Abstract
Background
Epidemiological studies typically diagnose heart failure (HF) at the time of hospitalization, and have not evaluated the prevalence of HF symptoms in CKD patients without a prior HF diagnosis.
Methods and Results
We modified the Kansas City Cardiomyopathy Questionnaire (KCCQ) to detect and quantify symptoms characteristic of HF (dyspnea, edema, and fatigue) among 2,883 CKD patients without diagnosed heart failure in the Chronic Renal Insufficiency Cohort (CRIC). The KCCQ is a 23-item instrument that quantifies the impact of dyspnea, fatigue and edema on physical, social, and emotional functions (scored 0–100). The median KCCQ score was 92, and 25% had KCCQ scores < 75. Compared with cystatin C-based eGFR >50ml/min/1.73m2 (reference), eGFR 40–50, 30–40, and <30 were independently associated with lower KCCQ scores (<75); adjusted odds ratios and (95% CI): 1.38 (1.06–1.78), 1.39 (1.09–1.82), and 2.15 (1.54–3.00), respectively. Lower hemoglobin (Hb) levels also had independent associations with KCCQ <75: Hb > 14 g/dL (reference), Hb 13–14 g/dL (1.03; 0.76–1.40), Hb 12–13 g/dL (1.41; 1.04–1.91), Hb 11–12 g/dL (1.56; 1.12–2.16); and Hb<11 g/dL (1.65; 1.15–2.37).
Conclusion
CKD patients without diagnosed HF have a substantial burden of symptoms characteristic of HF, particularly among those with lower eGFR and hemoglobin levels.
Keywords: hemoglobin, glomerular filtration rate
INTRODUCTION
In prior studies, chronic kidney disease (CKD) has been associated with an increased incidence of heart failure, even in subjects with early declines in kidney function. Studies using serum creatinine have found elevated risk of heart failure among elderly persons in the Cardiovascular Health Study (CHS) with creatinine >1.4 mg/dL)1, and with estimated GFR <60 ml/min/1.73m2 in middle aged adults2 and women with coronary heart disease.3 More recently, studies using cystatin C, an alternative marker of kidney function, have demonstrated a broader association between kidney function and heart failure risk across a spectrum ranging from preclinical kidney disease to advanced CKD.4–6
The actual pathogenesis of heart failure onset in persons with CKD has not been well characterized, as epidemiological studies typically detect heart failure only after hospitalization for decompensated health status. Symptoms of heart failure likely begin insidiously long before the requirement for hospitalization, but may be under-recognized particularly in patients with kidney disease. Dyspnea and fatigue are hallmarks of the clinical syndrome of heart failure, and persistent symptoms despite medical management are associated with high mortality.7 In addition, the symptoms of dyspnea and fatigue are common in the end-stage renal disease (ESRD) population. To date, however, no studies have evaluated the association of kidney function with symptoms characteristic of heart failure, such as dyspnea and fatigue, in persons with chronic kidney disease who do not have diagnosed heart failure. We hypothesized that these would be common symptoms among persons with CKD, and may be early indicators of heart failure long before clinical diagnosis.
In the Chronic Renal Insuffiency Cohort (CRIC), we surveyed nearly all participants for the presence and severity of symptoms characteristic of heart failure, including dyspnea, fatigue, and edema using a modified version of the Kansas City Cardiomyopathy Questionnaire (KCCQ). The primary objective of this paper was to describe the prevalence and severity of symptoms characteristic of heart failure among persons with CKD who did not have previously diagnosed heart failure. Additional goals were to determine whether estimated glomerular filtration rate (eGFR), hemoglobin levels, and other clinical characteristics were associated with a higher likelihood of participants reporting clinical significant symptoms.
METHODS
Participants
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established CRIC in 2001 as an observation study to evaluate the determinants of progression to ESRD and CVD among persons with CKD.8, 9 Participants were recruited from 7 clinical centers between July 2003 and March 2007. Inclusion criteria were an estimated GFR between 20–70 ml/min/1.73m2 for persons aged 21–44, 20–60 ml/min/1.73m2 for persons aged 45–64, and 20–50 ml/min/1.73m2 for those aged 65–74. Exclusion criteria included prior transplantation, polycystic kidney disease, multiple myeloma, use of immunosuppression, and severe comorbid illnesses such as cirrhosis, HIV disease, and severe heart failure. The diagnosis of heart failure at entry into CRIC was assessed by participant response to the question at baseline: “Have you ever been diagnosed with or has a doctor or other health professional ever told you that you have heart failure?” At follow-up visits, participants were asked: “Have you been hospitalized, including an ER visit, since the last CRIC study contact for heart failure or fluid in the lungs?”
Predictors
The primary predictors for this paper were estimated glomerular filtration rate (eGFR) using creatinine or cystatin C and hemoglobin levels. Creatinine-based estimated GFR was determined using the modified MDRD equation (eGFRcrea)10, and cystatin C-based eGFR by the CKD-EPI equation (eGFRcys)11. Estimated GFR was categorized as <30, 30–39, 40–49, ≥ 50 ml/min/1.73m2 for each marker, since all participants had established CKD. Hemoglobin levels were categorized as <11, 11–11.9, 12–12.9, 13–13.9, ≥ 14 g/dL.
Secondary predictors included demographic characteristics (age, sex, and race); clinical characteristics (body mass index, current smoking, asthma, hypertension, diabetes, coronary artery disease (prior myocardial infarction or revascularization), stroke, and peripheral vascular disease); and lipoprotein levels (LDL, HDL).
Outcome
The KCCQ was designed and validated as an instrument to capture longitudinal health status among persons with heart failure. The survey is a 23-item self-administered instrument that quantifies the importance of dyspnea, fatigue and edema on physical, social and emotional functions; the responses are combined to give an overall summary score of 0–100, with 100 representing the least burden of symptoms. Studies demonstrated that the KCCQ was sensitive to changes in health status among heart failure patients and predicted longitudinal outcomes better than serological biomarkers, such as BNP.12 In a stable population with advanced heart failure, >75 has been used to define good health status, and <75 as clinically significant symptoms.13–15 For the CRIC Heart Failure Study, we made minor modifications to the KCCQ to allow its administration to persons either with or without diagnosed heart failure; the scoring was not changed by this modification, which eliminated any reference to the participant having existing heart failure. The KCCQ was administered to participants at the Year 1 clinical visit of CRIC.
Analysis
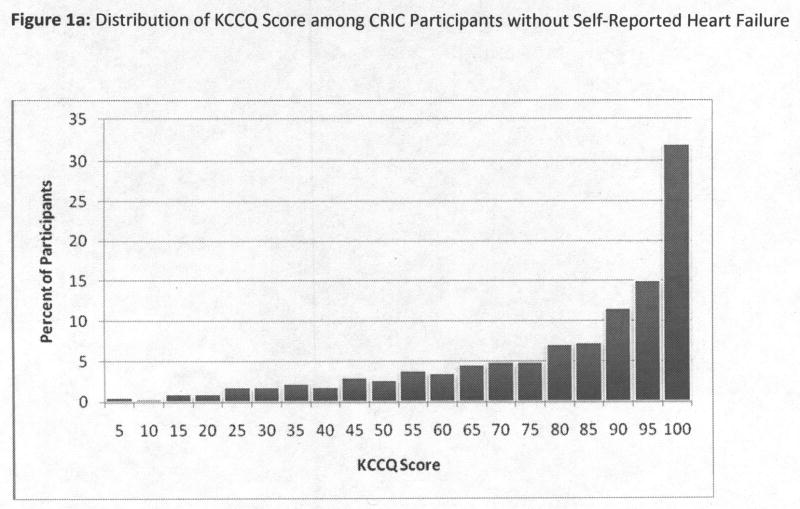
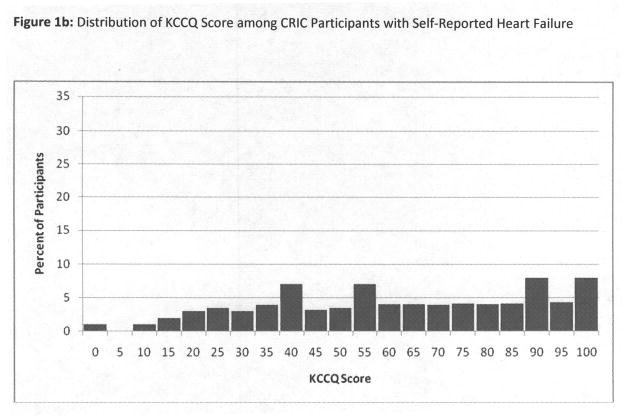
We first described the distribution of overall KCCQ scores among CRIC participants without self-report of clinical heart failure, using a frequency histogram, and found the distribution of KCCQ scores to be highly skewed left-ward (Figure 1a). We therefore chose to dichotomize the KCCQ score, and defined <75 as “clinically significant” based on prior literature.13–15 This cutpoint was also approximately the threshold for the lowest quartile in CRIC. For comparison, we also displayed the KCCQ distribution among those with self-reported heart failure (Figure 1b). However, all subsequent analyses were limited to participants without self-reported heart failure. We next tabulated characteristics among participants by KCCQ scores above or below 75, including demographics, clinical risk factors, eGFR, hemoglobin, and medication use. Tests for differences were conducted using the t-test or chi-squared test as appropriate.
Figure 1.
Figure 1a and 1b: Distribution of KCCQ Score among CRIC Participants without and with Self-Reported Heart Failure
The associations of eGFRcrea, eGFRcys and hemoglobin with overall KCCQ score were portrayed by Lowess plots, which are non-parametric approximations of splines. We also determined each predictor’s correlation with overall KCCQ score, physical limitation score, and symptom burden. We then tabulated the proportions with overall KCCQ score <75 by category of eGFR and hemoglobin. We used multivariable logistic regression models to determine whether observed associations were independent of potential confounding factors. Candidate variables included clinical sites and the covariates listed above, which were evaluated using a stepwise selection procedure with a p-value of 0.20 as the threshold for entry into the model, and 0.10 as criterion for retention. Interactions of eGFR and hemoglobin by sex and race categories were investigated using a cross-product term entered into the model, but none was significant. Because dyspnea is characteristic, but not specific, for heart failure, we conducted a sensitivity analysis in which we excluded participants most likely to have pulmonary causes of dyspnea, persons with a history or smoking. SAS version 9.1 was used for the analysis.
RESULTS
3,232 subjects completed the KCCQ at the Year 1 visit, of which 2,883 had no previously diagnosed heart failure. The mean age was 59.3±10.9, and levels of eGFRcrea, eGFRcys and hemoglobin were 42 ±15 ml/min/1.73m2, 56 ±22 ml/min/1.73m2, and 13±2 g/dL. The distribution of the KCCQ overall summary scores is depicted in Figures 1a and 1b, stratified by absence or presence of heart failure. Among participants with established heart failure, the range of KCCQ scores was much lower and spread across the range of possible scores (Figure 1b). The remainder of results are limited to participants without self-reported heart failure.
In those without heart failure, 23% reported no symptoms consistent with heart failure, KCCQ=100, and another 31% had scores between 90–99. Among the remaining participants, scores ranged across the spectrum, and 25% had KCCQ <75. Among the 2,200 in the restricted analysis (no asthma or current smoking), 21% (N=464) had KCCQ <75. The mean scores were similar for the overall summary (83±20), and for the subscores on physical limitation (81±24) and symptom burden (84±20), and quality of life (82±23).
Participants with KCCQ scores <75 were similarly aged to those with higher scores, but were more likely female and Black. Cardiovascular risk factors were associated with lower KCCQ scores, including hypertension, diabetes, higher BMI, and lower HDL levels, as were prevalent coronary artery disease, stroke, and peripheral vascular disease. Self-reported asthma and current smoking were also associated with a greater likelihood of low KCCQ scores (Table 1).
Table 1.
Baseline Characteristics of CRIC Participants without Prevalent Heart Failure by KCCQ Categories
| Characteristic | KCCQ <75 N=725 | KCCQ 75–100 N=2158 | p-value |
|---|---|---|---|
| Age | 59.6 (9.7) | 59.1 (11.3) | 0.297 |
| Female sex | 410 (56.6%) | 912 (42.3%) | <.0001 |
| Race | |||
| White | 277 (38.2%) | 1212 (56.2%) | <.0001 |
| Black | 414 (57.1%) | 824 (38.2%) | |
| Other | 34 (4.7%) | 122 (5.7%) | |
| Hypertension | 675 (93.4%) | 1856 (86%) | <.0001 |
| Diabetes | 411 (56.7%) | 825 (38.2%) | <.0001 |
| Coronary Artery Disease | 195 (26.9%) | 347 (16.1%) | <.0001 |
| Stroke | 109 (15%) | 169 (7.8%) | <.0001 |
| Peripheral Vascular Disease | 86 (11.9%) | 100 (4.6%) | <.0001 |
| Current Smoking | 137 (18.9%) | 220 (10.2%) | <.0001 |
| Asthma | 148 (20.4%) | 219 (10.1%) | <.0001 |
| Body Mass Index (BMI) (kg/m2) | |||
| <27 | 113 (15.9%) | 703 (32.7%) | <.0001 |
| 27-<30 | 97 (13.7%) | 429 (20%) | |
| 30-<35 | 159 (22.4%) | 557 (25.9%) | |
| >35 | 341 (48%) | 460 (21.4%) | |
| LDL-cholesterol (mg/dL) | 99.0 (35.5) | 100.6 (33.6) | 0.279 |
| HDL-cholesterol (mg/dL) | 47.3 (15.2) | 50.0 (16.0) | <.0001 |
| Hemoglobin (mg/dL) | 12.4 (1.8) | 13.1 (1.7) | <.0001 |
| eGFRcrea (ml/min/1.73m2) | 39.8 (15.9) | 43.1 (14.1) | <.0001 |
| eGFRcys (ml/min/1.73m2) | 49.0 (19.3) | 57.9 (22.5) | <.0001 |
LDL= low density lipoprotein
HDL= high density lipoprotein
eGFRcreat= estimated GFR by creatinine
eGFRcys= estimated GFR by cystatin C
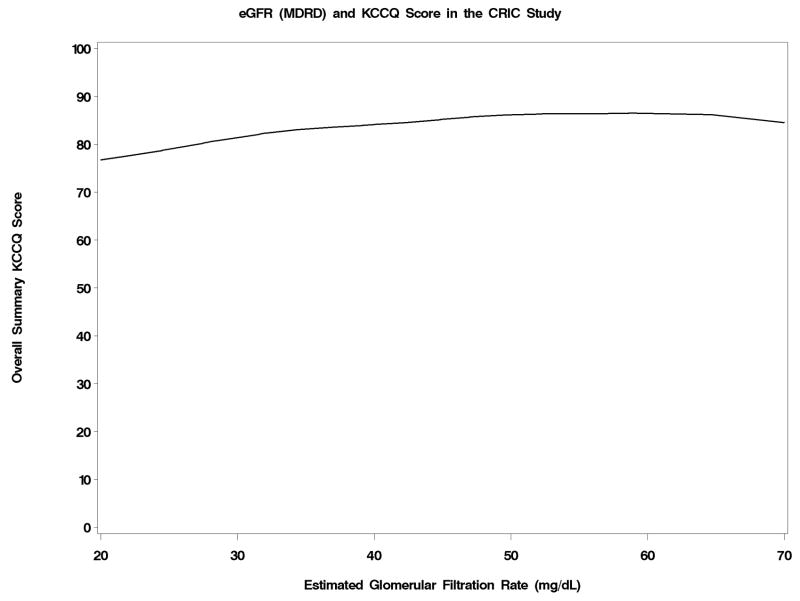
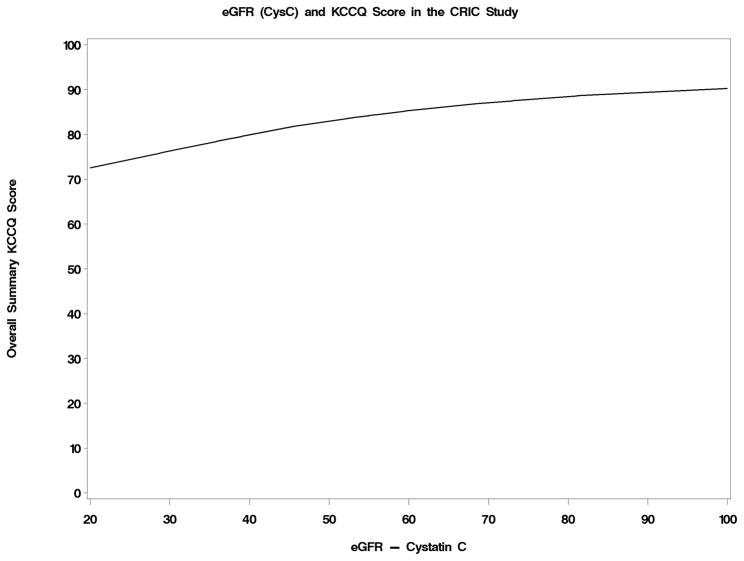
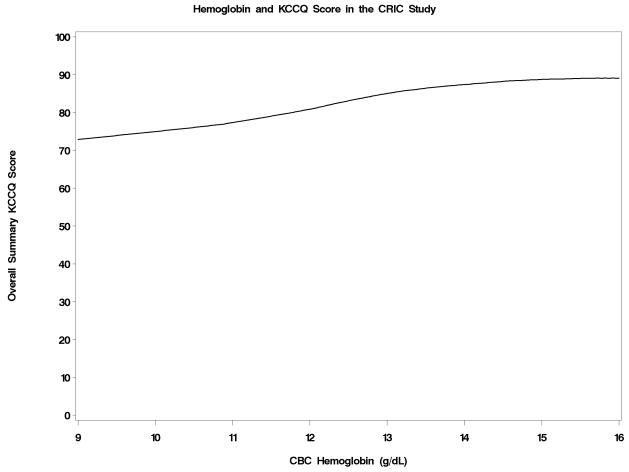
The level of the KCCQ overall score was portrayed as a function of eGFRcrea, eGFRcys and hemoglobin in Figures 2a–2c. Below an eGFRcrea of approximately 50 ml/min/1.73m2, reduced kidney function was associated with lower KCCQ score. The association of reduced kidney function and lower KCCQ score was much more linear when eGFRcys was used. Hemoglobin levels were inversely associated with KCCQ score below a level of about 15 g/dL. The correlations with the overall KCCQ score were 0.19 (p < 0.001) for eGFRcrea, 0.28 (p< 0.001) for eGFRcys, and 0.28 (p< 0.001) for hemoglobin, and were similar for each KCCQ domain.
Figure 2.
Figure 2a–2c: Joint Associations with KCCQ Overall Score of creatinine-based estimated GFR (Figure 2a), cystatin C-based estimated GFR (Figure 2b), and hemoglobin levels (Figure 2c)
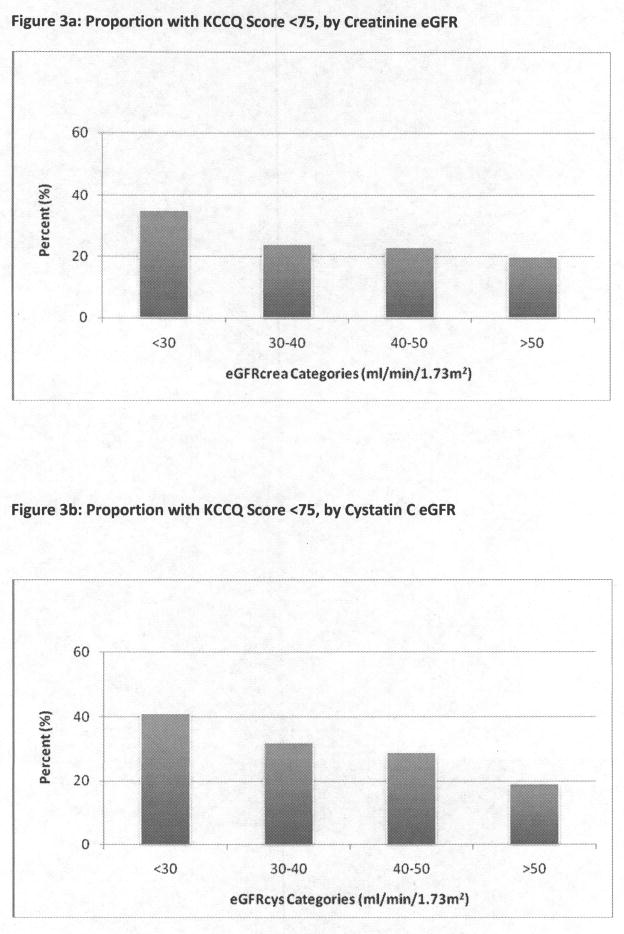
The prevalence of clinically significant symptoms (KCCQ< 75) was higher among participants with lower eGFRcrea, ranging from 20% to 35% (Figure 3a); this association appeared more incremental by eGFRcys categories (Figure 3b). After multivariate adjustment, the association of eGFRcreat ≤ 30 ml/min/1.73m2 was attenuated, but remained statistically significant. Intermediate categories of eGFRcrea were not significantly associated with heart failure-related symptoms after multivariate adjustment (Table 2), and the association of eGFRcrea ≤ 30 ml/min/1.73m2 was no longer significant in the restricted analysis. Categories of eGFRcys had stronger associations with KCCQ <75; in the multivariate analysis, eGFR < 30 ml/min/1.73m2, 30–40 ml/min/1.73m2, and 40–50 ml/min/1.73m2 were all significantly associated with the outcome.
Figure 3.
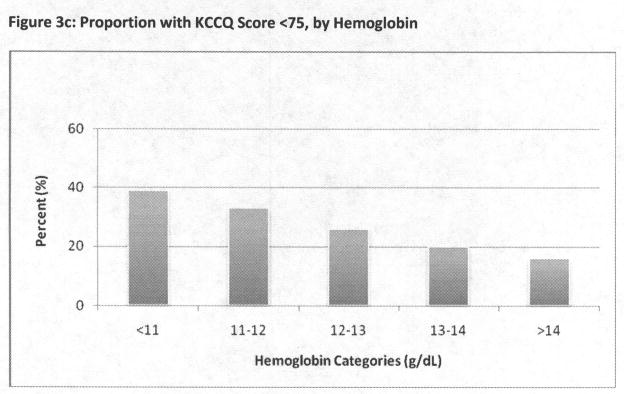
Figure 3a–3c: Proportion with KCCQ Score <75, by Categories of Creatinine eGFR (Figure 3a), cystatin C eGFR (Figure 3b), and hemoglobin (Figure 3c)
Table 2.
Associations of Estimated GFR categories with Advanced Heart Failure Symptoms in Logistic Regression Analyses
| Estimated GFR (ml/min/1.73m2) | ||||
|---|---|---|---|---|
| >50 | 40–50 | 30–40 | <30 | |
|
Creatinine | ||||
| Unadjusted OR | 1.0 (ref) | 1.18 (0.93–1.50) | 1.27 (1.00–1.63) | 2.10 (1.66–2.65) |
| Adjusted OR1 | 1.0 (ref) | 1.05 (0.80–1.38) | 1.03 (0.77–1.37) | 1.53 (1.14–2.05) |
| Adjusted OR2 | 1.0 (ref) | 1.09 (0.78– 1.51) | 1.08 (0.76– 1.53) | 1.37 (0.96–1.97) |
|
Cystatin C | ||||
| Unadjusted OR | 1.0 (ref) | 1.55 (1.23–1.95) | 2.08 (1.66–2.60) | 2.64 (2.05–3.40) |
| Adjusted OR | 1.0 (ref) | 1.38 (1.06–1.78) | 1.39 (1.09–1.82) | 2.15 (1.54–3.00) |
| Adjusted OR2 | 1.0 (ref) | 1.54 (1.13–2.11) | 1.56 (1.12–2.18) | 2.09 (1.38– 3.15) |
adjusted for sex, race, current smoking status, coronary artery disease, diabetes, asthma, peripheral vascular disease, hemoglobin, BMI and clinical site
excludes persons with history of asthma or current smoking
Hemoglobin levels also had strong associations with heart failure-related symptoms, with a more than doubling in prevalence from higher to lower hemoglobin levels (Figure 3c). Although largely attenuated by multivariate adjustment, hemoglobin levels below 13 g/dL remained independently associated KCCQ <75 (Table 3). This finding was not attenuated by the restricted analysis.
Table 3.
Associations of Hemoglobin categories with Advanced Heart Failure Symptoms in Logistic Regression Analyses
| Hemoglobin (g/dL) | |||||
|---|---|---|---|---|---|
| >14 | 13–14 | 12–13 | 11–12 | <11 | |
| Unadjusted OR | 1.0 (ref) | 1.35 (1.02–1.79) | 1.96 (1.50–2.55) | 2.70 (2.05–3.54) | 3.43 (2.58–4.56) |
| Adjusted OR1 | 1.0 (ref) | 1.03 (0.76–1.40) | 1.41 (1.04–1.91) | 1.56 (1.12–2.16) | 1.65 (1.15–2.37) |
| Adjusted OR2 | 1.0 (ref) | 0.97 (0.65–1.43) | 1.67 (1.15–2.43) | 1.52 (1.01–2.31) | 1.84 (1.18–2.87) |
adjusted for sex, race, current smoking status, coronary artery disease, diabetes, asthma, peripheral vascular disease, hemoglobin, BMI and clinical site
excludes persons with history of asthma or current smoking
In the full multivariate model, Black race was associated with KCCQ <75 (Table 4). Higher BMI categories had the strongest associations with symptoms characteristic of heart failure, particularly for persons with BMI>35. Diabetes, coronary artery disease, and peripheral vascular disease were each independently associated with the outcome, as were current tobacco smoking and asthma. When participants who reported smoking or asthma were excluded, the other covariates were strengthened, particularly the BMI categories.
Table 4.
Characteristics independently associated with advanced heart failure-related symptoms among persons with CKD
| Unadjusted OR | Adjusted OR1 | Adjusted OR2 | |
|---|---|---|---|
| Female sex | 1.78(1.50– 2.11) | 1.45 (1.17–1.79) | 1.57 (1.21– 2.04) |
| Race | |||
| White | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Black | 2.20 (1.84–2.62) | 1.42 (1.15–1.76) | 1.61 (1.25–2.09) |
| Other | 1.22 (0.82–1.82) | 1.53 (0.97–2.43) | 1.82 (1.07– 3.11) |
| BMI | |||
| <27 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 27- <30 | 1.41 (1.05– 1.89) | 1.52 (1.10– 2.10) | 1.69 (1.10– 2.59) |
| 30- <35 | 1.78 (1.36– 2.32) | 1.78 (1.33– 2.38) | 2.44 (1.67– 3.55) |
| >35 | 4.61 (3.62– 5.88) | 3.99 (3.03– 5.27) | 5.56 (3.89– 7.96) |
| Current smoker | 2.05 (1.63– 2.59) | 2.25 (1.71– 2.95) | N/A |
| Asthma | 2.27 (1.81– 2.85) | 2.02 (1.55– 2.64) | N/A |
| Coronary Artery Disease | 1.92 (1.57– 2.35) | 1.77 (1.41– 2.23) | 1.84 (1.39– 2.43) |
| Diabetes | 2.11 (1.78– 2.51) | 1.35 (1.10– 1.66) | 1.36 (1.06– 1.75) |
| Peripheral Vascular Disease | 2.77 (2.05– 3.75) | 2.29 (1.61– 3.26) | 2.98 (1.96– 4.53) |
adjusted for sex, race, current smoking status, coronary artery disease, diabetes, asthma, peripheral vascular disease, hemoglobin, BMI and clinical site
excludes persons with history of asthma or current smoking
DISCUSSION
Among CRIC participants with moderate to severe CKD, we assessed symptoms characteristic of heart failure - dyspnea, fatigue, and edema - among those who did not report a prior diagnosis of heart failure. To assess these symptoms, we used the KCCQ, a well-established instrument for monitoring the burden of heart failure-related quality of life, but we modified it to make the instrument applicable to persons without a heart failure diagnosis. The most striking finding of this study was the high prevalence of symptoms characteristic of heart failure, which remained even after excluding persons with asthma or current smoking. Over one-fourth of the cohort had a KCCQ score below 75, which is a threshold that has been used in clinical trials of established heart failure to denote at least a moderate burden of symptoms. Lower levels of eGFRcys and hemoglobin were independently associated with a higher likelihood of clinically significant symptoms, as were obesity, diabetes, and prevalent cardiovascular disease. During follow-up in CRIC, we will evaluate the extent to which the KCCQ score portends future diagnosis and hospitalization for heart failure.
CKD and heart failure commonly co-exist as each condition is a risk factor for the other. Among the adverse cardiovascular disease outcomes linked with CKD, the strongest association is consistently with heart failure.16 CKD complicates the management of heart failure, and is among the strongest predictors of adverse outcomes and mortality in acute decompensated or chronic heart failure.17 This challenging clinical scenario of co-existing CKD and heart failure has been labeled the “cardio-renal syndrome.”18 Despite general recognition that heart failure is a major complication of CKD, no systematic approach has been developed for detecting the clinical syndrome of heart failure in CKD patients. Because we suspected that heart failure symptoms would be common in CKD patients, we designed and implemented this survey assessment in CRIC.
To our knowledge, this is the first study designed to evaluate the prevalence of symptoms characteristic of heart failure and their clinical correlates in persons with CKD without known heart failure. Prior studies have found CKD to be associated with reduced general health status and health-related quality of life19, but have not specifically evaluated the characteristic symptoms of heart failure. Our rationale for applying a heart failure instrument to a CKD population was that heart failure often develops insidiously, particularly in persons with other chronic diseases like CKD, diabetes, obesity, and cardiovascular disease. Based upon the low KCCQ scores observed in our study and the known high incidence of clinical heart failure in CKD patients, a targeted screening for symptoms of heart failure may be warranted in clinical practice. We believe that these symptoms indicate the presence of early heart failure in many of our participants.
The challenge, however, in interpreting our results is that we cannot be certain whether the substantial burden of symptoms reported in this study is attributable to undiagnosed or impending heart failure, to the participants’ underlying CKD, or to another chronic condition. Although fluid retention and fatigue are common in persons with CKD, dyspnea is not a hallmark symptom; in fact, half of CRIC participants reported minimal if any symptom burden despite having advanced CKD. In our study, most of the risk factors for low KCCQ scores are known risk factors for clinical heart failure. As we hypothesized, both lower eGFR and hemoglobin levels had independent associations, as did obesity, diabetes, and prevalent cardiovascular disease conditions. On the other hand, the associations of smoking and asthma with low KCCQ scores are likely attributable to their direct effects on dyspnea, rather than an association with heart failure risk. For that reason, we validated our findings after excluding persons with smoking or asthma. A major objective of CRIC follow-up will be to determine the strengths of association for each of these risk factors with the development of clinical heart failure.
A major strength of this study is the application of a novel symptom assessment tool to a large, multi-center, well-characterized and diverse cohort of persons with established CKD. This study does have important limitations that should influence the interpretation of our findings. Most importantly, as mentioned above, the modified KCCQ instrument assesses symptoms that are characteristic, but not specific, to heart failure. Only after longitudinal follow-up will we know the extent to which the low KCCQ scores are a harbinger of risk for clinical heart failure. A second limitation is that prevalent heart failure was assessed by self-report, so some participants may have been inappropriately classified as not having heart failure. The focus of this analysis was on symptoms of heart failure. A third limitation is that our study did not have serological predictors of heart failure, such as BNP; however, BNP is not an established screening test for heart failure in this population. In future years, we hope to compare the strengths of association among the KCCQ scores and BNP levels as predictors of incident heart failure. Our hypothesis is that the KCCQ score will be at least as strong as BNP, and likely stronger.
In conclusion, we observed a remarkable burden of symptoms characteristic of heart failure in a CKD cohort without prior diagnosis of heart failure. Several risk factors for heart failure were also associated with a greater likelihood of advanced heart failure symptoms, including lower eGFR and hemoglobin, obesity, diabetes, and prevalent cardiovascular disease. Future studies will evaluate the KCCQ as a predictor of future heart failure risk in CRIC. If validated as an indicator of early heart failure symptoms, the KCCQ could be a useful clinical tool to monitor the risk and progression of heart failure in persons with CKD.
Acknowledgments
Funding Sources
This project was supported by M.S.’s R01 DK066488 award (principal investigator M.S.). In addition, we would like to acknowledge the CRIC GCRC and CTSA awards: University of Pennsylvania (UL1 RR-024134), Johns Hopkins University (UL1 RR-025005), University of Maryland (M01 RR-16500), Case Western Reserve University (UL1 RR-024989), University of Michigan (M01 RR-000042, UL1 RR-024986), and University of Illinois at Chicago (UL1 RR-029879).
Footnotes
Disclosures:
None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 2.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, et al. Predictors of heart failure among women with coronary disease. Circulation. 2004;110(11):1424–1430. doi: 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, et al. Cystatin-C as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Kritchevsky SB, et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166(13):1396–1402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 7.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 9.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 13.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 14.Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, et al. Patient health status and costs in heart failure: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) Circulation. 2009;119(3):398–407. doi: 10.1161/CIRCULATIONAHA.108.820472. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41(8):1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003;138(11):917–924. doi: 10.7326/0003-4819-138-11-200306030-00013. [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation. 2004;110(12):1514–1517. doi: 10.1161/01.CIR.0000143547.55093.17. [DOI] [PubMed] [Google Scholar]
- 19.Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1997;29(6):888–896. doi: 10.1016/s0272-6386(97)90463-7. [DOI] [PubMed] [Google Scholar]