Abstract
Background.
Recent European studies suggest that vitamin D deficiency may be associated with increased odds of cognitive impairment in older persons, although findings from the United States are equivocal. Our objective was to investigate the association between vitamin D deficiency and cognitive impairment in the elderly U.S. population.
Methods.
Three thousand and three hundred twenty-five adults aged 65 years or more completed cognitive assessments, medical examinations, and physical performance measures and provided blood samples in the Third National Health and Nutrition Examination Survey, a nationally representative cross-sectional study of the U.S. noninstitutionalized population. We determined whether low levels of serum 25-hydroxyvitamin D (25(OH)D) were associated with increased odds of cognitive impairment using logistic regression models. Cognitive impairment was assessed using measures of immediate and delayed verbal memory, orientation, and attention (impairment was defined as the worst 10% of the distribution of combined scores).
Results.
The multivariate adjusted odds ratios (95% confidence interval) of cognitive impairment in participants who were 25(OH)D insufficient (≥50 < 75 nmol/L), deficient (≥25 < 50 nmol/L), and severely deficient (<25 nmol/L) in comparison with those sufficient (≥75 nmol/L) were 0.9 (0.6–1.3), 1.4 (1.0–2.1), and 3.9 (1.5–10.4), respectively (p for linear trend = .02). Log-transformed levels of 25(OH)D were also significantly associated with the odds of cognitive impairment (p = .02).
Conclusions.
These findings suggest that vitamin D deficiency is associated with increased odds of cognitive impairment in the elderly U.S. population. Further exploration of a possible causal relationship between vitamin D deficiency and cognitive impairment is warranted.
Keywords: Cognitive impairment, Cognition, Dementia, Vitamin D, Serum 25-hydroxyvitamin D
VITAMIN D may be neuroprotective as it influences neurogenesis, calcium homeostasis, the expression of neurotrophic factors, detoxification, and amyloid-beta clearance (1–3). Recent population-based studies have linked low serum 25-hydroxyvitamin D (25(OH)D) levels to cognitive dysfunction in older adults in England (4) and across Europe (5). However, the evidence from studies in the United States is less consistent. Low 25(OH)D levels were associated with cognitive dysfunction in homebound elderly adults from Boston (6). However, those with the highest levels of serum 25(OH)D were more likely to be impaired on a test of delayed verbal memory in older U.S. adults from the Third National Health and Nutrition Examination Survey (NHANES III) (7). We hypothesized that the inconsistency between the results from NHANES III and other studies might reflect the minimal adjustment for potential confounders or the limited use of available cognitive measures. We therefore examined the multivariate adjusted association between vitamin D and cognitive impairment using data from NHANES III.
METHODS
Participants
NHANES III is designed to assess the health and nutritional status of the noninstitutionalized U.S. population. NHANES III is a large, nationally representative, cross-sectional population-based study of persons aged 2 months and older living in the United States between 1988 and 1994 selected using a stratified multistage probability sampling design. Data were collected via detailed household interviews, including demographic, socioeconomic, dietary, and health-related questions. Participants also completed medical and dental examinations, physiological measurements, and laboratory tests administered by trained medical personnel. The overall household interview response rate was high (86%). Five thousand two hundred and fifty-two adults aged 65 years and older were eligible for inclusion in the present analyzes. Of these, 1,238 (23.6%) did not have serum 25(OH)D measurements, and a further 618 (11.8%) had missing data relating to cognitive impairment or other variables selected as potential confounders. In comparison with the 3,396 participants with complete data, participants with missing data were older, had fewer years of education, and were more likely to be women (all p < .01), though were no more likely to be white (p = .2). NHANES III was approved by the National Center for Health Statistics Institutional Review Board, and written informed consent was obtained from all participants.
Serum 25(OH)D Concentration
Blood samples were collected as part of the nutrition biomarker component of NHANES III. 25(OH)D assays were performed at the primary environmental and nutritional health laboratory at the National Center for Environmental Health (CDC, Atlanta, GA) by radioimmunoassay (RIA kit; DiaSorin, formerly Incstar Corp., Stillwater, MN). The assay coefficient of variation was 10%–25% for lower values (20–62.5 nmol/L) and 12%–18% for higher values (85–147.5 nmol/L). Full details of the assay method for this measurement and correlates of 25(OH)D in NHANES III have been published elsewhere (8–10).
Neuropsychological Tests
Six neuropsychological tests incorporated in NHANES III were designed for administration in the participants’ preferred language (English or Spanish) and were selected to assess cognitive functions typically affected in dementia (see Table 1). The evaluation included measures of orientation, immediate and delayed verbal memory, and attention. Orientation in time and place was assessed using six items from the Mini-Mental State Examination (11). Immediate and delayed verbal memory was assessed using object and story recall tasks. The object recall task incorporates three words (“apple,” “table,” and “penny”) and is also taken from the Mini-Mental State Examination (11). In the test, the interviewer repeats the three words up to three times until they have been successfully learned (participants were scored on the first trial only). Participants were then asked to recall the objects again after a short delay. The story recall task was taken from the East Boston Memory Test (12) and involved the immediate and delayed recall of the following story: “Three children were alone at home and the house caught on fire. A brave fireman managed to climb in a back window and carry them to safety. Aside from minor cuts and bruises, all were well.” Participants were asked to repeat the story immediately after it was read to them and again after a few minutes of answering unrelated questions. Points were allocated for recall of each of the six following ideas: three children, house on fire, fireman climbed in, children rescued, minor injuries, and everyone well. Attention was assessed by asking the participant to count backward from 20 in intervals of three. The series of digits were selected from the Weschler Adult Intelligence Scale (13).
Table 1.
Neuropsychological Test Details
| Cognitive Domain | Number of Items/Tasks | Source of Items/Tasks | Range of Raw Scores | Mean (SD) of Raw Scores |
| Orientation (time and place) | 6 | MMSE (11) | 0–6 | 3.3 (2.1) |
| Immediate verbal memory (object) | 3 | MMSE (11) | 0–3 | 2.7 (0.8) |
| Immediate verbal memory (story) | 6 | East Boston Memory Test (12) | 0–6 | 3.4 (1.7) |
| Delayed verbal memory (object) | 3 | MMSE (11) | 0–3 | 2.2 (1.0) |
| Delayed verbal memory (story) | 6 | East Boston Memory Test (12) | 0–6 | 3.3 (1.8) |
| Attention (serial 3s) | 5 | Weschler Adult Intelligence scale (13) | 0–5 | 3.8 (1.8) |
Note: MMSE = Mini-Mental State Examination.
We obtained a global cognitive function score by summing the standardized scores from each of the six neuropsychological tests (Table 1). Composite scores are commonly used because they integrate information from multiple sources and provide a more stable representation of cognitive function than a single test (14). We also derived a separate memory composite score by summing the standard scores from each of the four memory tests as previous results from the same sample suggested no association with a limited measure of delayed verbal memory (7). We defined cognitive impairment as the lowest 10% of the distribution of cognitive performance. Such a population-based cutoff point is a sensitive and specific indicator of cognitive impairment (15) and has been used previously (16–18).
Statistical Analyses
Multivariate logistic regression models were used to determine the relationship of serum 25(OH)D to cognitive impairment. We divided the levels of serum 25(OH)D into clinically relevant groups to aid interpretation: severely 25(OH)D deficient (defined as <25 nmol/L), 25(OH)D deficient (defined as ≥25 < 50 nmol/L), 25(OH)D insufficient (defined as ≥50 < 75 nmol/L), and sufficient 25(OH)D (defined as ≥75 nmol/L) (19,20). In fully adjusted models, we controlled for the following variables that have previously been identified as potential confounders in studies of 25(OH)D and cognition (4): age in years, sex, race/ethnicity (white, black, or other), education in years, season during which blood samples were obtained (to account for seasonal variation in sunlight exposure), current smoking status, body mass index (kg/m2), alcohol consumption (0 drinks/month, 1–10 drinks/month, or ≥11 drinks/month), serum vitamin E (alpha tocopherol [μmol/L]), total combined family income during the last 12 months (<$20,000, $20,000–34,999, or ≥$35,000), impaired mobility (mean gait speed ≤0.4 m/s during two-timed 4-m walks at normal pace) (21), and limited physical activity (not having walked a mile, cycled, swam, or undertaken gardening/yard work in the past month).
In a series of secondary analyses, we examined whether any observed association was mediated by medical conditions that are thought to be associated with both 25(OH)D deficiency (22–24) and cognitive impairment (25): diabetes (using antidiabetic agent, self-reported physician diagnosis, or having fasting plasma glucose ≥126 mg/dL), stroke (self-reported physician diagnosis), and untreated and treated hypertension (using antihypertensive drugs, self-reported physician diagnosis, and mean systolic ≥140 mmHg or diastolic ≥90 mmHg from three readings). We also examined whether 25(OH)D levels were associated with memory impairment in a series of further logistic regression models. Sufficient 25(OH)D is also commonly defined as ≥50 nmol/L (19). We therefore compared those who were severely deficient (defined as <25 nmol/L) or deficient (defined as ≥25 < 50 nmol/L) with this broader reference group. We examined whether natural log-transformed levels of vitamin D were also associated with the odds of cognitive impairment. Finally, we investigated possible two-way interactions between 25(OH)D and age or sex by including product terms in a fully adjusted model.
Because differential participation, the deliberate oversampling of minority groups, and similarities between related observations have the potential to bias results, we performed weighted logistic regression analyses using official NHANES III population weights, clusters, and strata. Two-sided statistical tests were used throughout, and the Type I error rate for statistical significance was preset at .05. All analyses were performed using Stata SE version 10.1 (StataCorp, College Station, TX).
RESULTS
The study population is described in Table 2. Participants who were cognitively impaired were more likely to be 25(OH)D deficient or severely deficient in comparison with those who were cognitively normal when unadjusted frequencies were compared. A number of further differences were observed between the characteristics of participants who were cognitively impaired and those who were cognitively normal. Those who were cognitively impaired were also more likely to be older, of black or other race/ethnicity, tested between September and November, physically inactive, have impaired mobility and diabetes, and have lower education, body mass index, alcohol consumption, serum vitamin E, and income.
Table 2.
Baseline Characteristics of Elderly Participants in Third National Health and Nutrition Examination Survey According to Cognitive Status*
| Characteristic | All Participants (n = 3,396) | Cognitively Normal (n = 3,056) | Cognitively Impaired (n = 340) | p† |
| Serum 25-hydroxyvitamin D (nmol/L) | ||||
| ≥75 | 1,104 (37.5) | 1,019 (37.9) | 85 (30.1) | <.001 |
| ≥50 < 75 | 1,306 (39.0) | 1,194 (39.4) | 112 (31.3) | |
| ≥25 < 50 | 894 (21.8) | 770 (21.2) | 124 (31.7) | |
| <25 | 92 (1.8) | 73 (1.5) | 19 (7.0) | |
| Age (y), M (SD) | 73.7 (10.9) | 72.6 (11.3) | 76.6 (12.6) | <.001 |
| Women | 1,736 (55.2) | 1,571 (55.4) | 165 (52.7) | .4 |
| Race/ethnicity | ||||
| White | 2,744 (90.9) | 2,521 (91.9) | 223 (74.0) | <.001 |
| Black | 594 (7.6) | 488 (6.7) | 106 (23.0) | |
| Other | 58 (1.5) | 47 (1.4) | 11 (3.0) | |
| Education (y), M (SD) | 10.7 (8.1) | 11.3 (8.2) | 7.7 (7.9) | <.001 |
| Season tested | ||||
| December to February | 700 (13.4) | 615 (13.4) | 85 (13.8) | .04 |
| March to May | 898 (29.5) | 823 (29.7) | 75 (25.6) | |
| June to August | 824 (31.5) | 780 (32.1) | 44 (21.9) | |
| September to November | 974 (25.6) | 838 (24.9) | 136 (38.8) | |
| Current smoker | 424 (12.8) | 379 (12.7) | 45 (14.8) | .6 |
| Body mass index (kg/m2), mean (SD) | 26.6 (6.7) | 26.8 (9.1) | 25.2 (8.0) | .001 |
| Alcohol consumption | ||||
| 0 drinks/mo | 2,314 (62.0) | 2,049 (61.4) | 265 (72.1) | .03 |
| 1–10 drinks/mo | 571 (18.4) | 523 (18.6) | 48 (16.2) | |
| ≥11 drinks/mo | 511 (19.6) | 484 (20.1) | 27 (11.8) | |
| Serum vitamin E (μmol/L), mean (SD) | 32.8 (20.8) | 33.4 (21.1) | 27.6 (18.8) | <.001 |
| Combined annual family income | ||||
| <$20,000 | 2,137 (52.6) | 1,855 (51.4) | 282 (74.6) | <.001 |
| $20,000–34,999 | 781 (26.8) | 742 (27.4) | 39 (15.2) | |
| ≥$35,000 | 478 (20.6) | 459 (21.2) | 19 (10.2) | |
| Impaired mobility | 289 (5.9) | 213 (5.1) | 76 (20.7) | <.001 |
| Physically inactive | 1,107 (25.9) | 949 (24.9) | 158 (44.2) | <.001 |
| Stroke | 245 (6.2) | 211 (6.1) | 34 (8.3) | .1 |
| Diabetes | 695 (16.7) | 612 (16.3) | 83 (24.0) | .02 |
| Hypertension | ||||
| Untreated | 1,109 (30.9) | 982 (30.7) | 127 (34.1) | .6 |
| Treated with antihypertensive drugs | 1,207 (34.6) | 1,098 (34.8) | 109 (31.8) | |
Notes: *Data are expressed as number and percentage unless otherwise indicated. Means and percentages are weighted to adjust for the sampling design.
p Value for χ2 test for categorical variables and analysis of variance for continuous variables.
In an unadjusted logistic regression model, participants who were severely 25(OH)D deficient were more likely to suffer from cognitive impairment (Table 3). Significant linear trends between groups suggested a monotonic relationship. These associations were attenuated but remained significant following adjustment for age, sex, race/ethnicity, education, season tested, current smoking status, body mass index, alcohol consumption, serum vitamin E, combined family income, impaired mobility, and physical inactivity. In fully adjusted models, those who were severely 25(OH)D deficient were around four times more likely to be cognitively impaired. Additional adjustment for stroke, diabetes, and hypertension slightly attenuated the association between 25(OH)D and cognitive impairment (Table 3). Post hoc analyses revealed that the observed attenuation was due to the influence of diabetes rather than of stroke or of hypertension, perhaps suggesting partial mediation of the association by diabetes (data not shown).
Table 3.
Logistic Regression Models Illustrating Odds of Cognitive Impairment (95% Confidence Intervals) by Serum 25-hydroxyvitamin D3 (25(OH)D) Levels*
| Unadjusted Model | Fully Adjusted Model† | Fully Adjusted Model Plus Medical Conditions‡ | |
| Serum 25(OH)D (nmol/L) | |||
| ≥75§ | 1.0 | 1.0 | 1.0 |
| ≥50 < 75 | 1.00 (0.66–1.51) | 0.86 (0.59–1.26) | 0.83 (0.57–1.22) |
| ≥25 < 50 | 1.88 (1.30–2.73) | 1.42 (0.96–2.09) | 1.33 (0.90–1.97) |
| <25 | 5.97 (2.82–12.61) | 3.94 (1.49–10.43) | 3.68 (1.37–9.90) |
| p for linear trend | <.001 | .017 | .033 |
Notes: *Cognitive impairment was defined as the worst 10% of the distribution of scores. Population weights are used to adjust for the sampling design.
Adjusted for age, sex, race/ethnicity, education, season tested, current smoking status, body mass index, alcohol consumption, serum vitamin E, combined family income, impaired mobility, and physical inactivity.
Adjustment as for fully adjusted model plus medical conditions that may mediate the association between 25(OH)D and cognitive impairment (stroke, diabetes, untreated hypertension, and hypertension treated with antihypertensive drugs).
Participants with serum 25(OH)D concentrations ≥75 nmol/L served as the reference group.
In a further unadjusted logistic regression model, participants who were severely 25(OH)D were more likely to have impaired memory (Table 4), and there was evidence for a monotonic relationship. However, participants who were 25(OH)D insufficient were less likely to have impaired memory than those who were sufficient. The association became less clear in the fully adjusted model, and there was no longer the suggestion of a monotonic association. Additional adjustment for stroke, diabetes, and hypertension did not change the pattern of associations.
Table 4.
Logistic Regression Models Illustrating Odds f Memory Impairment (95% confidence intervals) by Serum 25-hydroxyvitamin D3 (25(OH)D) Levels*
| Unadjusted Model | Fully Adjusted Model† | Fully Adjusted Model Plus Medical Conditions‡ | |
| Serum 25(OH)D (nmol/L) | |||
| ≥75§ | 1.0 | 1.0 | 1.0 |
| ≥50 < 75 | 0.69 (0.52–0.92) | 0.59 (0.43–0.83) | 0.58 (0.42–0.80) |
| ≥25 < 50 | 1.45 (1.01–2.08) | 1.18 (0.78–1.81) | 1.12 (0.73–1.72) |
| <25 | 4.46 (2.07–9.59) | 3.18 (1.20–8.44) | 2.98 (1.13–7.88) |
| p for linear trend | .011 | .18 | .26 |
Notes: *Memory impairment was defined as the worst 10% of the distribution of scores. Population weights are used to adjust for the sampling design.
Adjusted for age, sex, race/ethnicity, education, season tested, current smoking status, body mass index, alcohol consumption, serum vitamin E, combined family income, impaired mobility, and physical inactivity.
Adjustment as for fully adjusted model plus medical conditions that may mediate the association between 25(OH)D and cognitive impairment (stroke, diabetes, untreated hypertension, and hypertension treated with antihypertensive drugs).
Participants with serum 25(OH)D concentrations ≥75 nmol/L served as the reference group.
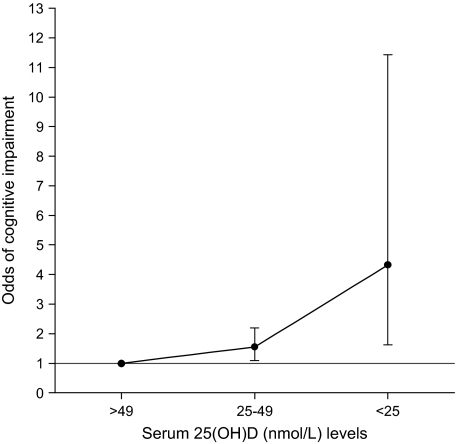
When a broader definition of 25(OH)D sufficiency was adopted, a highly similar pattern of associations was observed (Figure 1). Participants who were 25(OH)D deficient (≥25 < 50 nmol/L) and severely deficient (<25 nmol/L) in comparison with those sufficient (≥50 nmol/L) were again more likely to be cognitively impaired (p for linear trend = .002). A fully adjusted logistic regression model incorporating natural log-transformed levels of 25(OH)D rather than predefined categories also indicated that low levels of 25(OH)D were associated with greater odds of cognitive impairment (p = .015). No significant two-way interactions were observed between 25(OH)D levels and sex (p = .34) or age (p = .92).
Figure 1.
Odds of cognitive impairment by serum 25-hydroxyvitamin D [25(OH)D] levels. Bars indicate 95% confidence intervals. Cognitive impairment was defined as the worst 10% of the distribution of scores. Population weights are used to adjust for the sampling design. Results are also adjusted for age, sex, race/ethnicity, education, season tested, current smoking status, body mass index, alcohol consumption, serum vitamin E, combined family income, impaired mobility, and physical activity.
DISCUSSION
In this large population-based study, we found that elderly people with low levels of 25(OH)D were at increased risk of cognitive impairment, and there was evidence of a monotonic relationship. The association remained significant following adjustment for a wide range of potential confounders. The association between 25(OH)D and memory impairment was unclear. This is one of the first population-based studies to show that 25(OH)D deficiency is associated with a greater risk of cognitive impairment.
The strengths of this study include the statistical adjustment for a wide range of potentially confounding variables, such as sociodemographics, health behaviors, clinical status, and dietary factors. Response rates were high, and minimal bias is likely due to attrition. The assessment of cognitive impairment included a range of neuropsychological tests that assess different cognitive domains. NHANES III is a large well-characterized study, which incorporates a diverse sample that is representative of the U.S. population. These results are therefore more likely to generalize to other populations. A number of limitations should be considered when interpreting our findings. Low levels of 25(OH)D may reflect the limited outdoor activity of participants who are cognitively impaired and it is not possible to discount the possibility of reverse causality on the basis of cross-sectional evidence. However, we adjusted for impaired mobility and physical inactivity, which did not greatly attenuate the association. Although the response rate was high, it is still possible that missing data and nonresponse biased our results. We therefore conducted weighted analyses to allow for nonrandom attrition.
It is not likely that the difference observed between our findings and those reported in a previous analysis of NHANES III (7) reflects differences in adjustment for potential confounders as both our unadjusted and fully adjusted models suggested a robust association with cognitive impairment. More likely, the differences observed reflect the different cognitive measures incorporated. In the previous analysis, the raw scores of the delayed element of the object and story recall tasks were summed to derive a “memory and learning score.” In our analyses of cognitive impairment, we utilized a composite score, which incorporated the full range of NHANES III cognitive measures (additional tests of orientation, immediate verbal memory, and attention). By integrating information from multiple sources in this way, we obtained a more stable representation of cognitive function than a single measure (14). It is also possible that 25(OH)D levels are primarily associated with cognitive dysfunction in domains other than memory as we have previously hypothesized (4). We observed no clear association with memory impairment utilizing a composite score, which incorporated the full range of NHANES III memory measures.
Our findings are consistent with recent European studies that have suggested that low 25(OH)D levels are associated with increased odds of cognitive impairment (4) and poor sustained attention but not memory or visuospatial ability (5). Similarly, low 25(OH)D levels in homebound elderly adults from Boston were associated with poor executive function and processing speed but not memory (6). Further research incorporating a comprehensive neuropsychological test battery is therefore needed to confirm whether vitamin D deficiency is primarily associated with frontal lobe deficits, such as impaired executive function, processing speed, and attention. In the meantime, caution should be exercised when interpreting results from studies that only incorporate measures of memory or otherwise limited measures of cognitive function.
Several mechanisms have been proposed to explain why vitamin D deficiency may increase the odds of cognitive impairment. Vitamin D deficiency may increase the risk of stroke (23), diabetes (22), and hypertension (26), and these conditions may in turn be associated with cognitive impairment (25). Our findings provide some support for the possibility that diabetes may partially mediate the association, although stroke and hypertension appear unlikely mediators. Vitamin D receptors are present in a wide variety of cells, including neurons and glial cells, and genes encoding the enzymes involved in the metabolism of vitamin D are also expressed in the brain (1). A series of animal and in vitro studies suggest that vitamin D influences antioxidative mechanisms, neurogenesis, neuronal calcium homeostasis, and the expression of neurotrophic factors and enhances nerve conduction and detoxification (1–3). Vitamin D may play a role in brain detoxification pathways by reducing cellular calcium, inhibiting the synthesis of nitric oxide synthase and protecting neurons from reactive oxygen species by increasing levels of glutathione (27). Vitamin D stimulates neurogenesis and regulates the synthesis of neurotrophic factors, which are important for cell differentiation and survival (1,28). Vitamin D is also an immunosuppressor and inhibits autoimmune damage to the nervous system (27). Vitamin D stimulates amyloid-beta clearance and phagocytosis while protecting against programmed cell death (3). Vitamin D deficiency may also be associated with an increased risk of neurological diseases, such as multiple sclerosis (29) and Parkinson’s (30).
We discovered that low levels of 25(OH)D were associated with increased odds of cognitive impairment and that this association remained after adjusting for a wide range of potential confounders. Taken together, the results from several large population-based studies in Europe and the United States suggest that vitamin D deficiency is associated with cognitive impairment. Further research is needed to clarify the association between vitamin D deficiency and memory. Neuroimaging and neuropathological studies would also be useful to identify the mechanisms underlying these associations. The correction of vitamin D deficiency may have immense potential for the prevention of cognitive impairment and dementia, given the high prevalence of deficiency and the easy, inexpensive, and safe way in which vitamin D can be supplemented. Future prospective studies and randomized controlled trials to more clearly identify a potential causal relationship between Vitamin D deficiency and cognitive impairment are therefore warranted.
FUNDING
NHANES III was supported by the U.S. government. Dr. Lang was supported by the National Health Service South-West Region Public Health Training Scheme. Dr. Langa was supported by the National Institute on Aging (grant R01 AG027010).
CONFLICT OF INTEREST
None reported.
Acknowledgments
The sponsors played no part in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
References
- 1.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 2.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29(6):415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoumi A, Goldenson B, Ghirmai S, et al. 1Alpha, 25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2009;17(3):703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin d concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(3):188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatr. 2009;80(7):722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 6.Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64(8):888–895. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29(1–2):49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 8.Gunter EW, Lewis BL, Koncikowski SM. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. [Google Scholar]
- 9.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 10.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Gfeller JD, Horn GJ. The East Boston Memory Test: a clinical screening measure for memory impairment in the elderly. J Clin Psychol. 1996;52(2):191–196. doi: 10.1002/(SICI)1097-4679(199603)52:2<191::AID-JCLP10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D. San Antonio, TX: The Psychological Corporation; 1997. WAIS-III Administration and Scoring Manual. [Google Scholar]
- 14.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48(4):M152–M161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 16.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60(2):198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 17.Llewellyn DJ, Lang IA, Langa KM, Naughton F, Matthews FE. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. BMJ. 2009;338:b462. doi: 10.1136/bmj.b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Melzer D. Neighbourhood deprivation and incident mobility disability in older adults. Age Ageing. 2008;37(4):403–410. doi: 10.1093/ageing/afn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 23.Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 25.Hendrie HC, Albert MS, Butters MA, et al. The NIH cognitive and emotional health project: report of the critical evaluation study committee. Alzheimers Dement. 2006;2(1):12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Forman JP, Bischoff-Ferrari HA, Willett WC, Stampfer MJ, Curhan GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. 2005;46(4):676–682. doi: 10.1161/01.HYP.0000182662.82666.37. [DOI] [PubMed] [Google Scholar]
- 27.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 28.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-Dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 29.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 30.Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65(10):1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]