Abstract
Background.
Hospitalization represents a stressful and potentially hazardous event for older persons. We evaluated the value of the Short Physical Performance Battery (SPPB) in predicting rates of functional decline, rehospitalization, and death in older acutely ill patients in the year after discharge from the hospital.
Methods.
Prospective cohort study of 87 patients aged 65 years and older who were able to walk and with a Mini-Mental State Examination score ≥18 and admitted to the hospital with a clinical diagnosis of congestive heart failure, pneumonia, chronic obstructive pulmonary disease, or minor stroke. Patients were evaluated with the SPPB at hospital admission, were reevaluated the day of hospital discharge, and 1 month later. Subsequently, they were followed every 3 months by telephone interviews to ascertain functional decline, new hospitalizations, and vital status.
Results.
After adjustment for potential confounders, including self-report activity of daily living and comorbidity, the SPPB score at discharge was inversely correlated with the rate of decline in activity of daily living performance over the follow-up (p < .05). In a multivariable discrete-time survival analysis, patients with poor SPPB scores at hospital discharge (0–4) had a greater risk of rehospitalization or death (odds ratio: 5.38, 95% confidence interval: 1.82–15.9) compared with those with better SPPB scores (8–12). Patients with early decline in SPPB score after discharge also had steeper increase in activity of daily living difficulty and higher risk of rehospitalization or death over the next year.
Conclusions.
In older acutely ill patients who have been hospitalized, the SPPB provides important prognostic information. Lower extremity performance-based functional assessment might identify older patients at high risk of poor outcomes after hospital discharge.
Keywords: SPPB, Hospitalization, Disability, Mortality, Prognosis
HOSPITALIZATION for an acute medical event represents a stressful and potentially hazardous event for older persons. After discharge from the hospital, older patients often experience negative short- and long-term consequences, including functional decline, disability, rehospitalization, nursing home admission, and mortality (1–4). For example, the Hospital Outcomes Projects for the Elderly estimated that in a sample of acutely ill medical patients, as many as 19% had activity of daily living (ADL) decline and 40% had instrumental activity of daily living (IADL) decline in the 3 months after hospital discharge compared with their disability status prior to the index hospitalization (5).
Identification of patients at high risk of functional decline or other adverse events after hospital discharge is of paramount importance for posthospital discharge assessment and management and for the prevention of these common negative outcomes. Very old frail patients and those with preadmission functional limitation are at higher risk of posthospital complications; nevertheless, the ability of medical diagnoses and traditional clinical assessment to discriminate high- and low-risk groups is limited (6). Objective measures of physical performance may prove an additional and useful clinical tool for risk stratification.
Despite a large body of evidence demonstrating the predictive value of different mobility performance tests in terms of various adverse outcomes (7–11), the use of physical performance measures in the acute care clinical setting has received little attention so far (12). The Short Physical Performance Battery (SPPB), a set of objective measures of lower extremity physical performance, was highly predictive of subsequent disability, hospitalization, institutionalization, and mortality in community-dwelling elders in epidemiological studies and outpatient clinics (13,14). Recently, we have demonstrated that the SPPB can be feasibly and safely used to evaluate the functional status of acutely ill geriatric patients admitted to the hospital for serious medical conditions and that the SPPB score can provide important short-term prognostic information (15). What has not been evaluated is whether this simple battery of performance tests can capture change in function in the immediate period posthospitalization and predict long-term outcomes after hospital discharge.
The aim of the present project was to determine whether SPPB score assessed at hospital discharge and sequential SPPB assessment in the first month after discharge are predictive of subsequent functional decline and risk of rehospitalization and mortality over a 12-month follow-up. We hypothesized that, even after adjusting for the presence of multiple medical conditions, patients with poorer SPPB score at discharge and those with immediate decline in SPPB score after discharge would be at greater risk of ADL decline, rehospitalization, and death.
METHODS
Study Design and Data Collection
Between October 1, 2004 and December 31, 2006, patients, admitted to the academic center of internal medicine and geriatrics (University of Ferrara, Italy), were screened for eligibility for a 1-year longitudinal observational study, as previously described (15). Briefly, inclusion criteria for the study were as follows: (a) age 65 years and older; (b) ability to stand and walk for a few meters at the time of study enrollment; (c) a clinical diagnosis of one of the following conditions: congestive heart failure, chronic obstructive pulmonary disease, pneumonia, and minor stroke. Patients were considered ineligible for the study if they had severe cognitive impairment (Mini-Mental State Examination score <18), acute coronary syndrome, if they were living more than 25 km (15.5 miles) from the medical center, or if they refused to participate in the study. The local institutional review board reviewed and approved the study protocol. Of those eligible, 92 (74%) agreed to participate, signed the informed consent, and were enrolled in the study. Two patients died before hospital discharge, and three were lost immediately after discharge; these patients where therefore excluded from the present analysis. Participants were evaluated by three trained research physicians by means of a comprehensive geriatric assessment at hospital admission, were reevaluated the day of hospital discharge, then 1 month after hospital discharge by in-home visits, and subsequently, every 3 months by telephone interviews. The performance-based measures of physical function were assessed at hospital admission, within 24 hours before hospital discharge, and during the home visit 1 month after hospital discharge. Of the 87 patients included in the analysis, 11 had died and only 1 was lost during the follow-up. Three patients died before the 3-month follow-up visit, three patients died before the 6-month follow-up, two patients died before the 9-month follow-up, and three patients died before the end of the 1-year follow-up. One patient was lost to follow-up at the 9-month follow-up.
Measures
Performance-based measures of physical function.—
The trained research physicians administrated all performance-based measures. The SPPB includes usual walking speed over 4 m, five chair–stands test, and balance test. A score (scale: 0–4) was assigned to performance on time to rise five times from a seated position, standing balance, and 4-m walking velocity. Individuals received a score of 0 for each task they were unable to complete. Participants coded in the “unable to perform” category included (a) those who tried but were unable and (b) the interviewer or participant felt it was unsafe. Scores of 1–4 for each task were assigned based on quartiles of performance for more than 5,000 participants in the Established Populations for the Epidemiologic Study of the Elderly (8,13). Summing the three individual categorical scores, a summary performance score was created for each participant (range: 0–12), with higher scores indicating better lower body function. In the walking test, the patients could use a cane, a walker, or other walking aid but the aid of another person. The SPPB has been shown to be reliable, valid, and sensitive to change. Intraclass correlation coefficients ranged from .88 to .92 for measures made 1-week apart, with a 6-month average correlation coefficient of .78 (16).
Self-report measures of physical function.—
Information on six IADLs was obtained using a modified version of the Lawton and Brody scale (17). The six activities included were as follows: using the telephone, traveling via car or public transportation, shopping, housecleaning, handling money, and taking medications. Participants were asked if they had any difficulty performing each task without help from another person or special equipment. If they said they did have difficulty, they were then asked how much difficulty, with response options of “some difficulty, a lot of difficulty, or unable to do without help.” At hospital admission, the patients were asked if they had any difficulty performing each task during the preceding 2 weeks. The same information was collected at home visits and during the four telephone interviews.
Disability in basic ADL was measured according to the participants’ self-reported difficulty in performing each of six activities: getting in and out of a bed, bathing, dressing, eating, personal hygiene, and using the toilet (18). For ADL activities, the format was the same used for IADLs. Based on the results of previous work (6,19), showing a high rate of decline in ADL function in the weeks preceding hospital admission, information on ADL was queried for both hospital admission and the 2 weeks prior to admission. Prevalent IADL and ADL disabilities were defined as a lot of difficulty or inability to do at least one IADL or ADL 2 weeks before hospital admission, respectively
Outcomes.—
The three outcome domains for the study were decline in functional status, hospitalization, and mortality over the 12-month follow-up. The measures of decline in functional status were ADL disability and the ADL summary scale (20). Incident ADL disability was defined as a lot of difficulty or inability to do at least one ADL over the follow-up. For the ADL summary score, each item has a range of 0–3, where 0 indicates able to do without difficulty, 1 indicates able to do with some difficulty, 2 indicates able to do with a lot of difficulties, and 3 indicates unable to do without help. The ADL summary score has a range of 0–18, with higher score representing increased difficulty. Cronbach’s alpha for the scale at hospital admission was high (.95). Previous work demonstrated that the ADL summary scale is valid, reliable, and sensitive to change (20). Information on new hospitalization and vital status was collected from the home visits and phone interviews.
Other covariates.—
Sociodemographic information, including gender, marital status, living arrangement, educational level, and smoking habits, was collected at baseline by standardized interview. Cognitive functioning was assessed using the Mini-Mental State Examination (21). Depressive symptoms were measured using the Center for Epidemiological Studies-Depression scale (22) (range from 0 to 60, with higher scores indicating more depressive symptomatology). Comorbidity was assessed by using the Cumulative Illness Rating Scale (23), a validated (24) physician-rated index derived by means of patient history as well as physical examination and laboratory findings. The Cumulative Illness Rating Scale is divided into 14 categories or disorders. This index measures the chronic medical illness burden while taking into consideration the severity of chronic diseases. Each system is rated as follows: (a) 0, none—no impairment to that organ or system; (b) 1, mild impairment—normal activity; (c) 2, moderate impairment—interferes with normal activity; (d) 3, severe impairment—disabling; and (e) 4, extremely severe impairment—life threatening. The final score of the Cumulative Illness Rating Scale is the sum of each of the 14 individual system scores, with higher values indicating greater disease burden severity.
Analysis
The statistical analyses were designed to assess the relationship between SPPB and three major outcomes: (a) incident ADL disability, (b) change in ADL summary scale over the 12-month follow-up, and (c) incidence of a composite outcome in which new hospitalizations and death were combined. The predictive value of SPPB score was investigated using two analytical approaches. First, we analyzed the relationship between SPPB score measured at hospital discharge with study outcomes, and second, we estimated the predictive values of early change in SPPB score after hospital discharge. This specific analysis included 77 patients with SPPB assessment at both hospital discharge and at 1 month after discharge (2 patients died in the first month after discharge and 8 patients had missing SPPB data). Patients were classified in one of three mutually exclusive categories according to their SPPB trajectory during the first month after hospital discharge. Patients who declined one or more points in the SPPB score (25) at 1 month after discharge were classified as “declining”; patients who improved one or more points were classified as “improving” and those with identical SPPB score at hospital discharge and 1 month later were classified as “stable.” Preliminary descriptive analyses of the baseline data according to SPPB score assessed at hospital discharge were used to characterize the sample. We evaluated change over time in ADL summary scale score as a function of SPPB score using a random effect model. Separate models were fitted for SPPB score assessed at hospital discharge and for SPPB change at 1 month after hospital discharge. Random effect models were adjusted for demographic characteristics. Discrete-time survival analysis with logistic regression was used to estimate the association between SPPB score and the likelihood of incident disability and new hospitalization or mortality. Each participant potentially contributed an observation for each 3-month follow-up interval (for a maximum of four). In fact, each patient contributed data up to the round at which he/she first reported a new hospitalization, died, or was lost to follow-up and not evaluated thereafter (censored). Separate models were fitted for SPPB score assessed at hospital discharge and for SPPB score change at 1 month after hospital discharge. Logistic models were adjusted for demographic characteristics (Model 1) and additional potential confounders, including cognitive status, comorbidity level, IADL disability 2 weeks before hospital admission, ADL summary scale score 2 weeks before hospital admission, and ADL summary scale score at hospital discharge (Model 2). All analyses were performed using STATA (26).
RESULTS
The study sample consisted of 87 patients; the mean age was 77.4 years (range: 65–93), and 49% were women. Patients with better SPPB score (8–12) were younger, more likely to be men, had better cognitive function, lower burden of comorbidity (total Cumulative Illness Rating Scale score), and better IADL and ADL status before hospital admission and at hospital admission (Table 1). During the first month after hospital discharge, 18.2% of the patients experienced decline in SPPB score, 50.1% improved their SPPB performance, and the other 31.1% remained stable (Table 2). Patients with improving SPPB score tended to have greater level of comorbidity and disability and poorer lower extremity performance at hospital admission and at hospital discharge.
Table 1.
Selected General and Clinical Characteristics of the Sample by SPPB Score at Hospital Discharge
| SPPB | p* | |||
| Low (0–4) | Intermediate (5–7) | High (8–12) | ||
| N = 16 | N = 27 | N = 44 | ||
| Age (y, M ± SD) | 81.7 (5.7) | 78.1 (7.9) | 75.4 (4.8) | .001 |
| Women, n (%) | 11 (68.8) | 18 (66.7) | 14 (31.8) | .003 |
| Education (>5 y), n (%) | 1 (6.3) | 4 (14.8) | 10 (22.7) | .051 |
| Smoking, n (%) | ||||
| Former | 6 (37.5) | 13 (48.2) | 31 (70.5) | .543 |
| Current | 1 (6.3) | 4 (14.8) | 4 (9.1) | .608 |
| Body mass index (kg/m2, M ± SD) | 27.0 (8.0) | 28.2 (7.3) | 27.5 (4.6) | .815 |
| MMSE score (M ± SD) | 23.4 (2.7) | 25.8 (2.0) | 26.5 (2.2) | .001 |
| Admission diagnosis, n (%) | ||||
| Congestive heart failure | 13 (81.2) | 20 (74.1) | 24 (54.6) | |
| COPD | 3 (18.8) | 3 (11.1) | 9 (20.5) | |
| Pneumonia | 0 (0) | 3(11.1) | 7 (15.9) | |
| Minor stroke | 0 (0) | 1 (3.7) | 4 (9.1) | .307 |
| CES-D (M ± SD) | 17.3 (8.1) | 17.6 (9.2) | 15.6 (7.6) | .965 |
| Total Cumulative Illness Rating Scale score (M ± SD) | 9.9 (2.4) | 9.3 (3.7) | 8.2 (2.1) | .008 |
| IADL disability 2 weeks before admission, n (%) | 13 (81.3) | 13 (48.2) | 8 (18.2) | .003 |
| ADL disability 2 weeks before admission, n (%) | 5 (31.3) | 3 (11.1) | 1 (2.3) | .044 |
| ADL disability at admission, n (%) | 10 (62.5) | 12 (44.4) | 8 (18.2) | .019 |
Notes: COPD = chronic obstructive pulmonary disease; ADL = activity of daily living; CES-D = Center for Epidemiological Studies-Depression scale; IADL = instrumental activity of daily living; MMSE = Mini-Mental State Examination; SPPB = Short Physical Performance Battery.
p Values from age- and gender-adjusted analysis of covariance or age- and gender-adjusted logistic regression analysis.
Table 2.
Selected Clinical and Functional Characteristics of the Sample by SPPB Score Change From Discharge to 1 Month After Discharge
| SPPB | ||||
| Declining | Stable | Improving | ||
| N = 14 | N = 24 | N = 39 | p* | |
| Age (y, M ± SD) | 75.6 (4.4) | 77.3 (5.4) | 76.5 (7.0) | .718 |
| Women, n (%) | 9 (64.3) | 11 (45.8) | 19 (48.7) | .516 |
| MMSE score (M ± SD) | 27.0 (1.8) | 26.2 (2.0) | 25.3 (2.5) | .051 |
| Total Cumulative Illness Rating Scale score (M ± SD) | 8.5 (4.2) | 8.3 (2.7) | 9.7 (3.1) | .294 |
| ADL disability 2 weeks before admission, n (%) | 1 (7.1) | 1 (4.2) | 5 (12.8) | .130 |
| ADL disability at admission, n (%) | 3 (21.4) | 6 (25.0) | 14 (35.9) | .318 |
| SPPB score at admission (M ± SD) | 6.71(2.7) | 7.13 (2.3) | 5.51 (2.5) | .041 |
| SPPB score at discharge (M ± SD) | 8.93(2.2) | 8.25 (2.5) | 6.16 (2.9) | .001 |
| SPPB score 1 month after discharge (M ± SD) | 7.57(2.4) | 7.13 (2.3) | 8.28 (2.5) | .638 |
Notes: ADL = activity of daily living; MMSE = Mini-Mental State Examination; SPPB = Short Physical Performance Battery.
p Values from age- and gender-adjusted analysis of covariance or age- and gender-adjusted logistic regression analysis.
Risk of ADL Disability
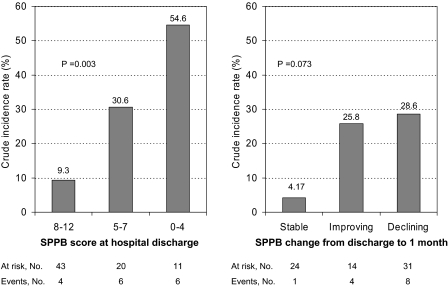
Among the 74 patient free of ADL disability at hospital discharge, 16 (21.6%) developed new incident ADL disability over the follow-up after hospital discharge. Figure 1 depicts unadjusted incidence rates as a function of SPPB score at hospital discharge (left panel) and SPPB trajectory during the first month after discharge (right panel). There was a clear gradient in the risk of disability in the activities of daily living across the full range of performance scores at hospital discharge (p = .003); higher scores, indicating better functional status, were associated with a lower risk of subsequent disability. When the risk of disability was analyzed as a function of SPPB change after hospital discharge, patients with stable SPPB score had the lowest incidence, whereas those with declining lower extremity performance had the greatest risk of new ADL disability. For both analyses, results were statistically significant after adjustment for age and gender (p = .005 and p = .037, respectively), but adjustment for other potential confounders was not performed because of the limited number of events.
Figure 1.
Crude incidence rate of new incident activity of daily living disability over the follow-up according to Short Physical Performance Battery (SPPB) score at hospital discharge (left panel) and SPPB trajectory during the first month after discharge from the hospital (right panel).
Change Over Time in ADL Summary Scale
Estimated changes over time in ADL summary score as a function of SPPB score at hospital discharge are reported in Table 3. After adjustment for demographic characteristics, patients with lower (0–4) and intermediate (5–7) SPPB score at discharge experienced a significant increase of ADL summary scale score over time, indicating worsening ADL functional status, whereas those with better SPPB score (8–12) had a trivial and nonsignificant change in ADL summary scale score (p = .182). A formal test for interaction between time and SPPB categories suggested that the magnitude of the change in ADL score was larger in patients with lower (p = .108) and intermediate (p = .01) SPPB score compared with those with better SPPB performance (Table 3, section A). Patients with stable and improving SPPB score in the first month after discharge were characterized by the lowest increase in ADL difficulty over the 12-month of follow-up (Table 3, section B). Conversely, for the group of patients who experienced a SPPB decline, the ADL summary scale score tended to increase over time, indicating worsening ADL functional status, and the estimated slope was statistically significant (p = .016) . Nevertheless, when this slope was formally compared with that of patients with stable (p = .124) or improving SPPB score (p = .126, data not shown), the difference was not statistically significant. Analyses performed after exclusion of 10 patients with scores either at the floor or at the ceiling for the SPPB range at hospital discharge showed similar results.
Table 3.
Mean Change in ADL Summary Scale Score Over Time According to SPPB Score at Discharge (A) and Change From Discharge to 1 Month After Discharge (B) Estimated From Random-Effect Models
| Model 1 | ||||
| Mean 3-Month ADL Score Change | SE | p | p* | |
| A. SPPB score at discharge | ||||
| SBBP categories | ||||
| 0–4 (n = 16) | 0.24 | 0.10 | .021 | .108 |
| 5–7 (n = 27) | 0.28 | 0.07 | .001 | .010 |
| 8–12 (n = 44) | 0.05 | 0.05 | .287 | — |
| All patients (n = 87) | 0.14 | 0.04 | .001 | — |
| B. SPPB change from discharge to 1 month | ||||
| Declining (n = 14) | 0.24 | 0.10 | .022 | .124 |
| Stable (n = 24) | 0.04 | 0.07 | .590 | .826 |
| Improving (n = 39) | 0.06 | 0.06 | .316 | — |
| All patients (n = 77) | 0.09 | 0.04 | .04 | — |
Notes: Adjusted for age and gender. ADL = activity of daily living; SPPB = Short Physical Performance Battery.
p Value for the interaction term between time and SPPB categories at hospital discharge testing the hypothesis that the average rate of change in the dependent variable is statistically different compared with the 8–12 SPPB category and to the improving category, respectively.
Risk of Hospitalization or Death
Fifty-five percent of the cohort was admitted to the hospital at least one time and 12.6% died during the 12-month follow-up. Because of the small number of deaths (11), the analysis was performed using a unique composite outcome. There was a graded relationship between SPPB score at hospital discharge and the risk of the composite outcome, with patients with lower score at highest risk. Multivariate analysis (Table 4, section A) confirmed the inverse dose–response association between SPPB score and the likelihood of hospitalization or death and that the association was independent of potential confounders and self-reported measures of functional status. Compared with patients with better SPPB score, those with poorer score (0–4) had a fivefold increased risk of hospitalization or death, whereas those with intermediate score had a 2.6 time higher risk. Furthermore, when SPPB score was analyzed as a continuous variable, a 1-point increase of the SPPB score at discharge was associated with a 14% reduction of the risk of the composite outcome (95% confidence interval: 2%–25%). Finally, we investigated the risk of hospitalization or death over the 1-year follow-up as a function of SPPB change during the first month after discharge (Table 4, section B). Patients with stable SPPB score had the lowest risk, whereas those with declining SPPB score had an adjusted threefold risk of hospitalization or death, independent of comorbidity, ADL status, and SPPB score at hospital discharge. Patients improving in SPPB score had no difference in risk compared with stable patients. Nevertheless, after stratification for SPPB score at hospital discharge, patients improving in SPPB score and with performance above the median SPPB score (7 points) at discharge had a lower risk of rehospitalization or death compared with the group with stable SPPB (odds ratio: 0.33, 95% confidence interval: 0.09–1.25). Conversely, patients who improved in SPPB score but who had an SPPB score at discharge ≤7 had a significant increased risk of rehospitalization or death (odds ratio: 4.8, 95% confidence interval: 1.15–15.4).
Table 4.
Adjusted ORs for New Hospitalization or Death During the Follow-Up According to SPPB Score at Discharge (A) and Change From Discharge to 1 Month After Discharge (B)
| Proportion With Outcome (%) | Model 1 | Model 2 | |||
| OR | 95% CI | OR | 95% CI | ||
| A. SPPB score at discharge | |||||
| SBBP categories | |||||
| 0–4 (n = 16) | 75.0 | 3.72 | 1.52–9.08 | 5.38 | 1.82–15.9 |
| 5–7 (n = 27) | 65.4 | 2.95 | 1.38–6.28 | 2.63 | 1.16–6.01 |
| 8–12 (n = 44) | 52.3 | 1.0 | — | 1.0 | — |
| SBBP continuous score* | 0.87 | 0.78–0.97 | 0.86 | 0.75–0.98 | |
| OR† | 95% CI | OR† | 95% CI | ||
| B. SBBP change from discharge to 1 month | |||||
| Stable (n = 24) | 45.8 | 1 | — | 1 | |
| Declining (n = 14) | 71.4 | 3.50 | 1.29–9.50 | 3.59 | 1.20–10.0 |
| Improving (n = 39) | 59.0 | 1.28 | 0.53–3.10 | 1.35 | 0.56–3.28 |
Notes: Model 1: adjusted for age, gender, and education; Model 2: adjusted for age, gender, and education; Cumulative Illness Rating Scale; IADL disability 2 weeks before hospital admission; ADL summary scale 2 weeks before hospital admission; ADL summary scale at discharge; and MMSE score at hospital admission. ADL = activity of daily living; CI = confidence interval; IADL = instrumental activity of daily living; MMSE = Mini-Mental State Examination; ORs = odds ratio; SPPB = Short Physical Performance Battery.
Tested as a separate model.
Additional adjustment for SPPB score at hospital discharge.
DISCUSSION
In this study, among acutely ill, hospitalized patients aged 65 years and older, lower extremity performance was measured with the SPPB, a simple battery of objective tests involving gait speed, balance, and ability to rise from a chair. SPPB score measured at hospital discharge was predictive of subsequent decline in ADL status and subsequent rehospitalization and mortality. Patients with poor performance were significantly more likely to report increasing difficulties in basic ADL and to be readmitted to the hospital in the following year, even after adjustment for baseline comorbidity level and self-report indicators of functional status. Furthermore, early decline in physical performance after hospital discharge was also predictive of increasing ADL difficulties and increased risk of rehospitalization and death.
Our results extend to the acute care setting the findings of previous epidemiological studies in which lower extremity performance predicted a number of different geriatric outcomes, including disability (7), hospitalization (9), nursing home admission (13) and death (27). In particular, our longitudinal results are in good agreement with the study of Purser and colleagues (28) who demonstrated, in a sample of frail male veterans, that walking speed is useful for the functional assessment of acutely ill hospitalized older adults, and measurement of walking speed over time may help predict those who will need and use more health-related services. Despite a large body of epidemiological evidence demonstrating the predictive value of different mobility performance tests in terms of various adverse outcomes in community-dwelling elders, the use of physical performance measures in the acute care clinical setting is still limited (12). In our study, the predictive value of objective lower extremity performance was independent of self-report ADL status, a measure traditionally used in routine clinical practice (29), indicating that these two assessment tools explore different and only partially overlapping domains of physical function and might provide useful complementary information (30). Furthermore, the finding that sequential assessment of physical performance during hospital stay and in the first weeks after discharge can provide additional information on future health risk in older acutely ill patients should support and encourage the systematic assessment of these objective tests in everyday clinical practice.
Why does poor SPPB score and decline in SPPB score after hospitalization predict future health outcomes? Objective measures of physical performance are likely to capture the integrated and multisystemic effects of aging, comorbidity, disease severity, malnutrition, motivation, and cognition (31–34) on the health status of older persons. Like other biomarkers (35,36), SPPB might be considered as a nonspecific but highly sensitive indicator of global health status reflecting several underlying physiological impairments (37). All these aspects might explain why, after adjustment for level and severity of comorbidity and self-report functional status, SPPB score remained an independent and strong predictor of functional decline, hospitalization, and mortality.
Improvement in SPPB score after hospital discharge was not associated with better ADL status or lower risk of rehospitalization or death over the follow-up. This finding was unexpected and somehow surprising because it has been demonstrated that 1-year improvement in gait speed is associated with a substantial reduction in long-term mortality (38). In our sample, compared with patients who remained stable, those who improved their lower extremity performance after discharge had higher level of comorbidity, poorer cognitive function, and poorer SPPB score at hospital admission and at discharge, indicating for this subgroup of patients a worse health profile before hospitalization. It is conceivable that, although being able to ameliorate their physical performance in the weeks after the acute medical event, some of these patients are likely to be more frail, vulnerable, and clinically unstable and therefore at greater risk of poor long-term outcomes. Although we adjusted for multiple health- and function-related variables, including SPPB at discharge, there may still be residual confounding that does not allow for fully adjusting the health differences between this group and the others. Indeed, in a separate analysis stratified according to SPPB score at hospital discharge, those who had better lower extremity performance (SPPB >7 points) at discharge and improvement in SPPB score after discharge had a lower risk of rehospitalization or death compared with those with stable SPPB score. On the other hand, persistent stability in lower extremity performance after hospitalization might represent a reliable marker of robustness and stabilized clinical condition.
The limitations of the study include the small sample size that reduced the statistical power of our analysis. For example, because of limited statistical power, we were not able to further investigate the association between change in SPPB and risk of incident disability after stratification for SPPB score at discharge. Furthermore, it was not possible to directly compare the predictive value of SPPB score to those of more traditional, and widely used, assessment tools, including self-report ADL status, and to compare the predictive values of SPPB score at discharge to the predictive value of early SPPB change after discharge. A second limitation is the enrollment of patients able to walk and admitted to the hospital with selected medical conditions (ie, congestive heart failure, chronic obstructive pulmonary disease, pneumonia, and minor stroke). Although we chose these diseases because they are common causes of hospitalizations (3,5) in older patients and because they are conditions that strongly affect physical function in older frail participants, these restricted inclusion criteria might have reduced the external validity, and our findings might not apply to the full spectrum of hospitalized older patients. Larger studies should be designed to confirm our findings and overcome these limitations.
Physical performance measures have been proposed as a potential “vital sign” in older adults (14,39). This study demonstrates that in the acute care setting, lower extremity performance assessment also provides useful prognostic information in terms of long-term functional decline, hospitalization, and mortality risk. The findings that sequential SPPB assessment during hospital stay and in the first weeks after hospital discharge also had long-term independent predictive validity might further encourage the use of physical performance for continuity of care and posthospital discharge assessment and management.
FUNDING
This research was supported in part by contracts from the National Institute on Aging, Intramural Research Program, National Institutes of Health.
References
- 1.Boyd CM, Xue QL, Guralnik JM, Fried LP. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women's Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2005;60(7):888–893. doi: 10.1093/gerona/60.7.888. [DOI] [PubMed] [Google Scholar]
- 2.Laniece I, Couturier P, Drame M, et al. Incidence and main factors associated with early unplanned hospital readmission among French medical inpatients aged 75 and over admitted through emergency units. Age Ageing. 2008;37(4):416–422. doi: 10.1093/ageing/afn093. [DOI] [PubMed] [Google Scholar]
- 3.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64(12):1296–1303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645–652. [PubMed] [Google Scholar]
- 6.Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med. 2007;22(5):668–674. doi: 10.1007/s11606-007-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 9.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol Med Sci. 2000;55(11):M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity—the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58(8):715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998;13(12):817–823. doi: 10.1046/j.1525-1497.1998.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quadri P, Tettamanti M, Bernasconi S, Trento F, Loew F. Lower limb function as predictor of falls and loss of mobility with social repercussions one year after discharge among elderly inpatients. Aging Clin Exp Res. 2005;17(2):82–89. doi: 10.1007/BF03324578. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 14.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 15.Volpato S, Cavalieri M, Guerra G, et al. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008;63(12):1393–1398. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55(9):916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 17.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 18.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 19.Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2010;65(8):866–872. doi: 10.1093/gerona/glq069. [DOI] [PubMed] [Google Scholar]
- 20.Ostir GV, Volpato S, Kasper JD, Ferrucci L, Guralnik JM. Summarizing amount of difficulty in ADLs: a refined characterization of disability. Results from the Women's Health And Aging Study. Aging (Milano) 2001;13(6):465–472. [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137(9):1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 23.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 24.Conwell Y, Forbes NT, Cox C, Caine ED. Validation of a measure of physical illness burden at autopsy: the Cumulative Illness Rating Scale. J Am Geriatr Soc. 1993;41(1):38–41. doi: 10.1111/j.1532-5415.1993.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 25.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.StataCorp. College Station, TX: StataCorp LP: 2005. Stata Statistical Software: Release 9. [Google Scholar]
- 27.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57(2):251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42(4):535–546. doi: 10.1682/jrrd.2004.07.0087. [DOI] [PubMed] [Google Scholar]
- 29.Sleiman I, Rozzini R, Barbisoni P, et al. Functional trajectories during hospitalization: a prognostic sign for elderly patients. J Gerontol A Biol Sci Med Sci. 2009;64(6):659–663. doi: 10.1093/gerona/glp015. [DOI] [PubMed] [Google Scholar]
- 30.Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59(10):1056–1061. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 31.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62(3):296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 32.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 34.Forrest KYZ, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: a 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1194–1200. doi: 10.1093/gerona/61.11.1194. [DOI] [PubMed] [Google Scholar]
- 35.Volpato S, Ble A, Metter EJ, et al. High-density lipoprotein cholesterol and objective measures of lower extremity performance in older nondisabled persons: the InChianti study. J Am Geriatr Soc. 2008;56(4):621–629. doi: 10.1111/j.1532-5415.2007.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpato S, Leveille SG, Corti MC, Harris TB, Guralnik JM. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49(9):1142–1147. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 37.Morley JE. Mobility performance: a high-tech test for geriatricians. J Gerontol A Biol Sci Med Sci. 2003;58(8):712–714. doi: 10.1093/gerona/58.8.m712. [DOI] [PubMed] [Google Scholar]
- 38.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 39.Hall WJ. Update in geriatrics. Ann Intern Med. 2006;145(7):538–543. doi: 10.7326/0003-4819-145-7-200610030-00012. [DOI] [PubMed] [Google Scholar]