SYNOPSIS
Frailty is an important geriatric syndrome characterized by multi-system dysregulation. Substantial evidence suggests heightened inflammatory state and significant immune system alterations in frailty. A heightened inflammatory state is marked by increases in levels of inflammatory molecules (IL-6 and CRP) and counts of white blood cell and its subpopulations, which may play an important role in the pathogenesis of frailty, directly or through its detrimental influence to other physiologic systems. Alterations in the innate immune system include decreased proliferation of the peripheral blood mononuclear cells (PBMCs) and upregulated monocytic expression of specific stress responsive inflammatory pathway genes. In the adaptive immune system, while little information is available about potential B-cell changes, significant alterations have been identified in the T-cell compartment including increased counts of CD8+, CD8+CD28−, CCR5+ T cells, above and beyond age-related senescent immune remodeling.
Keywords: Frailty, inflammation, IL-6, monocytic gene expression, T cells
INTRODUCTION
Frailty is an important and common geriatric syndrome. It is characterized as a state of decreased physiologic reserve and increased vulnerability for subsequent morbidity and mortality (1–3). Frailty is also described as a clinical phenotype in old age with a loss of complexity in resting dynamics involving multiple physiologic systems, manifested by maladaptive response to stressors, leading to a vicious cycle towards functional decline and other serious adverse health outcomes (2;4). The phenotypic characteristics of frail older adults are now recognized to be a syndrome consisting of three or more of the following: weakness (by grip strength), low physical activity, slowed motor performance (by walking speed), exhaustion, and unintentional weight loss (1). The frailty definition developed by a group of investigators at Johns Hopkins University defines frailty as a syndrome when these phenotypic characteristics are present. This status independently predicts a number of serious adverse health outcomes, including acute illness, falls, hospitalization, disability, dependency, and mortality, adjusting for comorbidities (1;5). This phenotype definition has been favorably evaluated and compared to other proposed frailty criteria, and has been validated in a number of large older adult cohorts as well as in various clinical and cultural settings (6–9). Based on this definition, the estimated prevalence of frailty is 7–10% among community-dwelling men and women age 65 and older, and up to one-third of those aged 80 years and older (1;10). Because of the profound functional, medical, and socioeconomic consequences of the frailty syndrome, it is imperative to advance our understanding of the pathogenesis and physiologic impact of this syndrome and, with this information, to develop interventional strategies.
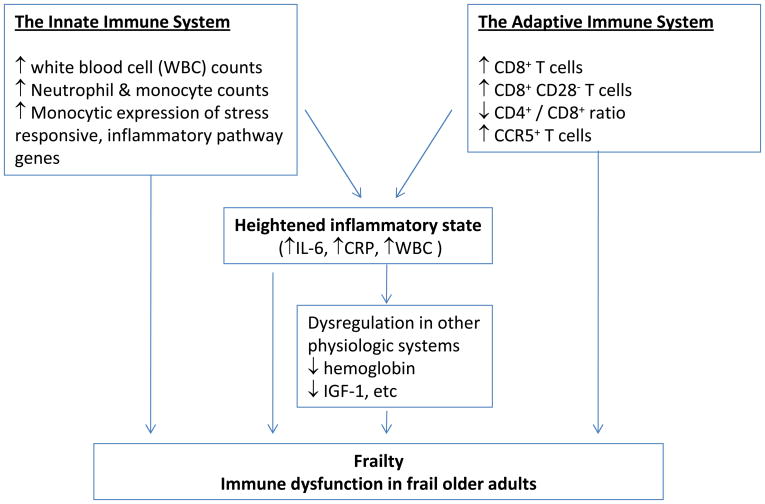
A large body of literature, most rapidly accumulated in the past few years, suggests that frail older adults manifest multi-system dysregulation including that in the musculoskeletal, immune, endocrine, hematologic, and cardiovascular systems, to just name a few (11;12). This article will focus on frailty-associated inflammation and immune system alterations which, for the ease of discussion, are categorized as heightened inflammation and alterations in the innate and adaptive immunity (Figure 1). As described below, this heightened inflammatory state appears to play an important role, directly or through its adverse influence on other intermediary pathophysiologic processes, in the pathogenesis of frailty. Importantly, alterations in the innate and adaptive immunity likely also lead to heightened inflammation as well as impairment in vaccine-induced immune protection and increased susceptibility to infections in frail older adults.
Figure 1.
Inflammation and Immune System Alterations in Frailty
THE INNATE IMMUNE SYSTEM AND FRAILTY
The innate immune system is the front line of defense against injury and infection in most living organisms. It provides an immediate response to external stressors, and as such is critical in the development and shaping of immune responses which, in turn, play a central role in inflammation and immune protection against infections. The major cellular components of the innate immune system include neutrophils, monocytes, and dendritic cells, although multiple other cell types such as fibroblasts and hepatocytes are also capable of mounting an inflammatory response to stressors. Emerging evidence from a variety of sources suggests that the innate immune system is altered in frailty. The cellular and molecular data presented below support that the innate immune system is overall more active in frail compared to non-frail older adults.
Elevated Markers of Inflammation in Frailty
The aging immune system is characterized by a low grade, chronic systemic inflammatory state, so-called “InflammAging” (13). This inflammatory phenotype is marked by elevated inflammatory molecules and is associated with increased morbidity and mortality in older adults (14;15). C-reactive protein (CRP) and pro-inflammatory cytokines IL-6 are well known such inflammatory molecules. Peripheral white blood cell (WBC) and its subpopulations are circulating immune cells and cellular inflammatory components. Clinically, increase in WBC counts is recognized as an important cellular marker of systemic inflammation. Recent studies have provided a large body of evidence suggestive of a heightened inflammatory state in frail older adults as marked by further increases of these molecular and cellular inflammatory markers compared to that observed in non-frail, robust older individuals.
IL-6 is a pro-inflammatory cytokine with increased circulating levels in older adults. Age-related increases in IL-6 levels are associated with several pathophysiologic processes, including atherosclerosis, osteoporosis, and sarcopenia, and with functional decline, disability, and all-cause mortality in older adults (16–19). In addition, increased IL-6 levels are associated with lower muscle mass and strength even in well-functioning older men and women (19;20). In a longitudinal study, Ferrucci and colleagues reported that elevated IL-6 levels at baseline predict a significantly higher risk for the development of physical disability and a steeper decline in muscle strength and motor performance during a follow-up period of 3.5 years in older women living in the community (21). This study and others have shown that chronic systemic inflammation marked by elevated IL-6 levels is associated with decreased muscle strength and power and slowed walking speed, two central components of the frailty syndrome. Direct evidence supporting the relationship of this molecular inflammatory marker with frailty came first from a pilot study in which community-dwelling frail older adults had significantly higher IL-6 levels than non-frail controls with similar age (22). A subsequent age, race, and sex-matched pair study has further demonstrated that frail older adults had significantly higher IL-6 production by the peripheral blood mononuclear cells (PBMCs), upon stimulation with lipopolysaccharide (LPS), compared to the non-frail controls (23). In addition, recent studies in large cohorts of older women have demonstrated that elevated IL-6 levels are independently associated with frailty (24;25). In an IL-10 knockout mouse model for frailty, older mice with phenotype mimicking human frailty had elevated IL-6 levels compared to the control wild type mice (26). These clinical, laboratory, and population studies have provided strong evidence for the association of this important proinflammatory cytokine with frailty in older adults living in the community.
C-Reactive Protein (CRP), discovered in 1930 as an acute phase reactant, is a classic circulating molecular marker of systemic inflammation (27). Elevated CRP levels are associated with cardiovascular (28). Clinically, CRP has now been integrated as part of the routinely measured panel of cardiovascular disease risk factors. Two large cohort studies have demonstrated the direct association of this molecular inflammatory marker with frailty. In the Cardiovascular Health Study (CHS), Walston and colleagues have shown that significant association of elevated CRP levels with frailty after excluding cardiovascular disease and diabetes and adjusting for basic demographic characteristics (29). Data from the Longitudinal Aging Study Amsterdam (LASA) have further confirmed these findings (30).
As part of the complete blood counts (CBC), WBC count is a standardized and widely available laboratory measurement. Acute and dramatic increase in total WBC counts (above the normal range) is recognized as a clinical indicator for systemic inflammation, frequently due to acute bacterial infections. A number of large cohort studies in older adults, have demonstrated that elevated WBC count, albeit within the normal range, is associated with cardiovascular and cerebrovascular events, cardiovascular and cancer mortality, as well as all-cause mortality in older adults (31–33). Recent studies have demonstrated direct relationship of frailty with elevated WBC counts of WBC as well as elevated counts of neutrophils and monocytes (24;34). A potential synergistic interaction between WBC and IL-6 in their associations with frailty has also been suggested (24). The potential synergy between these two commonly recognized cellular and molecular inflammatory components in frailty is further supported by the laboratory study cited above in which the PBMCs, the isolated WBC subpopulations, from frail older adults had significantly higher LPS-induced IL-6 production than that from matched non-frail controls (23). In addition, direct in vivo association between circulating IL-6 levels and WBC counts has been demonstrated in the same study cohort (35).
Molecular Inflammatory Pathway Activation in Frailty
Molecular evidence for inflammatory pathway activation in frailty has recently emerged through in-depth analyses of ex vivo expression of inflammatory pathway genes by purified monocytes (36;37). Utilizing molecular and genetic techniques including pathway-specific gene array analysis and quantitative real time reverse transcriptase-polymerase chain reaction (RT-PCR), these studies have shown frailty-associated upregulation in monocytic expression of CXCL10 gene that encodes a potent pro-inflammatory chemokine (36). Moreover, purified monocytes from frail older adults had consistent upregulation in ex vivo expression of seven stress-responsive inflammatory pathway genes upon LPS stimulation compared to those from matched non-frail older adult controls (37). These genes encode transcription factors, signal transduction proteins, as well as chemokines and cytokines. Findings from these in-depth molecular analyses have demonstrated frailty-associated upregulation in the expression of specific inflammatory pathway genes by monocytes, a major cell type of the innate immune system. As a potential underlying molecular and immune mechanism, upregulated expression of these inflammatory pathway genes could lead to the heightened inflammatory state in frail older adults described above. This possibility is further suggested by the correlation between frailty-associated CXCL10 upregulation and elevation of serum IL-6 levels, albeit the directionality of this association remains to be determined (36). In addition, frailty-associated upregulation in monocytic expression of hydrogen peroxide (H2O2)-induced clone 5 (hic-5), a transcription factor that is known to respond to oxidative stress and play an important role in cellular senescence (38), suggests a mechanistic link of oxidative stress and cellular senescence with frailty. Such mechanistic link can potentially open novel investigational avenues in frailty research. Moreover, the identified inflammatory pathway genes are stress-responsive genes whose products are known to play a role in stress responses in various study settings (38–42). This is consistent with the cardinal feature of frailty that frail elderly manifest increased vulnerability to stressors. Clinically, frail older adults may experience LPS exposure surge(s) during Gram (−) bacterial infections, such as urinary tract infection and urosepsis.
Potential role of Heightened Inflammation in Frailty
Although the consequences of this chronic activation is unclear, evidence is also emerging that chronic exposure to inflammatory mediators may be in part responsible for a host of tissue changes and susceptibility to the development of chronic disease states. As discussed above, the relationship between frailty and common molecular and cellular inflammatory markers are well documented. The critical question is whether this heightened inflammation plays a role in the pathogenesis of frailty. Individual inflammatory molecules, such as IL-6, may directly contribute to frailty or its central components (such as decreased muscle strength/power and slowed motor performance). In addition, frailty involves multiple physiologic organ systems, such as musculoskeletal system (sarcopenia, osteopenia), hematologic system (anemia), cardiovascular system (clinical or subclinical cardiovascular diseases), and endocrine system (decreased insulin-like growth factor-1 [IGF-1], decreased DHEA-S, and insulin resistance, etc.) (11;12;43). It is conceivable that heightened inflammation contributes to frailty through its detrimental effects (functional impairment and/or structural damage) to these physiologic organ systems. In fact, studies have shown that circulating IL-6 levels have inverse associations with hemoglobin concentration and IGF-1 levels in frail older adults, but not in non-frail controls; low hemoglobin and IGF-1 levels are each independently associated with frailty, as well (22;43). In addition, WBC counts have an inverse association with IGF- levels (44). Therefore, it is proposed that the heightened inflammatory state plays a key role in the pathogenesis of frailty, directly or through other intermediate pathophysiologic processes.
THE ADAPTIVE IMMUNE SYSTEM AND FRAILTY
Significant remodeling occurs in the adaptive immune system during aging. This includes age-related loss of CD28 expression, skewing immune repertoire to the memory phenotype, T cell clonal expansion, increased autoimmune antibody production, and altered cytokine expression. This is considered to be responsible, at least in part, for the inflammatory phenotype or “InflammAging”, poor immune response to vaccination, and overall immune functional decline observed in older adults. In the syndrome of frailty, increasing evidence supports significant alterations in the T cell compartment of the adaptive immune system. The first line of evidence comes from a post hoc analysis of the data from a nested-case control study evaluating the relationship between T cell subsets and mortality in community-dwelling older women (45). The results showed that frail older women had significantly higher counts of CD8+ and CD8+CD28− T cells compared to non-frail older women (n=24) matched by age and major comorbidities (cancer, arthritis, diabetes, cardiovascular disease, hypertension, and hormone replacement therapy). While no difference was observed in CD4+ T cell frequencies between the two study groups, the frail group had significantly lower CD4+:CD8+ ratio compared to the non-frail group.
The second line of evidence comes from studies in the Multi-center acquired immune deficiency syndrome (AIDS) Cohort Study (MACS) comprised of immunodeficiency virus (HIV) positive and negative gay men. In the MACS study cohort, Desquilbet and colleagues developed a frailty-related phenotype (FRP) which includes 4 of the 5 Fried’s frailty criteria with measured walking speed being substituted by self-reported difficulty in walking. The results showed that compared to HIV-uninfected men of similar age, ethnicity and education, HIV-infected men were more likely to have FRP for all durations of HIV infection (< 4, 4.01 – 8, and 8.01 – 12 years) prior to the era of highly active antiretroviral therapies (HAAT). In addition, among HIV serconverters, men HIV infected for ≤4 years had FRP prevalence comparable to HIV-uninfected men 10 years older (46). A subsequent study in the MACS cohort demonstrated that CD4+ T cell count predicted the development of a FRP among HIV-infected men, independent of HAART use and plasma HIV viral load (47). These findings suggest a role of CD4+ T cell dysregulation in the development of frailty in HIV infected patient population.
In addition, a pilot study in thirteen pairs of age, race, and sex-matched frail and non-frail older adults living in the community with mean age of 84 years (range: 72–94) has shown that frail participants had increased counts of T cells expressing chemokine CC receptor-5 (CCR5) compared to the matched non-frail controls (48). The increase of CCR5+ T-cell frequencies in the frail elderly cannot be contributed to the frailty-associated CD8+ T-cell expansion as such an increase was also observed in the CD8+ T-cell compartment. In addition, there was a trend toward graded increase in CCR5+ T-cell counts across the frailty scores in the frail participants (48). CCR5+ T cells have a type-1 pro-inflammatory phenotype and contribute significantly to several inflammatory conditions (49;50). Moreover, CCR5 is a well known co-receptor for type-1 HIV (HIV-1); active development of anti-CCR5-based therapies for HIV infection and AIDS has shown promising results (51;52). Therefore, findings from this pilot study, if validated, suggest that anti-CCR5-based strategies can potentially be developed for the prevention or delay and treatment of frailty in older adults.
Information about potential B-cell alteration in frailty is extremely limited. Utilizing spectratype analysis of the immunoglobulin (CDR)3 region, a recent study evaluated and compared B-cell repertoire diversity between elderly participants from the Swedish NONA Immune Study and young adults (53). The results showed an age-related decrease in B-cell diversity and a dramatic collapse of the B-cell repertoire in a subset of older individuals who were considered frail. However, details on how frailty was defined in that study were lacking. Ongoing studies indicate that frail older adults have significant impairment in their antibody response to influenza vaccination compared to the non-frail counterparts (Leng S, unpublished data). It is not clear, however, whether this impairment is caused by alterations in the B-cell compartment or secondary to the frailty-associated T-cell dysregulation.
In summary, emerging evidence suggests significant alterations in the adaptive immune system in frailty, particularly in the T-cell compartment, above and beyond age-related immune remodeling. Further investigations into B cell function and regulation in frailty are needed.
SUMMARY
This article provides an overview of current understanding of inflammation and immune system alterations in frailty. The immune system alterations observed in frailty are multi-facet, including the heightened inflammation and alterations in the innate and adaptive immune systems. The identified alterations indicate significant immune dysregulation that is likely responsible for the overall immune functional decline and increased susceptibility to infections in the frail older adult population. In addition, they may play an important role in the pathogenesis of frailty. Furthermore, they include molecular targets, such as CXCL10 and CCR5, for potential development of novel interventional strategies both for the treatment of frailty and for immune functional improvement in this vulnerable elderly population.
Acknowledgments
We would like to thank members of the Biology of Frailty Program at Johns Hopkins for their input and support. We would also like to thank Mrs. Denise Baldwin for her excellent secretarial support. Dr. Leng is a current recipient of the Paul Beeson Career Development Award in Aging Research funded by the National Institute on Aging and Private Foundations, K23 AG028963.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fried LP, Tangen C, Walston J, Newman A, Hirsch CH, Gottdiener JS, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001 March;56A(3):M1–M11. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Hadley EC, Walston JD, Newman A, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005 August 3;2005(31):e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 3.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006 June;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):115–25. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007 July;62(7):738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 7.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005 August;53(8):1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 8.Corapi KM, McGee HM, Barker M. Screening for frailty among seniors in clinical practice. Nat Clin Pract Rheumatol. 2006 September;2(9):476–80. doi: 10.1038/ncprheum0288. [DOI] [PubMed] [Google Scholar]
- 9.Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet. 2007 April 21;369(9570):1328–9. doi: 10.1016/S0140-6736(07)60613-8. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 March;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009 October;64(10):1049–57. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leng SX, Tian XP, Qu T. Pathophysiology of frailty: inflammatory, immune, or endocrine? Journal of General Medicine. 2008;(20):27–32. [Google Scholar]
- 13.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, De BG. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 June;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 14.De MM, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006 June;80(3):219–27. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003 October 15;115(6):429–35. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41(2):176–81. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 17.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006 June;61(6):575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999 May;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 19.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002 April;50(4):638–44. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 20.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 22.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002 July;50(7):1268–71. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 23.Leng S, Yang H, Walston J. Decreased Cell Proliferation and Altered Cytokine Production in Frail Older Adults. Aging Clin Exp Res. 2004 August;16:249–52. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 24.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007 June;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005 May;53(5):747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 26.Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008 April;63(4):391–8. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillet W, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. Journal of Experimental Medicine. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. 2007 September 18;50(12):1115–22. doi: 10.1016/j.jacc.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Walston J, McBurnie MA, Newman A, Tracy R, Kop WJ, Hirsch CH, Gottdiener JS, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002 November;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 30.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005 October;63(4):403–11. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 31.Leng SX, Xue QL, Huang Y, Ferrucci L, Fried LP, Walston JD. Baseline total and specific differential white blood cell counts and 5-year all-cause mortality in community-dwelling older women. Exp Gerontol. 2005 December;40(12):982–7. doi: 10.1016/j.exger.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005 March 14;165(5):500–8. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 33.Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007 May 8;49(18):1841–50. doi: 10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leng S, Xue Q, Tian J. Association of neutrophil and monocyte counts with fraily in community-dwelling older women. Experimental Gerontology. 2009;(44):511–6. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Leng S, Xue QL, Huang Y, Semba R, Chaves P, Bandeen-Roche K, Fried L, Walston J. Total and differential white blood cell counts and their associations with circulating interleukin-6 levels in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2005 February;60(2):195–9. doi: 10.1093/gerona/60.2.195. [DOI] [PubMed] [Google Scholar]
- 36.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009 June;46(3):319–24. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, Ferrucci L, Rose NR, Leng SX. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009 March;130(3):161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem. 2001 October 26;276(43):39911–8. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- 39.Li DP, Periyasamy S, Jones TJ, Sanchez ER. Heat and chemical shock potentiation of glucocorticoid receptor transactivation requires heat shock factor (HSF) activity. Modulation of HSF by vanadate and wortmannin. J Biol Chem. 2000 August 25;275(34):26058–65. doi: 10.1074/jbc.M004502200. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki T, Wada T, Kishimoto H, Irie-Sasaki J, Matsumoto G, Goto T, Yao Z, Wakeham A, Mak TW, Suzuki A, Cho SK, Zuniga-Pflucker JC, Oliveira-dos-Santos AJ, Katada T, Nishina H, Penninger JM. The stress kinase mitogen-activated protein kinase kinase (MKK)7 is a negative regulator of antigen receptor and growth factor receptor-induced proliferation in hematopoietic cells. J Exp Med. 2001 September 17;194(6):757–68. doi: 10.1084/jem.194.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barcellos-Hoff MH. How tissues respond to damage at the cellular level: orchestration by transforming growth factor-{beta} (TGF-{beta}) BJR Suppl. 2005;27:123–7. [Google Scholar]
- 42.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A. 2005 April 19;102(16):5808–13. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004 April;16(2):153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 44.Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009 April;64(4):499–502. doi: 10.1093/gerona/gln047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005 January;40(1–2):81–7. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007 November;62(11):1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 47.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, Jacobson LP. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009 March 1;50(3):299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De FU, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008 May;56(5):904–8. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391(6665):344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 50.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998 February 15;101(4):746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996 August 9;86(3):367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 52.Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der Ryst E. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11(11):1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 53.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009 February;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]