.350. [DOI] [PubMed] [Google Scholar]
- 20.Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 22.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008;168:1165–1173. doi: 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letz R, Gerr F. Covariates of human peripheral nerve function: I. Nerve conduction velocity and amplitude. Neurotoxicol Teratol. 1994;16:95–104. doi: 10.1016/0892-0362(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 25.Gerr F, Letz R. Covariates of human peripheral nerve function: II. Vibrotactile and thermal thresholds. Neurotoxicol Teratol. 1994;16:105–112. doi: 10.1016/0892-0362(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Morse CI, Wust RC, Jones DA, de Haan A, Degens H. Muscle fatigue resistance during stimulated contractions is reduced in young male smokers. Acta Physiol (Oxf) 2007;191:123–129. doi: 10.1111/j.1748-1716.2007.01721.x. [DOI] [PubMed] [Google Scholar]
- 27.Wust RC, Morse CI, de Haan A, Rittweger J, Jones DA, Degens H. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol. 2008;104:103–110. doi: 10.1007/s00421-008-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadsworth ME, Butterworth SL, Hardy RJ, et al. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med. 2003;57:2193–2205. doi: 10.1016/s0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]