Abstract
Obstructive sleep apnea (OSA) is recurrent obstruction of the upper airway leading to sleep fragmentation and intermittent hypoxia (IH) during sleep. There is growing evidence from animal models of OSA that IH is independently associated with metabolic dysfunction, including dyslipidemia and insulin resistance. The precise mechanisms by which IH induces metabolic disturbances are not fully understood. Over the last decade, several groups of investigators developed a rodent model of IH, which emulates the oxyhemoglobin profile in human OSA. In the mouse model, IH induces dyslipidemia, insulin resistance and pancreatic endocrine dysfunction, similar to those observed in human OSA. Recent reports provided new insights in possible mechanisms by which IH affects lipid and glucose metabolism. IH may induce dyslipidemia by up-regulating lipid biosynthesis in the liver, increasing adipose tissue lipolysis with subsequent free fatty acid flux to the liver, and inhibiting lipoprotein clearance. IH may affect glucose metabolism by inducing sympathetic activation, increasing systemic inflammation, increasing counter-regulatory hormones and fatty acids, and causing direct pancreatic beta cell injury. IH models of OSA have improved our understanding of the metabolic impact of OSA, but further studies are needed before we can translate recent basic research findings to clinical practice.
Keywords: Intermittent hypoxia, obstructive sleep apnea, metabolic syndrome, insulin resistance, dyslipidemia
Introduction
Obstructive sleep apnea (OSA) describes recurrent collapse of the upper airway during sleep.1 OSA is a common disorder affecting 4–24% of men and 2–9% of women in the US,2 but the prevalence of OSA in obese individuals exceeds 50%.3 OSA is particularly prevalent in individuals with central (visceral) obesity. Emerging evidence suggests that OSA leads to high cardiovascular mortality and morbidity.4–6 The cardiovascular risk imposed by OSA may be mediated through effects of OSA on glucose and lipid metabolism.
Several studies have shown that the impact of OSA on metabolic function is acutely or chronically reversible with continuous positive airway pressure (CPAP).7–8 The respiratory events associated with OSA lead to changes in intrathoracic pressure, hypercapnea, arousals from sleep, and intermittent hypoxia (IH). IH is the best studied aspect of OSA in terms of metabolic effects. A number of animal and human studies demonstrated that IH causes disturbances in lipid and glucose metabolism.9,10 In the present review, we will discuss experimental models of IH and effects of IH on metabolic function.
Models of Intermittent Hypoxia
Animal models of sleep disordered breathing were extensively reviewed elsewhere.9–10 IH models are the most commonly used research models of OSA. IH has been predominantly employed in rodents. IH is administered by cyclic delivery of nitrogen, oxygen and air to a sealed chamber. There are two different types of rodent models of IH. The first type is sleep-dependent IH.11 In this model, IH is delivered exclusively during sleep. Sleep-dependent IH requires implantation of EEG and EMG electrodes in mice and sophisticated monitoring techniques resulting in low throughput. The second type of IH system delivers the stimulus, regardless of sleep/wake state. However, investigators usually attempt to deliver the stimulus during the light phase, when rodent sleep primarily occurs. In the subsequent narrative we will discuss the latter sleep-independent IH model. IH models vary in both frequency and severity of the hypoxic stimulus. Protocols deliver "apneas" varying in frequency from 9 to 60 times/hr and varying in severity with the delivered oxygen nadir ranging from 3 to 10%.12–16 The main advantage of sleep-independent IH is high throughput. Therefore, this approach has been exclusively employed to study metabolic outcomes of IH and OSA. In our experiments we exposed mice to IH with a FiO2 nadir of 5% during the 12 hr light phase (9am – 9pm). According to our data, this regimen resulted in the mean PaO2 of 51.7 mm Hg18 and oxyhemoglobin saturation fluctuating between 99% and ~ 70%,19 which is similar to the oxyhemoglobin profile in severe OSA. In the following discussion, we will refer to this regimen, unless specified otherwise.
Finally, IH has recently been used in human experiments to study glucose metabolism in healthy subjects.19 In this study, volunteers were exposed to hypoxic gas mixtures that induced decreases in arterial oxygen saturation (SaO2) to 85% around 25 times/hr.19
Intermittent Hypoxia and Lipid Metabolism
We have recently reviewed relationships between OSA and dyslipidemia.20 Several cross-sectional studies suggest that OSA is independently associated with increased levels of total cholesterol, LDL and triglycerides, whereas others report no such relationships.21–24 Several studies show that OSA treatment with CPAP may have a beneficial effect on lipid profile.8,25,26 However, the majority of the studies were not specifically designed to evaluate the lipid profile, ignoring important confounding factors such as diet, physical activity and body composition.20 There are no long-term studies with follow-up beyond 6 months of CPAP treatment. Therefore, the clinical data are unclear.
Results from IH animal studies unambiguously show that IH is a direct cause of hyperlipidemia. IH causes increases in total cholesterol, HDL-C and triglycerides after 5 days, while an increase in LDL cholesterol is evident after 4 weeks.27,28 The severity of increases in lipids was proportional to the severity of the hypoxic stimulus.28 In C57BL/6J mice on a high cholesterol diet, exposure to IH for 12 wks predominantly increases VLDL and LDL.29 Similar changes in response to IH occurred in atherosclerosis-prone apolipoprotein E deficient mice.18
Mechanisms of dyslipidemia induced by IH
Up regulation of lipid biosynthesis
Early in our observations of IH-induced hyperlipidemia, we reported up-regulation of a key hepatic transcription factor of lipid biosynthesis, sterol regulatory element binding protein 1c (SREBP-1c) and a SREBP-1c -regulated enzyme, stearoyl coenzyme A desaturase 1 (SCD-1).27–30 These findings are in agreement with other studies of acute hypoxia.31 SCD-1 converts saturated fatty acids into monounsaturated fatty acids. Abundance of monounsaturated fatty acids increases the biosynthesis of cholesterol esters and triglycerides, which are incorporated into secreted VLDL particles.32,33 Indeed, we have shown that IH up-regulates lipoprotein secretion.28 Interruption of SREBP-1 signaling in transgenic mice and depletion of SCD-1 with anti-sense oligonucleotides in C57BL/6J mice prevented hyperlipidemia during IH.30,34 The increases in SCD-1 may be mediated through hypoxia inducible factor 1 (HIF-1), a master-regulator of metabolic responses to hypoxia.35 Mice with partial deficiency of functional HIF-1 are partially protected against hypertriglyceridemia and hepatic lipid accumulation during IH36 and show attenuated increases in the active nuclear isoform of SREBP-1 and SCD-1. Thus, dyslipidemia of IH may be a consequence of IH-induced up-regulation of lipid biosynthetic pathways in the liver.
Lipolysis
Under most circumstances, in the post-absorptive state, triglycerides are derived from the re-esterification of hepatic free fatty acids (FFA). Most FFA are derived from peripheral lipolysis, but in the absorptive state, can also be synthesized from carbohydrate "de novo" in the liver.37 Simultaneously, a tightly controlled cholesterol synthesis pathway manufactures lipoproteins that are used by the liver to export triglycerides in the form of VLDL. As previously discussed, SCD-1 is involved in the conversion of saturated fatty acids to monounsaturated fatty acids for triglyceride synthesis and VLDL export. VLDL particles enter the bloodstream and undergo hydrolysis by tissue enzymes including lipoprotein lipase (LpL) to liberate FFA (see lipoprotein clearance section). We have previously shown that IH increases hepatic triglyceride and hepatic VLDL secretion, but does not cause an increase in de novo fatty acid synthesis.27 These data suggest that peripheral lipolysis is supplying hepatic fatty acids for the synthesis of triglycerides and VLDL. Our experiments in apolipoprotein E-deficient mice have shown increases in serum FFA levels suggesting increased adipose tissue lipolysis during IH.18 IH activates the sympathetic nervous system,9–10 which could potentially induce lipolysis.38–40 In addition, more FFA may be available for triglycerides and cholesterol ester synthesis if FFA do not undergo beta-oxidation, the oxygen-dependent mitochondrial combustion of fatty acids.38–40 The confluence of increased FFA delivery and impaired beta oxidation may also underlie the association between OSA and accumulation of fat in the liver, liver injury, oxidative stress, and non-alcoholic steatohepatitis.41–48 Chronic IH resulted in liver injury and mild elevation of liver enzymes in lean mice.49 In mice fed a high cholesterol high fat diet, IH converted hepatic steatosis to steatohepatitis, increasing pro- inflammatory cytokines TNF-α, IL-1β, IL-6 and MIP-2 in liver tissue, lobular inflammation and fibrosis.17 Thus, IH may lead to dyslipidemia and steatohepatitis due to increased FFA flux from adipose tissue to the liver but additional studies are needed to explore this mechanism and its relevance for human disease.
Lipoprotein clearance
Another putative mechanism of dyslipidemia during IH is inhibited VLDL clearance. Mice exposed to IH show higher peak levels and prolonged decay of plasma chylomicrons (CM) after a fatty meal (Drager, Polotsky, Submitted). Triglyceride-rich lipoproteins, CM and VLDL, disproportionally elevated in IH are cleared from the circulation in a multi-step process, which begins with hydrolysis of triglycerides by LpL. LpL is a key enzyme in plasma lipoprotein metabolism that is preferentially expressed in adipose tissue, skeletal muscle and the heart.50,51 LpL is synthesized in adipocytes, myocytes and macrophages, secreted and transported to the luminal surface of blood vessels, where it is anchored by heparan sulfate proteoglycans and glycosylphosphatidylinositol-anchored HDL- binding protein 1.52 Our unpublished data show that IH selectively inhibits LpL in adipose tissue, but the mechanisms of this phenomenon are unknown.
In summary, IH may disrupt lipid metabolism by increasing adipose tissue lipolysis and FFA flux to the liver, up-regulating hepatic triglyceride biosynthesis and lipoprotein secretion, and suppressing lipoprotein clearance (Figure 1).
Figure 1.
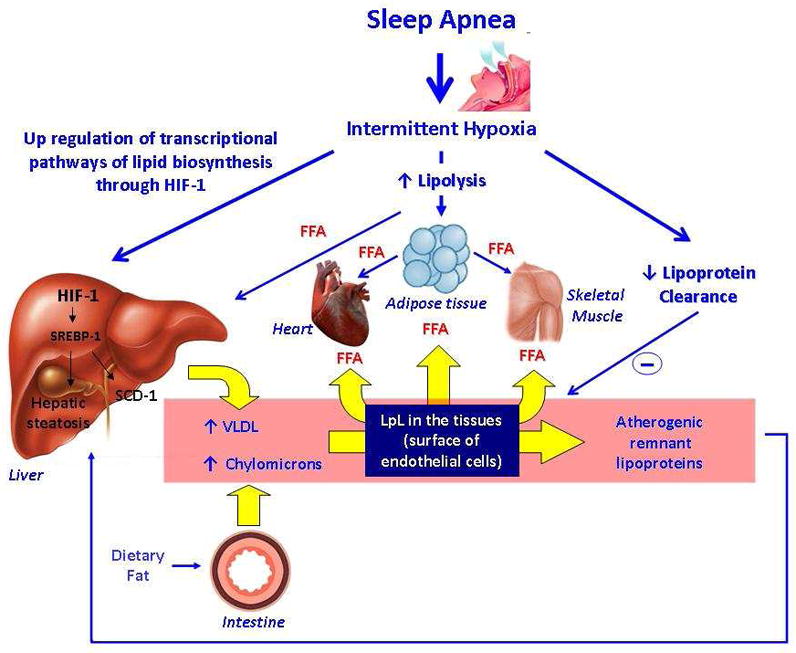
Main pathways by which intermittent hypoxia induces dysregulation of lipid metabolism.
Intermittent hypoxia up-regulates HIF-1 in the liver, which activates SREBP-1 and SCD-1. As a result of these processes, intermittent hypoxia leads to hepatic steatosis and an increase in lipoprotein secretion. Intermittent Hypoxia increases adipose tissue lipolysis increasing FFA flux to the liver. Intermittent hypoxia also inhibits lipoprotein clearance. The increase in lipoprotein secretion and inhibition of lipoprotein clearance lead to dyslipidemia with a rise in VLDL.
FFA: Free Fatty Acids.
HIF-1: Hypoxia inducible factor 1.
SREBP1: Sterol regulatory element binding protein 1.
SCD-1: Stearoyl coenzyme A desaturase 1.
Intermittent hypoxia and Glucose Metabolism
OSA is associated with increased prevalence of type 2 diabetes53 and has recently been shown to be a risk factor for incident diabetes.54 In non-diabetics, OSA is associated with insulin resistance in proportion to the degree of nocturnal hypoxemia.47,55–57 CPAP can reverse the insulin resistance of OSA both acutely (within 2 days) and chronically (after 4 months).7,58 In patients with type 2 diabetes, OSA may worsen glycemic control,59 which improves after CPAP.60,61 We will focus on the mechanisms that might explain these observations, drawing from IH experiments.
IH induces acute insulin resistance. In lean C57BL/6J mice, IH (60 cycles/hr with an FiO2 nadir 5–6%, 9 hr) resulted in a significant increase in insulin resistance measured by hyperinsulinemic euglycemic clamp.62 The insulin resistance rapidly normalized after cessation of each diurnal IH exposure.63 Chronic IH greatly exacerbates insulin resistance and glucose intolerance in obese leptin deficient ob/ob mice64 and in C57BL/6J mice with diet induced obesity (Polotsky, unpublished data). Recently, healthy human volunteers were exposed to IH simulating moderate OSA (at an average rate of 24.3 events/h).19 Alveolar hypoxia was established by inspiring hypoxic N2 –O2 gas mixture until the oxyhemoglobin saturation dropped to 85%.19 After 5 hours, an intravenous glucose tolerance test demonstrated a decrease in both insulin sensitivity and glucose effectiveness by minimal modeling methods. Moreover, IH did not induce an appropriate increase in insulin secretion, demonstrating inadequate pancreatic responses to insulin resistance.
There are several potential mechanisms of insulin resistance and impaired insulin secretion during IH. First, activation of hepatic lipid biosynthesis discussed in the previous section may lead to hepatic insulin resistance. Second, IH activates the sympathetic nervous system, which is a potent stimulator of lipolysis. FFAs reduce insulin-mediated whole body glucose uptake within hours due to interruption of insulin signaling in skeletal muscle.65,66 In addition, catecholamines directly stimulate the mobilization of glycogen and inhibit glucose uptake from muscle, stimulate secretion of glucagon, inhibit secretion of insulin, and increase gluconeogenesis in the liver.9 Up-regulation of pro-inflammatory pathways by cellular hypoxia may also lead to insulin resistance.67,68 IH also activates the hypothalamic-pituitary-adrenal axis.63 The resulting release of corticosteroids have well-defined effects leading to insulin resistance,69 including an increase in lipolysis, inhibition of insulin-dependent translocation of glucose transporter type 4 (Glut4) to the cell surface in the muscle, suppression of glycogen synthesis and an increase in gluconeogenesis. Steroid hormones also inhibit insulin secretion.70 IH may also lead to insulin resistance altering production of adipokines, hormones produced in adipose tissue. IH increases leptin gene expression and circulating protein levels levels. Leptin acts both centrally and peripherally, to inhibits insulin secretion while increasing glucose uptake.71–77
The development of insulin resistance during IH despite high leptin levels observed in OSA patients78–80 suggests a currently inexplicable phenomenon of leptin resistance.81 Finally, IH may suppress secretion of adiponectin, an insulin-sensitizing hormone.82
Diabetes is the final manifestation of insulin resistance and a failure of compensatory pancreatic beta cell insulin secretion. Experimental evidence on the effect of IH on pancreatic β-cells is scant and presented only in two publications. Immunohistochemistry of pancreatic islets showed that short-term IH increases both apoptosis and proliferation of β-cells.63,83 Xu et al. have shown that overexpression of antioxidant enzyme superoxide dismutase in pancreatic islets protected them against IH-induced apoptosis, whereas IH-induced β-cell proliferation was not affected.83 The net effect of IH exposure was increased turnover83 or increased replication of pancreatic β-cells. In contrast, human data in healthy volunteers suggest that IH may impair pancreatic endocrine function.19 Thus, IH induces insulin resistance and may have a detrimental effect on pancreatic endocrine function.
Limitations and Future Directions
OSA is associated with dysregulation of lipid and glucose metabolism, but studying of these phenomena in patients with OSA has been challenging due to confounding effects of obesity. Animal and human work has determined that IH has an impact on metabolism that parallel findings in patients with OSA. However, mechanisms of metabolic effects of IH are still poorly understood. Potential mechanisms include tissue hypoxia/reoxygenation, systemic catecholamine-mediated lipolysis and lipotoxicity, hepatic transcriptional upregulation of lipid synthesis, impaired lipid clearance, systemic inflammation and disruption of hypothalamic-pituitary-adrenal and adipokine hormonal balance. Future clinical, basic and translational studies are warranted to determine effects of IH and OSA on metabolic function and identify the mechanisms.
Practice Points.
Evidence from animal models of obstructive sleep apnea (OSA) suggests that intermittent hypoxia (IH) causes dyslipidemia and insulin resistance.
IH-induced dyslipidemia may be due to excessive adipose tissue lipolysis and FFA flux to the liver, up-regulation of hepatic triglyceride biosynthesis and lipoprotein secretion, and suppression of lipoprotein clearance.
OSA is associated with increased prevalence of type 2 diabetes and has recently been shown to be a risk factor for incident diabetes.
In non-diabetics, OSA is associated with insulin resistance in proportion to the degree of nocturnal hypoxemia.
In patients with type 2 diabetes, OSA may worsen glycemic control, which improves after continuous positive airway pressure (CPAP).
IH induces insulin resistance by several mechanisms including up-regulation of hepatic lipid biosynthesis, lipolysis, activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis and systemic inflammation.
Acknowledgments
Sources and Funding
Luciano F. Drager and Jonathan Jun are Post-Doctoral Fellow at Johns Hopkins University. Dr. Drager is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 200032/2009-7) and Fundação Zerbini, Brazil. Dr.Jun is supported by the National Sleep Foundation/American Lung Association Pickwick Grant (SF-78568 N) and NIH T32 training grant (HL07534).
Vsevolod Y. Polotsky is supported by NIH (R01 HL80105, 5P50HL084945) and the United States Israel Binational Science Foundation (grant BSF No. 2005265). .
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1:167–186. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4*.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 7.Harsch IA, Schahin SP, Bruckner K, Radespiel-Troger M, Fuchs FS, Hahn EG, Konturek PC, Lohmann T, Ficker JH. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71(3):252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 8.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134(4):686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 9.Jun J, Polotsky VY. SLEEP DISORDERED BREATHING AND METABOLIC EFFECTS: EVIDENCE FROM ANIMAL MODELS. Sleep Med Clin. 2007;2(2):263–277. doi: 10.1016/j.jsmc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Jun J, Polotsky VY. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50(3):289–306. doi: 10.1093/ilar.50.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol. 2001;91(6):2758–66. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19(6 Pt 1):555–61. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 13.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7(1):7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Veasey SC, Zhan G, Fenik P, Pratico D. Long-term intermittent hypoxia: reduced excitatory hypoglossal nerve output. Am J Respir Crit Care Med. 2004;170(6):665–72. doi: 10.1164/rccm.200403-261OC. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100(17):10073–8. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G871–7. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 18.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209(2):381–6. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106(5):1538–44. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):161–5. doi: 10.1097/MED.0b013e3283373624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 22.Tsioufis C, Thomopoulos K, Dimitriadis K, Amfilochiou A, Tousoulis D, Alchanatis M, Stefanadis C, Kallikazaros I. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007;25(1):141–6. doi: 10.1097/HJH.0b013e32801092c1. [DOI] [PubMed] [Google Scholar]
- 23.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early Signs of Atherosclerosis in Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Bortolotto LA, Maki-Nunes C, Trombetta IC, Alves MJ, Fraga RF, Negrão CE, Krieger EM, Lorenzi-Filho G. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208:490–5. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Tokuda F, Sando Y, Matsui H, Koike H, Yokoyama T. Serum levels of adipocytokines, adiponectin and leptin, in patients with obstructive sleep apnea syndrome. Intern Med. 2008;47:1843–9. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 26.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59(9):777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Savransky V, Nanayakkara A, Smith PL, O'donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102(2):557–63. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 29*.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic Intermittent Hypoxia Induces Atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics. 2007;31(2):273–80. doi: 10.1152/physiolgenomics.00082.2007. [DOI] [PubMed] [Google Scholar]
- 31.Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 2009;118(6):401–10. doi: 10.1042/CS20090313. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;29;275(39):30132–8. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 33.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43(2):91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 34*.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103(10):1173–80. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13(2):167–71. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics. 2006;25(3):450–7. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadian M, Duncan RE, Sul HS. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol Metab. 2009;20(9):424–8. doi: 10.1016/j.tem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G1–4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48(5):275–97. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50(1):3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–1296. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 42.Jouët P, Sabaté JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, Coffin B. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17(4):478–85. doi: 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 43.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41(10):918–21. doi: 10.1097/01.mcg.0000225692.62121.55. [DOI] [PubMed] [Google Scholar]
- 44.Norman D, Bardwell WA, Arosemena F, Nelesen R, Mills PJ, Loredo JS, Lavine JE, Dimsdale JE. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep. 2008;31(1):121–6. doi: 10.1093/sleep/31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra P, Nugent C, Afendy A, Bai C, Bhatia P, Afendy M, Fang Y, Elariny H, Goodman Z, Younossi ZM. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28:1080–1086. doi: 10.1111/j.1478-3231.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 46.Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1274–81. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS, Schwartz AR. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179(3):228–34. doi: 10.1164/rccm.200804-608OC. Erratum in: Am J Respir Crit Care Med. 2009;180(9):910–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulitsky A, Ananthakrishnan AN, Komorowski R, Wallace J, Surapaneni SN, Franco J, Saeian K, Gawrieh S. A Noninvasive Clinical Scoring Model Predicts Risk of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Obes Surg. 2010 Mar 25; doi: 10.1007/s11695-010-0118-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45(4):1007–13. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 50.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 51.Pulawa LK, Jensen DR, Coates A, Eckel RH. Reduction of plasma triglycerides in apolipoprotein C-II transgenic mice overexpressing lipoprotein lipase in muscle. J Lipid Res. 2007;48(1):145–51. doi: 10.1194/jlr.M600384-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Ginsberg HN. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 2002;106:2137–42. doi: 10.1161/01.cir.0000035280.64322.31. [DOI] [PubMed] [Google Scholar]
- 53.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122(12):1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 56.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 57.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179(3):235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79(6):1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 59.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4(6):538–42. [PMC free article] [PubMed] [Google Scholar]
- 61.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 62.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175(8):851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008;586:899–911. doi: 10.1113/jphysiol.2007.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552(Pt 1):253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10(2):142–8. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 66.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98(13):7522–7. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001 Aug 31;293:1673–7. doi: 10.1126/science.1061620. Erratum in: Science 2002 Jan 11;295:277. [DOI] [PubMed] [Google Scholar]
- 69.Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010;316(2):154–64. doi: 10.1016/j.mce.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 70.Rosmond R. Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes? Med Sci Monit. 2003;9(2):RA35–9. [PubMed] [Google Scholar]
- 71.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98(5):1101–6. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest. 1997;100(11):2729–36. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci U S A. 1999;96(2):674–9. doi: 10.1073/pnas.96.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG. Effects of leptin on insulin sensitivity in normal rats. Endocrinology. 1997;138(8):3395–401. doi: 10.1210/endo.138.8.5327. [DOI] [PubMed] [Google Scholar]
- 76.Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100(12):3105–10. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kojima S, Asakawa A, Amitani H, Sakoguchi T, Ueno N, Inui A, Kalra SP. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides. 2009;30(5):962–6. doi: 10.1016/j.peptides.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118(3):580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 79.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakamura T, Nakao K, Ohi M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100(7):706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 80.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–7. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 81.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010 Feb 23; doi: 10.1016/j.tem.2010.01.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes. 2009;117(3):129–34. doi: 10.1055/s-2008-1078738. [DOI] [PubMed] [Google Scholar]
- 83.Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46(6):783–90. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]


