Abstract
Background.
Muscle power is related to mobility function in older adults, and effective power production requires rapid neuromuscular activation. Accordingly, this study examines the association of neuromuscular activation rate with muscle performance in persons of different age and mobility function.
Methods.
Participants were recruited to three experimental groups: middle-aged healthy adults (MH), older healthy adults (OH), and older adults with mobility limitations (OML). OH and OML were primarily differentiated by performance on the Short Physical Performance Battery (SPPB). Muscle performance (acceleration and power) and electromyography (EMG) were recorded during a maximal-effort leg press task at an absolute resistance (260 N) and at a relative resistance (70% of the one-repetition maximum [1RM]). Neuromuscular activation rate was quantified as pre-movement time (duration between EMG onset and movement onset) and the rate of EMG rise.
Results.
Pre-movement time, rate of EMG rise, leg press acceleration, and leg press power were lower in OML relative to MH and OH but did not differ between OH and MH, with the exception of power at 70% 1RM. Across all older participants, rate of EMG rise was positively associated with acceleration, power, and the SPPB score.
Conclusions.
Slowing of neuromuscular activation rate is associated with compromised dynamic muscle performance, which may contribute to mobility limitations in some older adults. Future research should identify the precise neurophysiological impairments that contribute to declines in neuromuscular activation rate and mobility function with aging.
Keywords: Aging, Electromyography, Strength, Mobility
MAXIMAL voluntary muscle power declines with aging, and the magnitude of this deterioration has been shown to be predictive of mobility function in older adults (1–3). Because power is quantified as the product of force and velocity, it depends in part on the rate of movement and may therefore be more sensitive than traditional measures of muscle strength (eg, static force or the one-repetition maximum [1RM]) to physiological changes in the motor system that occur with aging. For instance, slower muscle contractile properties (4,5), impaired excitation–contraction coupling (6), and alterations to muscle architecture (7) and musculotendinous stiffness (7,8) may have differential effects on rapid versus slow or static muscle contractions. Perhaps even more important to dynamic muscle performance is the effect of neuromuscular activation, which may directly limit muscle output and also exacerbate the peripheral impairments just described. Neuromuscular activation is operationally defined here as the process by which the nervous system produces muscular force through recruitment and rate coding of motor units. A widely used technique for assessing neuromuscular activation is surface electromyography (EMG) (9). EMG detects the bioelectrical activity associated with muscle contraction and has previously revealed important neural alterations associated with aging and physical function (10–12).
Given the relevance of muscle power to mobility function in older adults, a particularly important contributory neural factor may be the maximal rate of neuromuscular activation. Indeed, mobility tasks often require rapid alterations of muscle activation, and slowing of activation may pose a particular challenge when task demands are higher such as during fast walking, stair negotiation, and balance recovery after tripping (11,13,14). It is therefore notable that age-related declines in the maximal voluntary rate of neuromuscular activation have been reported at the onset of rapid muscle contractions as evidenced by lower EMG amplitude (15), slope of the EMG signal (16,17), motor unit firing rates (18), and incidence of doublet firing (18,19). This slowed neuromuscular activation rate has been linked to impaired capability of older adults to produce force rapidly under isometric conditions (15,16,18).
Although the existing evidence suggests a link between age-related declines in neuromuscular activation rate and static force production, the influence of neuromuscular activation rate on dynamic muscle performance (eg, muscle power) is not well understood. Furthermore, the association of rate of neuromuscular activation with mobility function in older adults has not been examined. The present study addresses these issues by investigating neuromuscular activation rate and dynamic muscle performance during a leg press task in three distinct cohorts: healthy middle-aged adults, healthy older adults and adults with mobility limitations. Unlike earlier studies that have compared older adults to a young control group (15,17,18), we chose to compare older adults with middle-aged controls with the expectation that this narrower age range would reveal differences that are more focused and relevant to age-related declines in mobility function. We hypothesized that, relative to middle-aged adults, neuromuscular activation rate in the quadriceps would be slightly reduced in healthy older adults and more substantially reduced in mobility-limited older adults. We further hypothesized that neuromuscular activation rate would be positively associated with dynamic muscle performance and with mobility function.
METHODS
Participants
Volunteers were recruited by local newspaper advertisements, direct mailing to volunteers from earlier studies at our center, and posting of flyers. Three specific groups were recruited: middle-aged healthy adults (MH, age 40–55 years), older healthy adults (OH, age 70–85 years), and older adults with mobility limitations (OML, age 70–85 years). Volunteers were screened by telephone using the following exclusion criteria: presence of unstable chronic disease, acute or terminal illness, myocardial infarction within 6 months (or other symptomatic coronary artery disease), uncontrolled hypertension (>150/90 mm Hg), fracture in the previous 6 months, diseases or medications affecting neuromuscular function, anticoagulation therapy (due to a muscle biopsy procedure, data not presented here), hormone replacement therapy, body mass index less than 19 kg/m2 or greater than 33 kg/m2, weight loss or gain within the previous 6 months, and participation in a strength or endurance training program within the previous 6 months. Volunteers being considered for MH and OH were also required to not be taking any prescription medications. Individuals who passed the telephone screening were further screened by a licensed physician or nurse practitioner, including assessment of the presence of lower extremity joint pain and administration of the Mini-Mental State Examination (MMSE) and Short Physical Performance Battery (SPPB). Persons with MMSE score less than 23 or with joint pain were excluded. The SPPB, which probes the domains of strength, ambulation, and balance, is predictive of future disability (20) and was used to classify the older adults into the OH and OML groups. Older adults with scoring 9 or less (out of a possible 12 points) were classified as OML, whereas those scoring greater than 9 and who were not taking prescription medication were classified as OH.
Protocol
Participants were positioned on a seated bilateral leg press apparatus such that the range of motion started with knees flexed to 90° and hips flexed to approximately 110° and ended with the knees almost fully extended and hips flexed to approximately 80°. Strength was measured using the 1RM technique (21). Peak voluntary EMG amplitude was measured during an isometric maximal voluntary contraction (MVC), with the participants’ legs constrained to the starting position. Participants then performed five maximal-effort trials at each of two resistance levels, where they were instructed to push “as fast and as hard as possible” through the concentric phase of the movement and then slowly return the footplate to the starting position. The resistance conditions included an absolute force level, 260 N, and a relative force level, 70% of the 1RM. The 260 N resistance was chosen because it was close to the minimal resistance setting on our leg press apparatus, which helped to ensure that even very weak participants would be capable of performing the task. Each trial was separated by 30 seconds of rest, and each resistance condition was separated by at least 2 minutes of rest. The data presented here are from the second of two identical testing sessions performed approximately 1 week apart. The first session allowed participants to become familiar with our testing equipment and procedures.
Instrumentation
The leg press apparatus provides adjustable resistance via pneumatic pistons attached to the footplate (leg press A420; Keiser Corp., Fresno CA). Force and position of each piston were sampled at 400 Hz and saved to disk for offline analysis. Using software provided by the manufacturer, these data were then converted to force, position, velocity, and acceleration at the footplate (Software Release 7.8; Keiser Corp.). Knee angle was measured using an electrogoniometer (ADInstruments, Colorado Springs, CO). Muscle activation was assessed by surface EMG using a commercially available data acquisition system (Delsys Bagnoli-8; Delsys, Boston, MA). Single differential surface electrodes (Delsys 2.1; Delsys) with 1-cm interelectrode distance were placed over the muscle bellies of the rectus femoris (RF), vastus medialis (VM), and vastus lateralis (VL). Signals were recorded at a sampling rate of 1 kHz using a Powerlab/16SP A/D system and Chart software (ADInstruments). A custom-built trigger indicated the point of movement initiation by changing output voltage when the footplate was lifted from the supporting base. The trigger signal was recorded along with the EMG and was used to synchronize the EMG data with the leg press mechanical data.
Data Analysis
Data were analyzed using a custom analysis program created in MATLAB (version 7.0; Mathworks, Natick MA). Acceleration for each leg press trial was calculated as the mean value during the first centimeter of displacement to capture the initial explosive period of movement. Power was calculated as the mean value between 55° and 35° of knee flexion during the concentric phase of the movement to measure sustained output from the region where peak power was typically observed. All raw EMG signals were debiased and filtered with a zero phase–lag first-order Butterworth band-pass filter (10–200 Hz). Peak EMG amplitude during isometric MVC was quantified as the root mean square over the 100 millisecond window containing the greatest amplitude. For each resistance condition, the rectified EMG signals were smoothed using a 100 millisecond sliding window average and each muscle was normalized to its own peak EMG from the MVC.
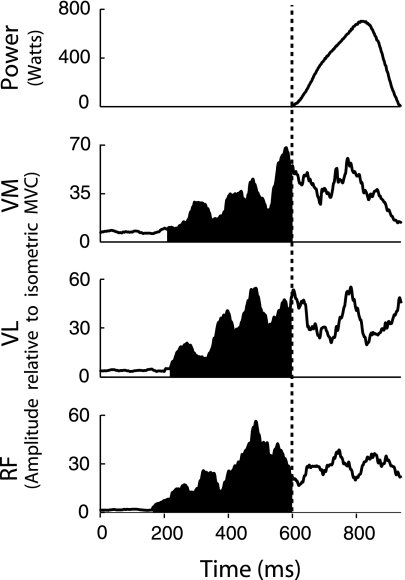
The phenomenon of neuromuscular activation rate was quantified using two measures. The first, pre-movement time, is the period of time between the first detection of activation in any muscle of the quadriceps group (EMG amplitude exceeding the resting mean plus 3 SDs in VM, VL, or RF) to the onset of movement. This measure reflects the amount of time that one or more muscles are contracting before sufficient force is generated to move the leg press load. The second measure, rate of EMG rise, was calculated for each muscle as the mean derivative of the normalized EMG signal between activation onset and movement onset. Typical data from a single trial are presented in Figure 1, with shaded areas representing the period from which both activation measures were calculated.
Figure 1.
Typical data from a leg press trial displaying leg press power and processed electromyography (EMG) from vastus medialis (VM), vastus lateralis (VL), and rectus femoris (RF). The vertical dashed line indicates the start of movement. The portion of EMG shaded in black indicates the time from the onset of muscle activation to the onset of movement and is the region from which pre-movement time and rate of EMG rise were calculated. MVC = maximal voluntary contraction.
Statistics.—
For each participant and condition, the average of trials 2, 3, and 4 was used for statistical analysis. Trial 1 was considered a practice trial at each new resistance condition, whereas trial 5 was eliminated due to the potential effects of short-term fatigue. Analyses were performed using JMP statistical software (version 7.0; SAS Institute Inc., Cary, NC). Acceleration, power, pre-movement time, and rate of EMG rise were analyzed at each resistance condition using a one-factor (group) analysis of variance. Resistance condition was not included as a factor in the model because the absolute force production at 70% 1RM varied widely among participants and thus comparison with the 260-N condition is not valid. Tukey’s post hoc test was used to examine significant effects, with significance set at α = .05. Pearson’s correlation analysis was used to assess associations between rate of EMG rise and acceleration and power, whereas Spearman’s correlation analysis was used to assess the association between SPPB score and rate of EMG rise.
RESULTS
Participants
Data were collected from 72 participants, including 24 MH, 25 OH, and 23 OML. Consistent with our recruitment criteria, OML had significantly lower SPPB scores than MH and OH (p < .0001). Full descriptive characteristics are presented in Table 1.
Table 1.
Participant Demographics
| Middle-Aged, Healthy | Older, Healthy | Older, Mobility Limited | |
| Age (y) | |||
| M ± SD | 47.5 ± 4.6 | 73.9 ± 3.5* | 77.4 ± 4.8* |
| Range | 41–55 | 70–82 | 70–85 |
| Weight (kg) | |||
| M ± SD | 75.9 ± 14.9 | 75.0 ± 16.2 | 71.6 ± 10.0 |
| Range | 54.5–115.5 | 47.4–104.6 | 50.2–95.5 |
| Body mass index (kg/m2) | |||
| M ± SD | 25.9 ± 3.0 | 25.1 ± 3.9 | 26.6 ± 2.9 |
| Range | 19.7–32.6 | 18.7–33.0 | 22.6–31.1 |
| Short Physical Performance Battery (out of 12) | |||
| M ± SD | 11.7 ± 0.5 | 11.0 ± 0.9 | 7.9 ± 1.3† |
| Range | 11–12 | 10–12 | 5–9 |
| Sex | |||
| Male/female | 12/12 | 14/11 | 11/12 |
Different from middle-aged healthy adults (p < .001).
Different from middle-aged healthy adults and older healthy adults (p < .001).
Leg Press Performance Measurements
A significant effect of group was present (p < .0001) for 1RM force, with post hoc analysis revealing that OML (963 ± 321 N) produced significantly less force than both MH (1,345 ± 322 N) and OH (1,202 ± 402 N). The same significant differences were observed for force production during the 70% 1RM condition (p < .0001), with MH, OH, and OML averaging 942, 841, and 674 N, respectively.
The 260-N resistance condition required a significantly (p = .0003) higher proportion of 1RM strength for OML (30.1 ± 10.4% of 1RM) compared with MH (20.6 ± 5.5%) and OH (24.4 ± 9.3%).
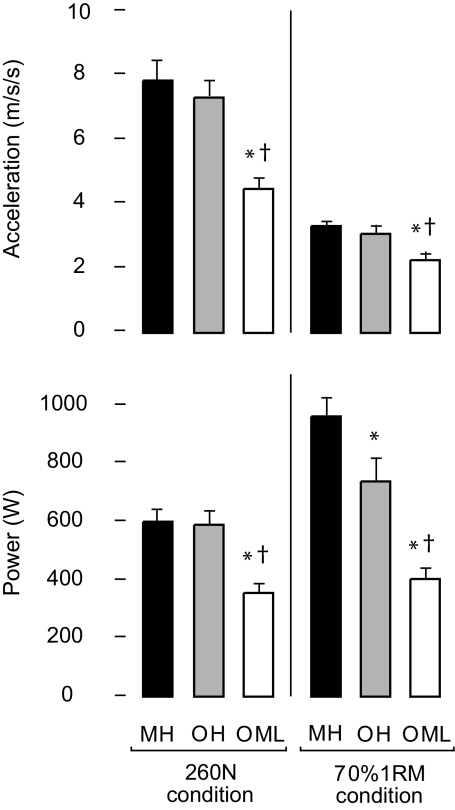
At each resistant condition, there was a significant effect of group for acceleration (p < .0003; Figure 2, top). Post hoc analysis revealed significantly lower values in OML compared with both MH and OH at 260 N (43% and 39% lower, respectively) and at 70% 1RM (31% and 26% lower, respectively).
Figure 2.
Measures of muscle performance during the leg press. Acceleration (top figure) and power (bottom figure) measured at the leg press footplate for each group at each resistance condition. Error bars indicate standard error of the mean. “*,” significantly different from MH; “†,”OML significantly different from OH. EMG = electromyography; MH = middle-aged healthy adults; MVC = maximal voluntary contraction; OH = older healthy adults; OML = older adults with mobility limitations.
There was also a significant effect of group for power (Figure 2, bottom) at each resistance condition (p < .0001). Post hoc analysis at 260 N showed that OML produced significantly less power than MH and OH (41% and 40% less, respectively). All three groups differed significantly at 70% 1RM. OH produced 23% less power than MH, whereas OML produced 58% and 45% less power than MH and OH, respectively.
EMG Measurements
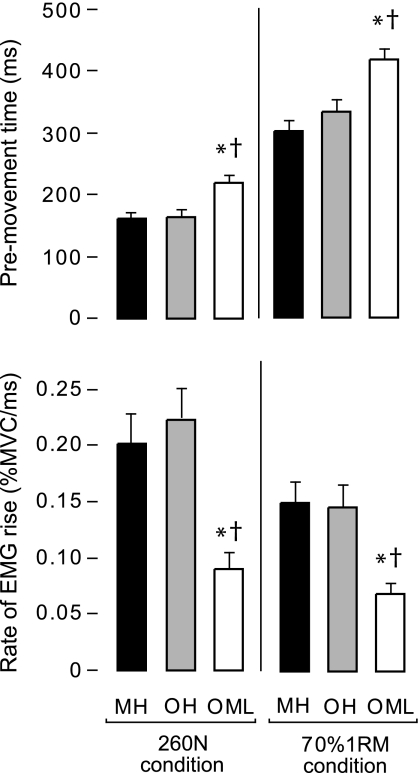
Non-normalized EMG amplitude (mV) during the isometric MVC did not significantly differ between the three experimental groups (p > .08). For pre-movement time (Figure 3, top) the effect of group was significant at each resistance condition (p < .001). Post hoc analysis revealed significantly lower values in OML compared with both MH and OH at 260 N (37% and 35% lower, respectively) and at 70% 1RM (38% and 25% lower, respectively).
Figure 3.
Measures of neuromuscular activation rate during the leg press. Pre-movement time (top figure) and rate of EMG rise (bottom figure) for each group at each resistance condition. Error bars indicate standard error of the mean. “*,” significantly different from MH; “†,” OML significantly different from OH. MH = middle-aged healthy adults; OH = older healthy adults; OML = older adults with mobility limitations.
For each group and resistance condition, the rate of EMG rise did not differ between VM, VL, and RF (p > .05). Accordingly, the data from the three muscles were averaged to form a composite measure of quadriceps rate of EMG rise, and this value was used for all subsequent analyses. A significant effect of group was revealed for rate of EMG rise (Figure 3, bottom) in both resistance conditions (p < .001). Post hoc analysis revealed significantly lower values in OML compared with both MH and OH at 260 N (55% and 60% lower, respectively) and at 70% 1RM (54% and 53% lower, respectively).
Association of Rate of EMG Rise to Acceleration, Power, and SPPB Score
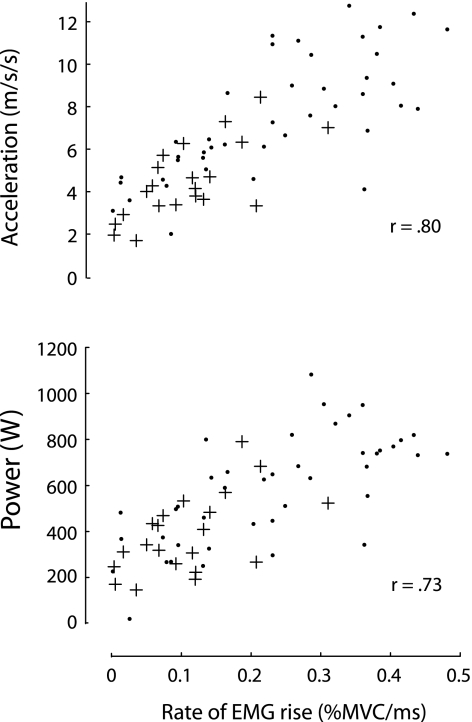
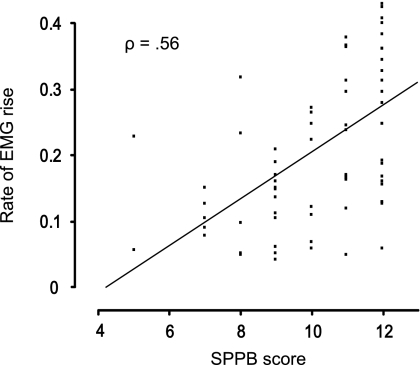
The rate of EMG rise was found to be strongly associated with acceleration (r = .80, p < .0001) and power (r = .73, p < .0001) at the 260-N resistance condition (Figure 4). Furthermore, we observed a significant positive association between the SPPB score and the rate of EMG rise across the MH and OH participants (ρ = .56, p = .02; Figure 5).
Figure 4.
Correlations between the rate of electromyography (EMG) rise and acceleration (top) and power (bottom) for the 260-N resistance condition. Healthy middle-aged and older adults are displayed as black circles, and mobility-limited older adults are displayed as crosses.
Figure 5.
Correlation between Short Physical Performance Battery (SPPB) score and rate of electromyography (EMG) rise for the 260-N resistance condition. Only participants belonging to OH and OML groups have been included to examine the association across persons of similar age. MH = middle-aged healthy adults; OH = older healthy adults; OML = older adults with mobility limitations.
DISCUSSION
This study demonstrates that the rate of neuromuscular activation is associated with dynamic muscle performance and mobility function in older adults. The two older groups studied, OH and OML, were generally healthy and of similar age, weight, and body mass index (Table 1), but OML exhibited a mobility deficit as detected by the SPPB. Consistent with our expectations, OML demonstrated marked impairment on the neuromuscular activation rate measures (pre-movement time and rate of EMG rise) compared with OH, despite having only a mild to moderate difference in mobility function. Contrary to our hypothesis, we found no difference between MH and OH for pre-movement time or rate of EMG rise. This finding suggests that slowing of neuromuscular activation rate is not purely a consequence of biologic aging, which is consistent with earlier studies showing that neuromuscular activation rate is higher in older adults who maintain an active lifestyle (22) and can be increased with resistance training (15,23). A slow rate of neuromuscular activation may therefore be an indicator of an emerging impairment within the nervous system that contributes to mobility dysfunction.
Neuromuscular activation rate and leg press performance (acceleration and power) were assessed at two different resistance levels: a relative resistance condition that was set at 70% of each participant’s own 1RM strength and an absolute resistance (260 N) that was biomechanically equivalent for all participants. The benefit of the relative resistance condition is that it partially accounts for intersubject differences in strength (eg, due to differences in muscle mass) to better isolate the effects of neuromuscular activation. The benefit of the absolute resistance condition is that it allows us to evaluate performance when task demands are identical. Despite the distinction between the two resistance conditions, both yielded similar evidence that leg press performance (Figure 2) and neuromuscular activation rate (Figure 3) were impaired in OML relative to MH and OH but similar between MH and OH. The one notable exception was that OH produced significantly less power than MH at 70% 1RM but not at 260 N. Given that this finding was not accompanied by differences in neuromuscular activation rate and that power differed at the relative force condition where muscle mass is less likely to affect the outcome, this finding may be related to slower muscle contractile properties (4,5), altered muscle architecture (7), or altered musculotendinous stiffness (7,8) in older adults.
Slowing of neuromuscular activation rate would be expected to result in a slower rate of force development and compromised dynamic muscle performance. We tested this by examining the association between rate of EMG rise to acceleration and power at the absolute (260 N) condition. We chose the 260-N condition because we believe that it is a more objective measure of true neuromuscular capacity than the 70% 1RM condition. The decision to use rate of EMG rise rather than pre-movement time is because the rate of EMG rise was more impaired in OML and therefore appears to be more sensitive to slowing of neuromuscular activation rate. This is likely due to the fact that it accounts for both the timing and the relative amplitude of activation. Consistent with our initial hypothesis, we demonstrate that rate of EMG rise is strongly associated with both variables (Figure 4). A significant positive association between SPPB score and rate of EMG rise was also revealed (Figure 5). However, a number of OH participants (SPPB >9) had low rates of EMG rise, whereas some OML participants (SPPB ≤9) had relatively high rates of EMG rise. It is important to remember that differences in mobility function across the range of SPPB scores can be subtle and that rate of neuromuscular activation is certainly not the only factor that may contribute to these subtle differences. Factors such as muscle mass, body composition, balance, and a variety of other conditions will likely also have an influence.
The precise mechanism linking maximal voluntary neuromuscular activation rate to compromised mobility function is not fully understood because mobility tasks such as walking may involve different neural systems (24) and generally require only submaximal levels of muscle activation. It may be that the observed slowing of neuromuscular activation rate manifests at the spinal (25) or motoneuronal (26,27) level, such that a robust descending command is not fully transmitted to the periphery. In this case, slow neuromuscular activation rate may generalize across all motor tasks. Alternatively, impaired descending drive from supraspinal motor regions may have widespread effects on control of both maximal-effort voluntary contractions and mobility tasks. Slower neuromuscular activation rate and compromised mobility function may then be independent symptoms of a general deterioration of the central nervous system (28–30). Regardless of the exact mechanism, the presence of impaired neuromuscular activation rate may limit mobility function by compromising the ability to produce acceleration and power at joints of the lower extremity. This impairment may prove particularly detrimental to performance of mobility tasks requiring rapid movement or high levels of effort.
There are some limitations to this study that should be considered. The use of surface EMG to assess neuromuscular activation has some inherent limitations (9), and there are two major factors affecting our measure of pre-movement time. The first is the ability to distinguish muscle activation onset from baseline signal noise, which was generally not a problem because the rapid maximal contractions used in our protocol resulted in a distinct burst of muscle activity. The second issue is that we did not record EMG from all the muscles that contributed to the leg press movement. However, the quadriceps muscle group is a primary agonist for the leg press task so its timing may be more critical than the timing of other muscles. Our measure of quadriceps rate of EMG rise involves the same timing issues as just discussed, as well as the issue of accounting for intersubject variability of activation amplitude due to factors such as subcutaneous adipose tissue and electrode placement. To help control for this, we expressed rate of EMG rise relative to the peak EMG from each participant’s own isometric MVC. Also, we do not know the habitual physical activity levels of our participants and whether potential differences could have affected the results, although we did exclude anyone who had been involved in an exercise training program in the 6 months preceding this study. Finally, although our participants were rigorously screened, we cannot completely rule out the possibility that unknown health conditions may have contributed to our findings.
This study establishes the importance of rapid neuromuscular activation for dynamic muscle performance and demonstrates that slowing of activation is associated with mobility function in older adults. Future research should identify the precise neurophysiological impairments that contribute to declines in neuromuscular activation rate and mobility function in older adults, and whether such impairments may be reversible.
FUNDING
This research was supported by the National Institute on Aging (AG-18844 to R.A.F.) and the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. D.J.C. was supported by U.S. Department of Veterans Affairs Career Development Award B4888M.
CONFLICT OF INTEREST
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture or U.S. Department of Veterans Affairs.
References
- 1.Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 2.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 3.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 4.Ng AV, Kent-Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci. 1999;54:B452–B458. doi: 10.1093/gerona/54.10.b452. [DOI] [PubMed] [Google Scholar]
- 5.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Boncompagni S, d’Amelio L, Fulle S, Fano G, Protasi F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance. J Gerontol A Biol Sci Med Sci. 2006;61:995–1008. doi: 10.1093/gerona/61.10.995. [DOI] [PubMed] [Google Scholar]
- 7.Narici MV, Maganaris CN. Plasticity of the muscle-tendon complex with disuse and aging. Exerc Sport Sci Rev. 2007;35:126–134. doi: 10.1097/jes.0b013e3180a030ec. [DOI] [PubMed] [Google Scholar]
- 8.Carroll CC, Dickinson JM, Haus JM, et al. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol. 2008;105:1907–1915. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 10.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brach JS, Kriska AM, Newman AB, VanSwearingen JM. A new approach of measuring muscle impairment during a functional task: quadriceps muscle activity recorded during chair stand. J Gerontol A Biol Sci Med Sci. 2001;56:M767–M770. doi: 10.1093/gerona/56.12.m767. [DOI] [PubMed] [Google Scholar]
- 13.Larsen AH, Puggaard L, Hamalainen U, Aagaard P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol. 2008;18:568–580. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Madigan ML. Age-related differences in muscle power during single-step balance recovery. J Appl Biomech. 2006;22:186–193. doi: 10.1123/jab.22.3.186. [DOI] [PubMed] [Google Scholar]
- 15.Barry BK, Warman GE, Carson RG. Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Exp Brain Res. 2005;162:122–132. doi: 10.1007/s00221-004-2127-3. [DOI] [PubMed] [Google Scholar]
- 16.Laroche DP, Knight CA, Dickie JL, Lussier M, Roy SJ. Explosive force and fractionated reaction time in elderly low- and high-active women. Med Sci Sports Exerc. 2007;39:1659–1665. doi: 10.1249/mss.0b013e318074ccd9. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RD, Brown JM. Influence of muscle activation dynamics on reaction time in the elderly. Eur J Appl Physiol Occup Physiol. 1994;69:344–349. doi: 10.1007/BF00392041. [DOI] [PubMed] [Google Scholar]
- 18.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 19.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 21.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 22.Leong B, Kamen G, Patten C, Burke JR. Maximal motor unit discharge rates in the quadriceps muscles of older weight lifters. Med Sci Sports Exerc. 1999;31:1638–1644. doi: 10.1097/00005768-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Patten C, Kamen G, Rowland DM. Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve. 2001;24:542–550. doi: 10.1002/mus.1038. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen JB. How we walk: central control of muscle activity during human walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- 25.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 26.Jankelowitz SK, McNulty PA, Burke D. Changes in measures of motor axon excitability with age. Clin Neurophysiol. 2007;118:1397–1404. doi: 10.1016/j.clinph.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Wang FC, de Pasqua V, Delwaide PJ. Age-related changes in fastest and slowest conducting axons of thenar motor units. Muscle Nerve. 1999;22:1022–1029. doi: 10.1002/(sici)1097-4598(199908)22:8<1022::aid-mus3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 30.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]